Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Microsatellite Genotyping
2.2. Construction of Core Collections
2.3. Indicators for Evaluating the Core Collections
3. Results
3.1. Comparison of Different Methods for Constructing Core Collections
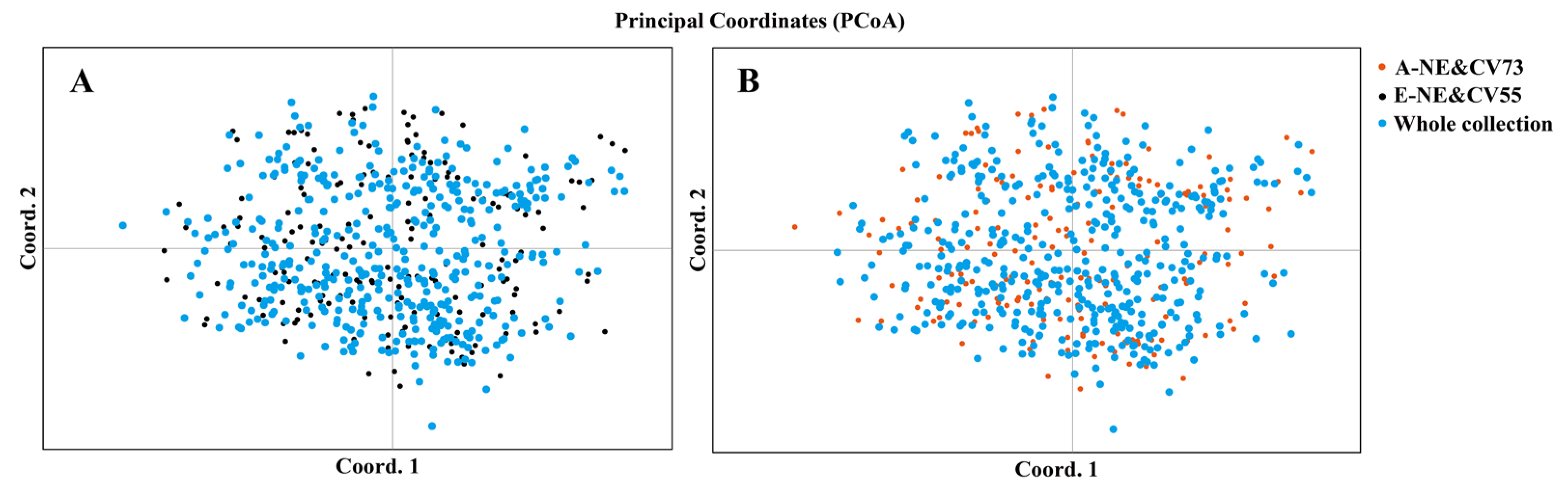
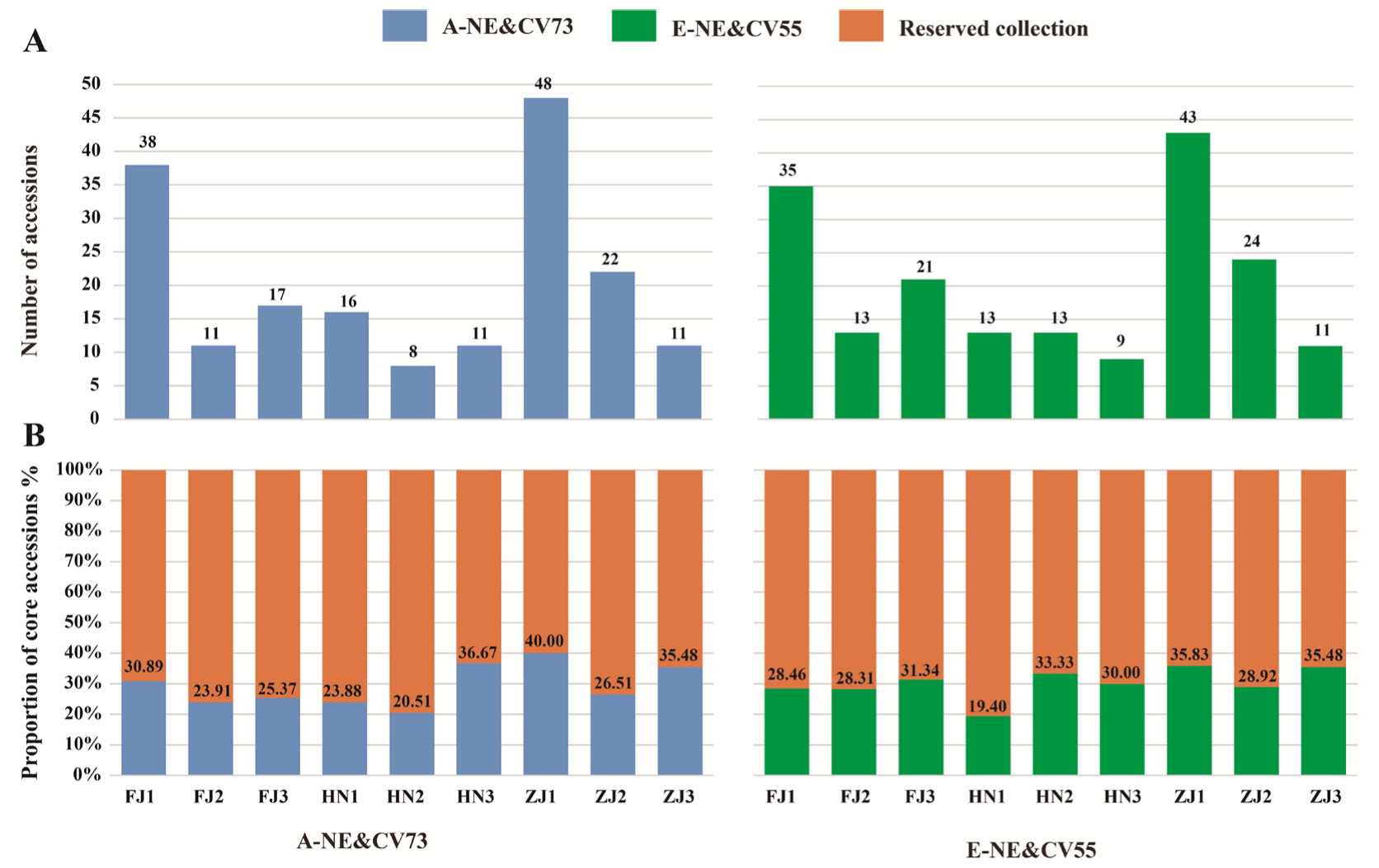
3.2. Construction of Core Collection
3.3. Evaluation of Core Collections
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Escribano, P.; Viruel, M.A.; Hormaza, J.I. Comparison of different methods to construct a core germplasm collection in woody perennial species with simple sequence repeat markers. A case study in cherimoya (Annona cherimola, Annonaceae), an underutilised subtropical fruit tree species. Ann. Appl. Biol. 2008, 153, 25–32. [Google Scholar] [CrossRef]
- Boccacci, P.; Aramini, M.; Ordidge, M.; van Hintum, T.J.L.; Marinoni, D.T.; Valentini, N.; Sarraquigne, J.-P.; Solar, A.; Rovira, M.; Bacchetta, L.; et al. Comparison of selection methods for the establishment of a core collection using SSR markers for hazelnut (Corylus avellana L.) accessions from European germplasm repositories. Tree Genet. Genomes 2021, 17, 48. [Google Scholar] [CrossRef]
- Grenier, C.; Deu, M.; Kresovich, S.; Bramel, P.; Hamon, P. Assessment of genetic giversity in three subsets constituted from the ICRISAT Sorghum collection using random vs non-random sampling procedures. Theor. Appl. Genet. 2000, 101, 197–202. [Google Scholar] [CrossRef]
- Hoshikawa, K.; Lin, Y.-P.; Schafleitner, R.; Shirasawa, K.; Isobe, S.; Nguyen, D.C.; Ohsawa, R.; Yoshioka, Y. Genetic diversity analysis and core collection construction for Amaranthus tricolor germplasm based on genome-wide single-nucleotide polymorphisms. Sci. Hortic. 2022, 307, 111428. [Google Scholar] [CrossRef]
- Frankel, O.H. Genetic perspectives of germplasm conservation. In Genetic Manipulation: Impact on Man and Society; Arber, W.K., Illