Evaporation of Promising Fire Extinguishing Agent Droplets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Evaporation of Fire Extinguishing Agent Droplets
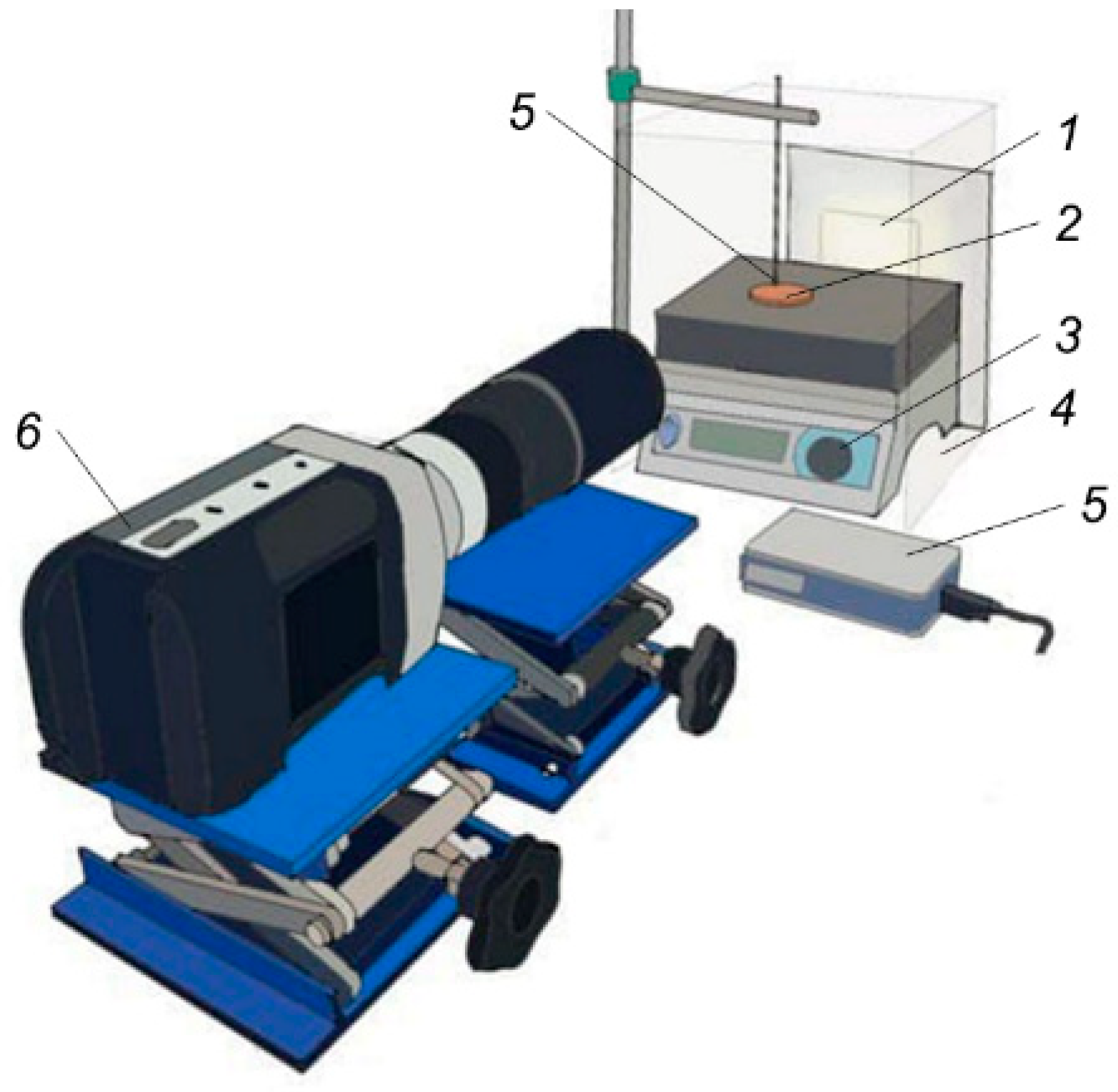
2.2.1. Evaporation of Fire Extinguishing Agent Droplets Using Conductive Heating
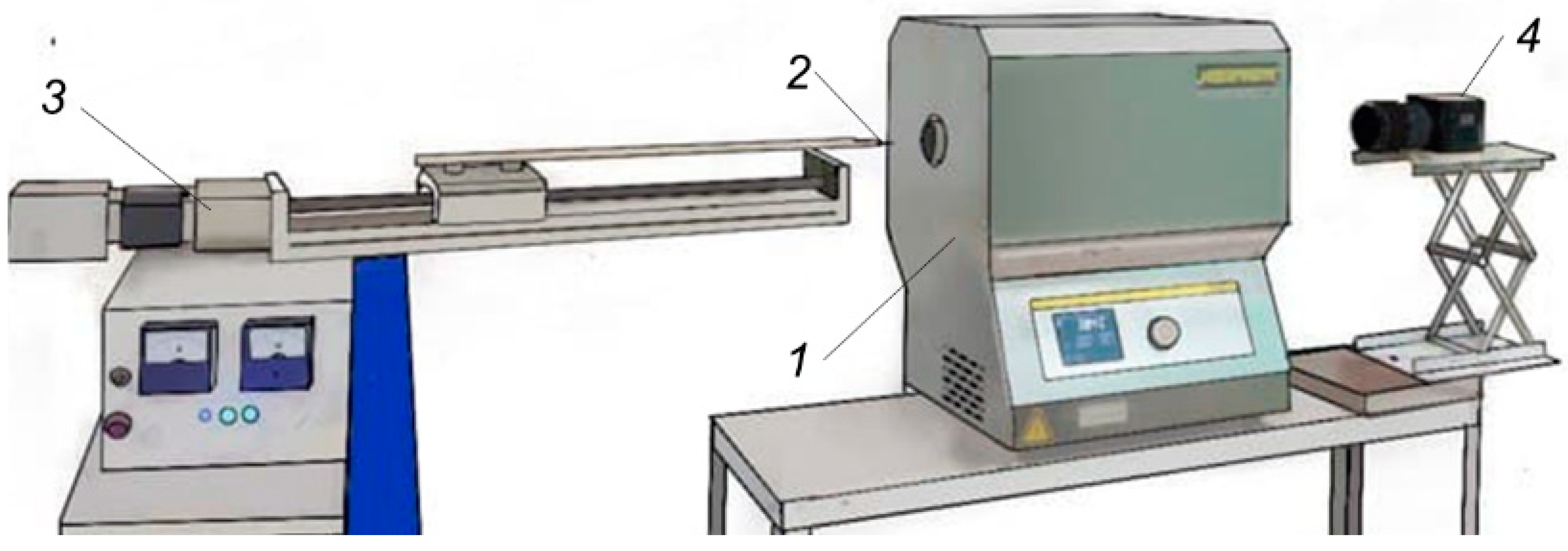
2.2.2. Evaporation of Fire Extinguishing Agent Droplets during Convective Heating
2.2.3. Evaporation of Fire Extinguishing Agent Droplets during Radiant Heating
2.2.4. Evaporation of Fire Extinguishing Agent Droplets during Open Flame Heating
3. Results
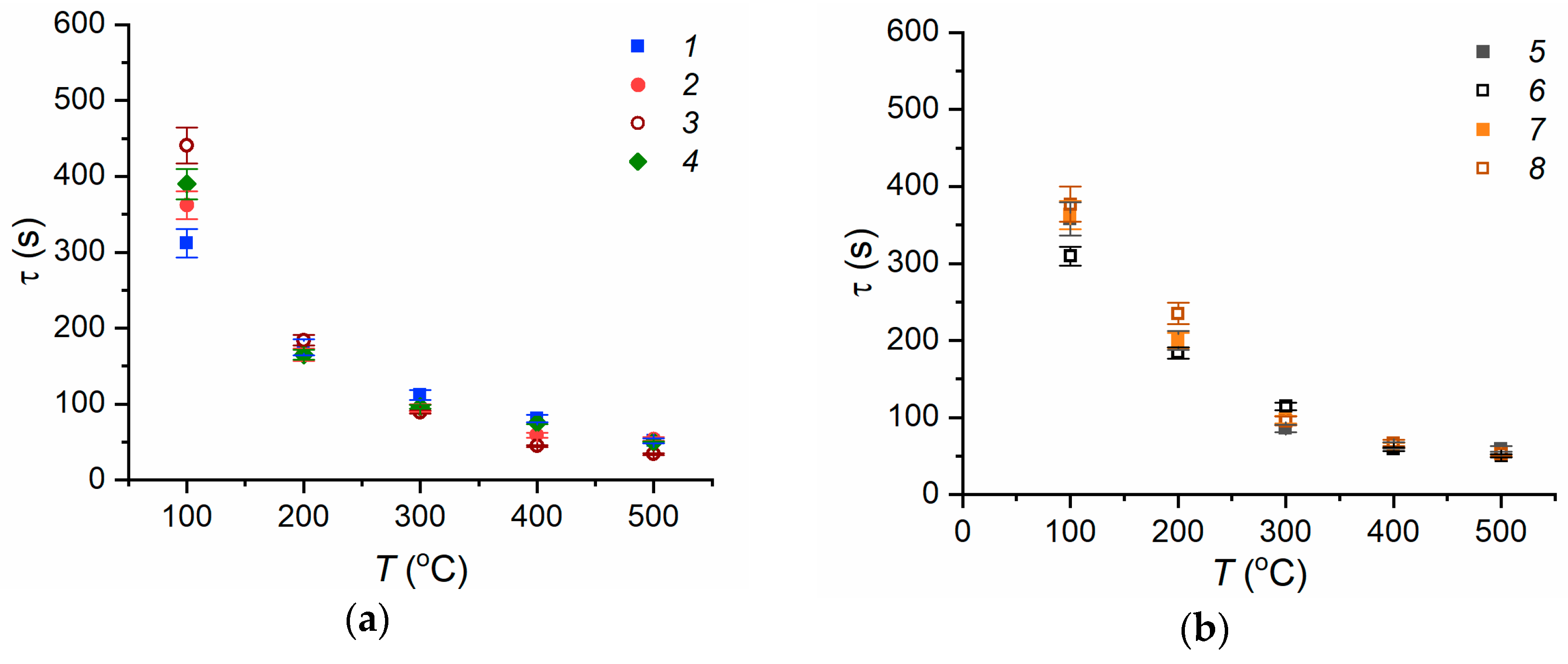
3.1. Heated Steel Surface Wetting by Fire Extinguishing Agents during Conductive Heating
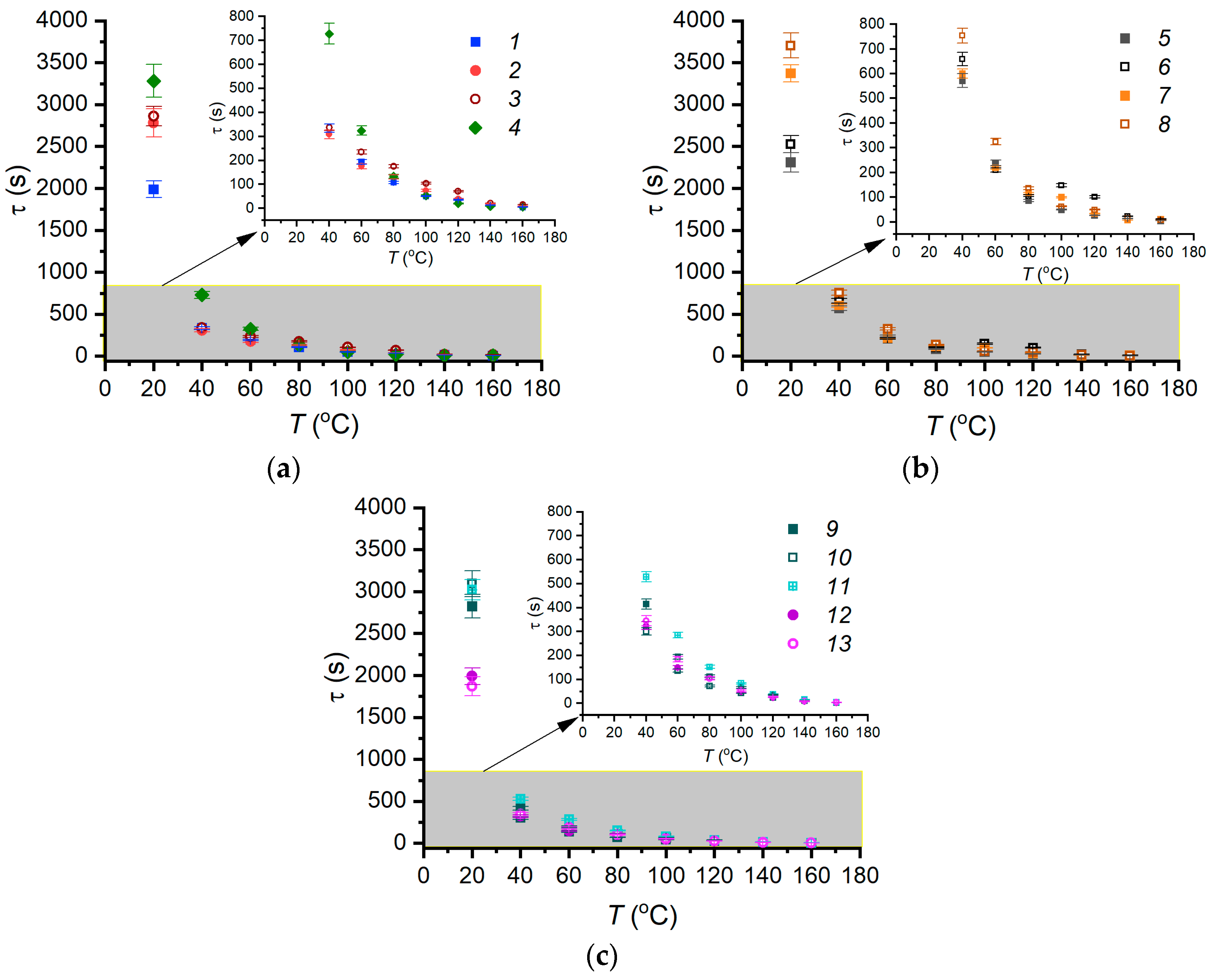
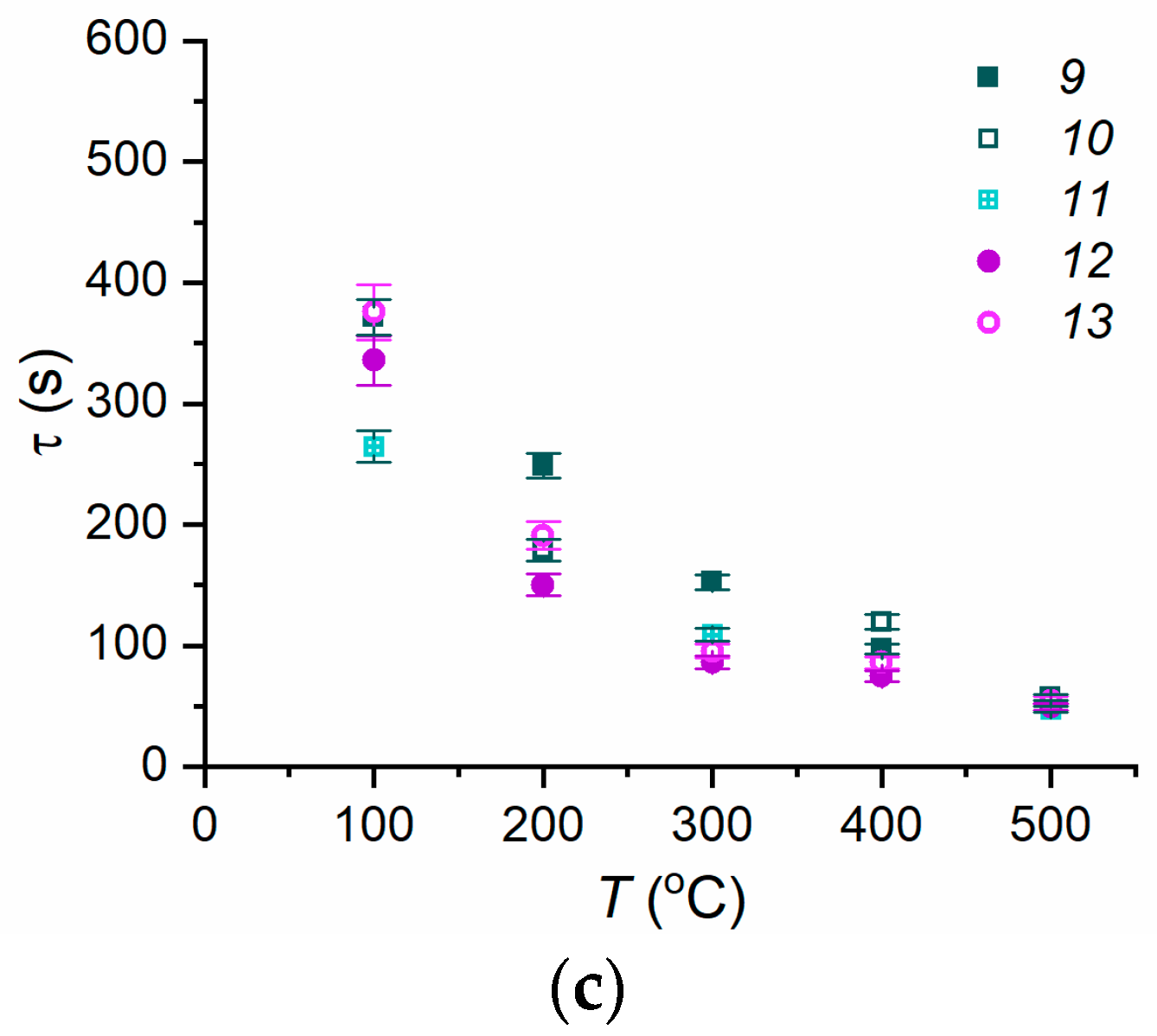
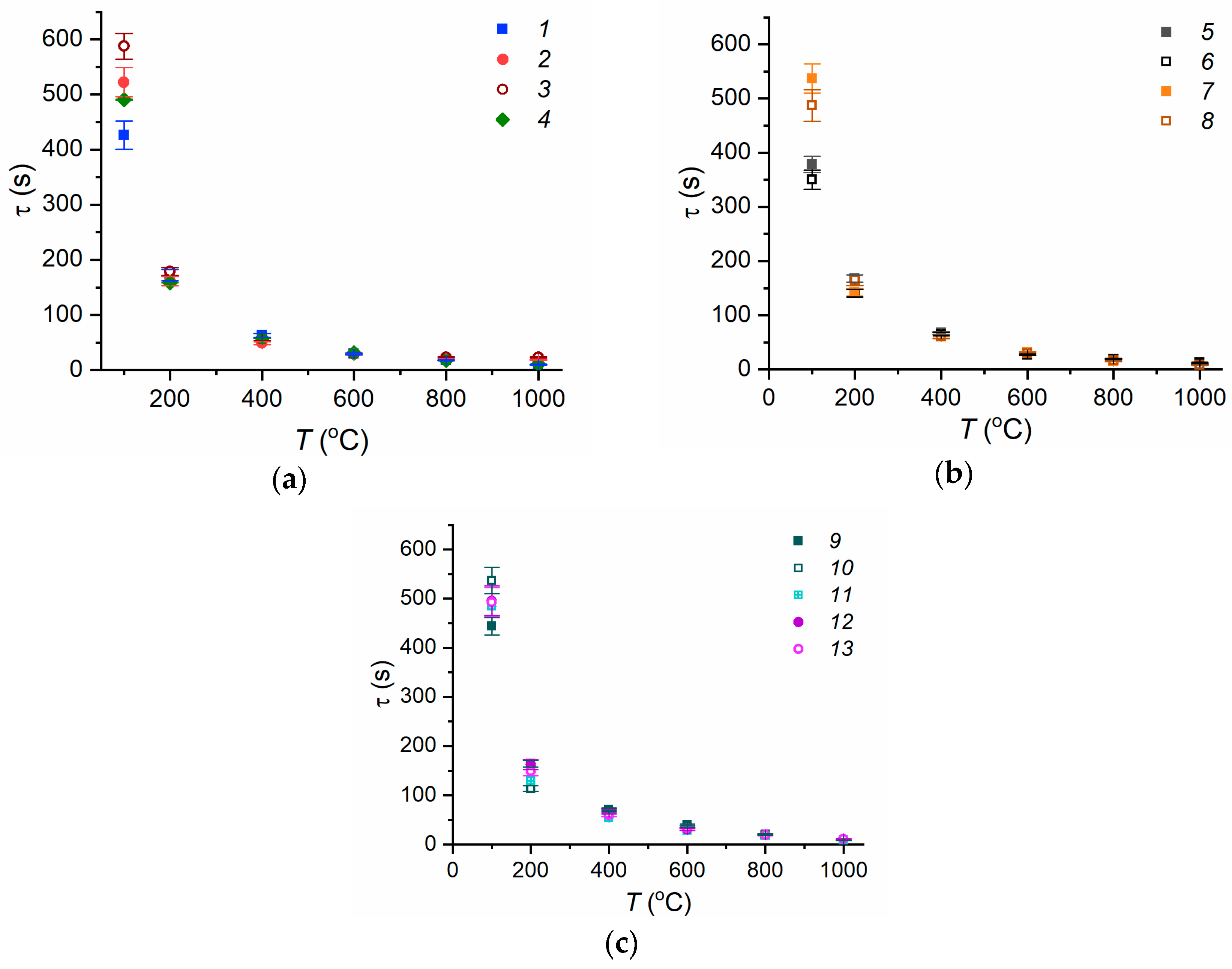
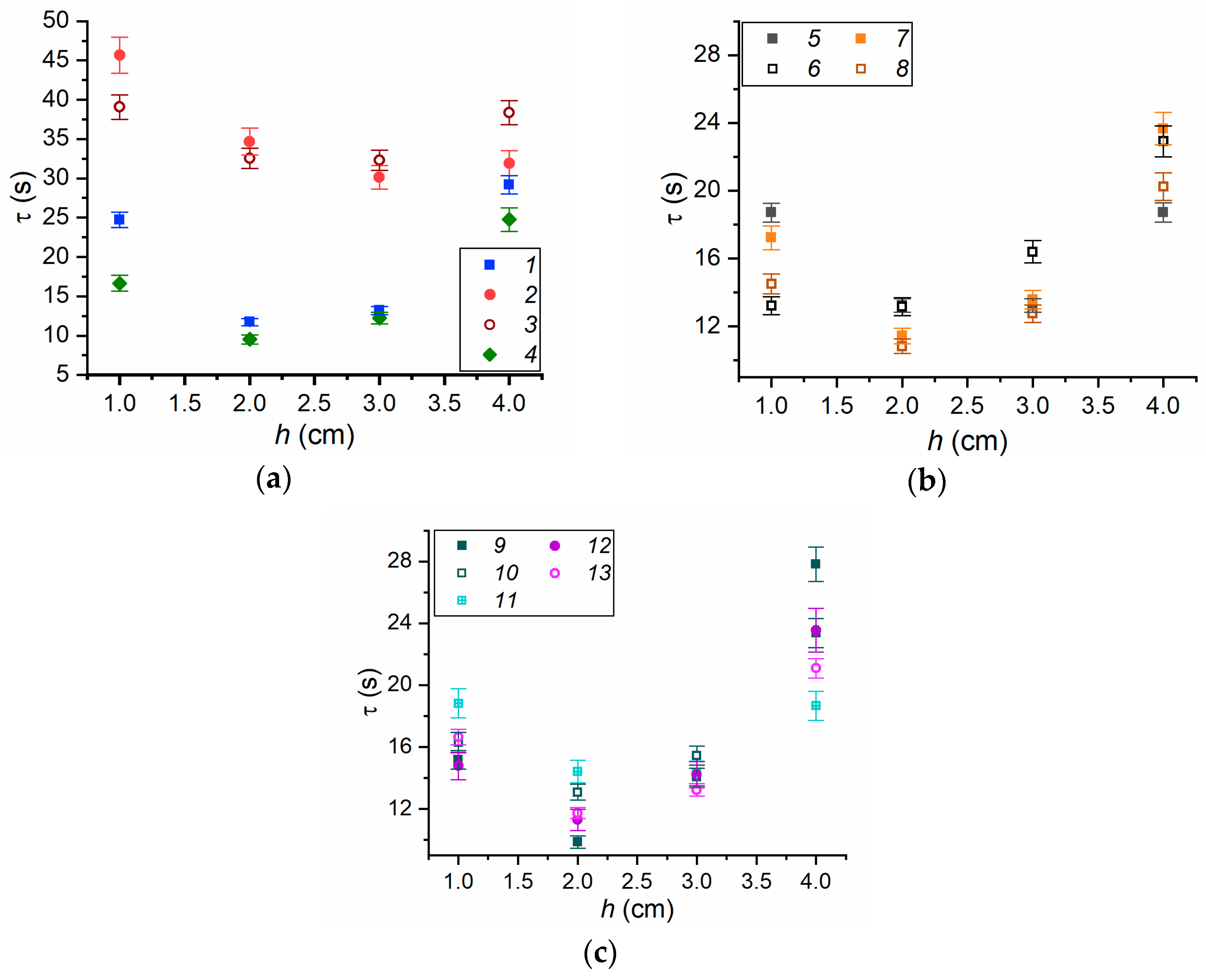
3.2. Evaporation of Fire Extinguishing Agent Droplets
3.3. Experimental Curve Fitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanotte, L.; Laux, D.; Charlot, B.; Abkarian, M. Role of red cells and plasma composition on blood sessile droplet evaporation. Phys. Rev. E 2017, 96, 053114. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, D.; Kim, W.; Horiike, S.; Nishimoto, T.; Se, H.L.; Ahn, C.H. Fully packed capillary electrochromatographic microchip with self-assembly colloidal silica beads. Anal. Chem. 2007, 79, 3214–3219. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, G.V.; Kralinova, S.S.; Voytkov, I.S.; Islamova, A.G. Rates of high-temperature evaporation of promising fire-extinguishing liquid droplets. Appl. Sci. 2019, 9, 5190. [Google Scholar] [CrossRef]
- Borodulin, V.Y.; Letushko, V.N.; Nizovtsev, M.I.; Sterlyagov, A.N. The Experimental Study of Evaporation of Water–Alcohol Solution Droplets. Colloid J. 2019, 81, 219–225. [Google Scholar] [CrossRef]
- Sobac, B.; Brutin, D. Thermocapillary instabilities in an evaporating drop deposited onto a heated substrate. Phys. Fluids 2012, 24, 032103. [Google Scholar] [CrossRef]
- Caddock, B.D.; Hull, D. Influence of humidity on the cracking patterns formed during the drying of sol-gel drops. J. Mater. Sci. 2002, 37, 825–834. [Google Scholar] [CrossRef]
- Shin, S.; Jacobi, I.; Stone, H.A. Bénard-Marangoni instability driven by moisture absorption. Europhys. Lett. 2016, 113, 24002. [Google Scholar] [CrossRef]
- Xu, W.; Leeladhar, R.; Kang, Y.T.; Choi, C.-H. Evaporation Kinetics of Sessile Water Droplets on Micropillared Superhydrophobic Surfaces. Langmuir 2013, 29, 6032–6041. [Google Scholar] [CrossRef]
- Koga, S.; Inasawa, S. Packing structures and formation of cracks in particulate films obtained by drying colloid–polymer suspensions. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 95–101. [Google Scholar] [CrossRef]
- Ryu, S.A.; Kim, J.Y.; Kim, S.Y.; Weon, B.M. Drying-mediated patterns in colloid-polymer suspensions. Sci. Rep. 2017, 7, 1079. [Google Scholar] [CrossRef] [Green Version]
- Yamamura, M.; Ono, H.; Uchinomiya, T.; Mawatari, Y.; Kage, H. Multiple crack nucleation in drying nanoparticle-polymer coatings. Colloids Surf. A Physicochem. Eng. Asp. 2009, 342, 65–69. [Google Scholar] [CrossRef]
- Harikrishnan, A.R.; Dhar, P. Optical thermogeneration induced enhanced evaporation kinetics in pendant nanofluid droplets. Int. J. Heat Mass Transf. 2018, 118, 1169–1179. [Google Scholar] [CrossRef]
- Yu, M.; Le Floch-Fouéré, C.; Pauchard, L.; Boissel, F.; Fu, N.; Chen, X.D.; Saint-Jalmes, A.; Jeantet, R.; Lanotte, L. Skin layer stratification in drying droplets of dairy colloids. Colloids Surf. A Physicochem. Eng. Asp. 2021, 620, 126560. [Google Scholar] [CrossRef]
- Hampton, M.A.; Nguyen, T.A.H.; Nguyen, A.V.; Xu, Z.P.; Huang, L.; Rudolph, V. Influence of surface orientation on the organization of nanoparticles in drying nanofluid droplets. J. Colloid Interface Sci. 2012, 377, 456–462. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Li, W.; Ji, W.; Sun, H.; Lan, D.; Wang, Y. Pattern Formation in Drying Sessile and Pendant Droplet: Interactions of Gravity Settling, Interface Shrinkage, and Capillary Flow. Langmuir 2019, 35, 113–119. [Google Scholar] [CrossRef]
- Daïf, A.; Bouaziz, M.; Chesneau, X.; Ali Chérif, A. Comparison of multicomponent fuel droplet vaporization experiments in forced convection with the Sirignano model. Exp. Therm. Fluid Sci. 1998, 18, 282–290. [Google Scholar] [CrossRef]
- Han, K.; Yang, B.; Zhao, C.; Fu, G.; Ma, X.; Song, G. Experimental study on evaporation characteristics of ethanol–diesel blend fuel droplet. Exp. Therm. Fluid Sci. 2016, 70, 381–388. [Google Scholar] [CrossRef]
- Hallett, W.L.H.; Beauchamp-Kiss, S. Evaporation of single droplets of ethanol–fuel oil mixtures. Fuel 2010, 89, 2496–2504. [Google Scholar] [CrossRef]
- Shringi, D.; Dwyer, H.A.; Shaw, B.D. Influences of support fibers on vaporizing fuel droplets. Comput. Fluids 2013, 77, 66–75. [Google Scholar] [CrossRef]
- Shih, A.T.; Megaridis, C.M. Suspended droplet evaporation modeling in a laminar convective environment. Combust. Flame 1995, 102, 256–270. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Shmakov, A.G.; Chernov, A.A.; Bol’shova, T.A.; Shvartsberg, V.M.; Kutsenogii, K.P.; Makarov, V.I. Fire suppression by aerosols of aqueous solutions of salts. Combust. Explos. Shock Waves 2010, 46, 16–20. [Google Scholar] [CrossRef]
- Mykhalichko, B.; Lavrenyuk, H.; Mykhalichko, O. New water-based fire extinguishant: Elaboration, bench-scale tests, and flame extinguishment efficiency determination by cupric chloride aqueous solutions. Fire Saf. J. 2019, 105, 188–195. [Google Scholar] [CrossRef]
- Rakowska, J.; Szczygieł, R.; Kwiatkowski, M.; Porycka, B.; Radwan, K.; Prochaska, K. Application Tests of New Wetting Compositions for Wildland Firefighting. Fire Technol. 2017, 53, 1379–1398. [Google Scholar] [CrossRef]
- Rakowska, J.; Prochaska, K.; Twardochleb, B.B.; Rojewska, M.; Porycka, B.B.; Jaszkiewicz, A. Selection of surfactants as main components of ecological wetting agent for effective extinguishing of forest and peat-bog fires. Chem. Pap. 2014, 68, 823–833. [Google Scholar] [CrossRef]
- Appah, S.; Zhou, H.; Wang, P.; Ou, M.; Jia, W. Charged monosized droplet behaviour and wetting ability on hydrophobic leaf surfaces depending on surfactant-pesticide concentrate formulation. J. Electrostat. 2019, 100, 103356. [Google Scholar] [CrossRef]
- Glenn, G.M.; Bingol, G.; Chiou, B.-S.; Klamczynski, A.P.; Pan, Z. Sodium bentonite-based coatings containing starch for protecting structures in wildfire emergency situations. Fire Saf. J. 2012, 51, 85–92. [Google Scholar] [CrossRef]
- Brahmia, F.Z.; Alpár, T.; Horváth, P.G.; Csiha, C. Comparative analysis of wettability with fire retardants of Poplar (Populus cv. euramericana I214) and Scots pine (Pinus sylvestris). Surf. Interfaces 2020, 18, 100405. [Google Scholar] [CrossRef]
- Jenkins, M.S.; Bedward, M.; Price, O.; Bradstock, R.A. Modelling bushfire fuel hazard using biophysical parameters. Forests 2020, 11, 925. [Google Scholar] [CrossRef]
- Robinne, F.-N.; Miller, C.; Parisien, M.-A.; Emelko, M.B.; Bladon, K.D.; Silins, U.; Flannigan, M. A global index for mapping the exposure of water resources to wildfire. Forests 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Islamova, A.; Kropotova, S.; Tkachenko, P.; Voitkov, I.; Kuznetsov, G. Transformation of Initially Unatomized Fire-Extinguishing Liquid Arrays at Free Fall from Different Heights. At. Sprays 2021, 31, 71–91. [Google Scholar] [CrossRef]
- Alsammak, I.L.H.; Mahmoud, M.A.; Aris, H.; Alkilabi, M.; Mahdi, M.N. The Use of Swarms of Unmanned Aerial Vehicles in Mitigating Area Coverage Challenges of Forest-Fire-Extinguishing Activities: A Systematic Literature Review. Forests 2022, 13, 811. [Google Scholar] [CrossRef]
- Kuznetsov, G.; Zhdanova, A.; Voitkov, I.; Strizhak, P. Disintegration of Free-falling Liquid Droplets, Jets, and Arrays in Air. Microgravity Sci. Technol. 2022, 34, 12. [Google Scholar] [CrossRef]
- Rodríguez-Veiga, J.; Ginzo-Villamayor, M.J.; Casas-Méndez, B. An integer linear programming model to select and temporally allocate resources for fighting forest fires. Forests 2018, 9, 583. [Google Scholar] [CrossRef]
- GOST R 50588-2012; Foaming Agents for Fire Extinguishing. General Technical Requirements and Test Methods. Standardinform: Moscow, Russia, 2012.
- GOST 16363-98; Fire Protective Means for Wood. Methods for Determination of Fire Protective Properties. Standardinform: Moscow, Russia, 1998.
- GOST 7759-73; Technical Magnesium Chloride (Bishofit). Specifications. Standardinform: Moscow, Russia, 1991.
- Shadrina, E.M.; Volkova, G.V. Determination of Thermophysical Properties of Gases, Liquids and Aqueous Solutions of Substances; Ivanovo University of Chemical Technology: Ivanovo, Russia, 2009. [Google Scholar]
- Kuznetsov, G.V.; Islamova, A.G.; Orlova, E.G.; Strizhak, P.A.; Feoktistov, D.V. Physicochemical features of the effect of special water-based fire retardants on forest materials. Fire Saf. J. 2021, 123, 103371. [Google Scholar] [CrossRef]
- Antonov, D.V.; Fedorenko, R.M.; Strizhak, P.A.; Nissar, Z.; Sazhin, S.S. Puffing/micro-explosion in composite fuel/water droplets heated in flames. Combust. Flame 2021, 233, 111599. [Google Scholar] [CrossRef]
- Soboleva, O.A. Enrichment and Leaning of the Area near the Line of Wetting by Surfactants, Msu Vestnik. Series 2. Chemistry 2003, 44, 337–341. [Google Scholar]
- Volkov, R.S.S.; Strizhak, P.A.A. Research of temperature fields and convection velocities in evaporating water droplets using Planar Laser-Induced Fluorescence and Particle Image Velocimetry. Exp. Therm. Fluid Sci. 2018, 97, 392–407. [Google Scholar] [CrossRef]

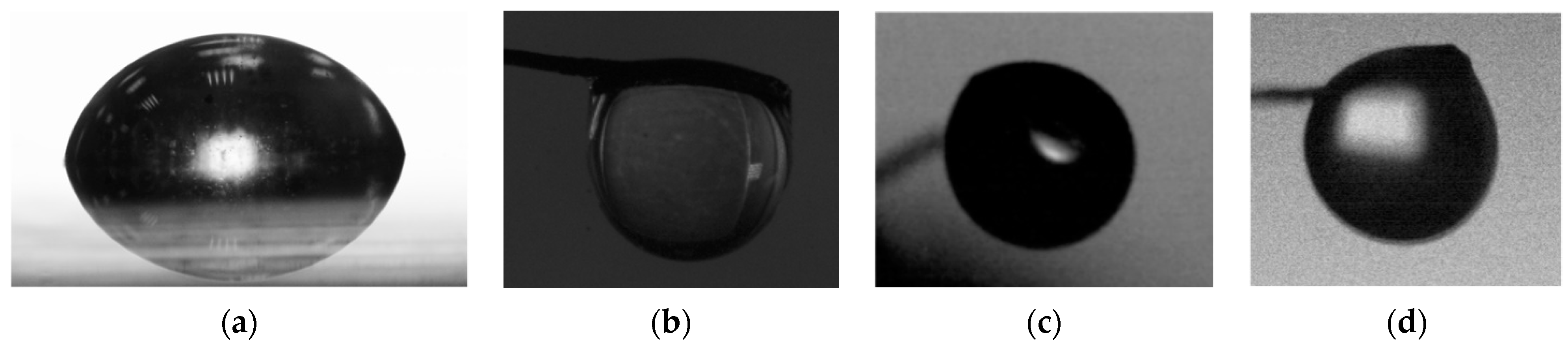


| Firefighting Compositions | Bischofite Solution | OS-5 Solution | FR-Les Solution | Bentonite Slurry | Foaming Agent Emulsion | Water | |
|---|---|---|---|---|---|---|---|
| C, % | 10 | 10 | 5 | 20 | 5 | 5 | - |
| γ, mN/m | 75.8 | 45 | 37.26 | 35.02 | 68.5 | 17.3 | 72.7 |
| ρ, kg/m3 | 1081.5 | 1099.1 | 1019 | 1047 | 1100 | 1002–1187 | 998.2 |
| λm, W/(m∙K) | 0.58 | 0.624 | 0.704 | 0.5952 | 0.599 | ||
| μ∙103, Pa∙s | 1.015 | 0.12 | 3.02 | 3.65 | 24.3 | 0.12 | 1.004 |
| Chemical composition (information from public sources) | MgCl2∙6H2O (93%); the rest are impurities | Carbamide (31%–32%); alkylbenzene sulfonate (2%–3%); acid dye (0.5%–1%); ammonium chloride (13%–15%); diammonium phosphate (the rest) | Ammonium polyphosphate solution, phosphorus pentoxide (31.5%); nitrogen (9%) | Montmorillonite Si8Al4O20(OH)4 × nH2O (60%–70%); the rest are sandy-aleuritic material impurities | Silicon dioxide (60%–65%); aluminum oxide and iron oxide (20%–25%) | Fluoridated surfactants | Binary inorganic compound whose molecule consists of two hydrogen atoms and one oxygen atom (the atoms are linked by covalent bonds) |
| T, °C | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| θ, ° | ||||||||||||
| 20 | 87.4 | 76.4 | 102.1 | 94.5 | 82.7 | 84.2 | 84.5 | 52.8 | 42.0 | 42.0 | 52.5 | 34.3 |
| 40 | 76.4 | 76.4 | 103.5 | 87.2 | 74.4 | 77.8 | 87.0 | 51.4 | 45.1 | 40.9 | 46.1 | 34.5 |
| 60 | 85.9 | 81.4 | 103.9 | 81.8 | 94.2 | 90.6 | 74.6 | 44.7 | 38.7 | 40.6 | 44.1 | 33.3 |
| 80 | 78.0 | 71.5 | 105.5 | 80.5 | 98.5 | 76.1 | 74.0 | 42.6 | 34.2 | 37.9 | 42.6 | 34.1 |
| 100 | 77.0 | 75.5 | 103.8 | 78.4 | 112.7 | 78.8 | 83.4 | 45.5 | 36.6 | 36.9 | 41.1 | 35.7 |
| d, mm | ||||||||||||
| 20 | 3.61 | 3.92 | 3.14 | 3.33 | 3.67 | 3.61 | 3.60 | 4.79 | 5.18 | 5.19 | 4.95 | 5.65 |
| 40 | 3.87 | 3.90 | 3.10 | 3.55 | 3.89 | 3.83 | 3.55 | 4.80 | 5.12 | 5.31 | 5.05 | 5.64 |
| 60 | 3.53 | 3.66 | 3.14 | 3.72 | 3.29 | 3.46 | 3.94 | 5.13 | 5.62 | 5.70 | 5.19 | 5.70 |
| 80 | 3.53 | 3.66 | 3.15 | 3.72 | 3.29 | 3.89 | 3.94 | 5.31 | 5.57 | 5.38 | 5.19 | 5.70 |
| 100 | 3.85 | 3.90 | 3.09 | 3.79 | 2.74 | 3.80 | 3.65 | 4.85 | 5.24 | 5.45 | 5.25 | 5.62 |
| h, mm | ||||||||||||
| 20 | 1.53 | 1.36 | 1.74 | 1.66 | 1.50 | 1.53 | 1.54 | 0.98 | 0.89 | 0.87 | 0.96 | 0.75 |
| 40 | 1.40 | 1.38 | 1.78 | 1.56 | 1.42 | 1.41 | 1.56 | 1.00 | 0.88 | 0.83 | 0.94 | 0.75 |
| 60 | 1.57 | 1.49 | 1.74 | 1.47 | 1.70 | 1.60 | 1.37 | 0.88 | 0.76 | 0.75 | 0.86 | 0.73 |
| 80 | 1.57 | 1.49 | 1.71 | 1.47 | 1.70 | 1.38 | 1.37 | 0.82 | 0.78 | 0.80 | 0.86 | 0.73 |
| 100 | 1.41 | 1.38 | 1.78 | 1.44 | 1.96 | 1.43 | 1.51 | 0.97 | 0.85 | 0.80 | 0.84 | 0.74 |
| Fire Extinguishing Agent | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Conductive heating (τ = aα∙Tbα) | |||||||||||||
| aλ∙106 | 2.54 | 3.63 | 3.75 | 4.38 | 3.25 | 3.40 | 4.46 | 4.93 | 3.72 | 4.02 | 4.01 | 2.63 | 2.48 |
| bλ | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 | −2.4 |
| R2 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.99 | 1.00 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 |
| Convective heating (τ = aα∙Tbα) | |||||||||||||
| aα∙105 | 0.84 | 0.91 | 1.06 | 0.97 | 0.93 | 0.84 | 0.95 | 1.00 | 1.04 | 0.76 | 0.75 | 0.85 | 0.97 |
| bα | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 | −1.2 |
| R2 | 0.95 | 0.99 | 0.97 | 0.99 | 0.97 | 0.94 | 0.98 | 0.94 | 0.87 | 0.89 | 0.83 | 0.99 | 0.99 |
| Radiant heating (τ = aε∙Tbε) | |||||||||||||
| aε∙105 | 6.93 | 8.22 | 9.23 | 7.78 | 6.25 | 5.72 | 19.92 | 7.76 | 7.17 | 8.24 | 7.55 | 7.88 | 7.77 |
| bε | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 | −1.6 |
| R2 | 0.99 | 0.99 | 0.99 | 0.99 | 0.98 | 0.98 | 0.99 | 0.99 | 0.99 | 0.98 | 0.99 | 0.98 | 0.99 |
| Heating over an open flame (τ = af∙T2 + bf∙T + cf) | |||||||||||||
| af | 7.2 | 3.2 | 3.1 | 4.9 | 2.7 | 1.7 | 4.0 | 2.8 | 4.8 | 2.8 | 2.2 | 3.2 | 3.2 |
| bf | −34.7 | −20.6 | −16.0 | −21.9 | −13.7 | −5.0 | −17.7 | −12.1 | −19.6 | −11.5 | −10.9 | −13.1 | −14.5 |
| cf | 52.2 | 63.0 | 51.9 | 33.6 | 29.6 | 16.6 | 30.9 | 23.8 | 30.0 | 25.1 | 27.6 | 24.7 | 28.0 |
| R2 | 0.98 | 1.00 | 0.95 | 0.92 | 0.95 | 0.97 | 0.98 | 0.98 | 0.95 | 1.00 | 0.99 | 0.98 | 0.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhdanova, A.; Islamova, A.; Kurapov, R.; Volkov, R. Evaporation of Promising Fire Extinguishing Agent Droplets. Forests 2023, 14, 301. https://doi.org/10.3390/f14020301
Zhdanova A, Islamova A, Kurapov R, Volkov R. Evaporation of Promising Fire Extinguishing Agent Droplets. Forests. 2023; 14(2):301. https://doi.org/10.3390/f14020301
Chicago/Turabian StyleZhdanova, Alena, Anastasia Islamova, Roman Kurapov, and Roman Volkov. 2023. "Evaporation of Promising Fire Extinguishing Agent Droplets" Forests 14, no. 2: 301. https://doi.org/10.3390/f14020301





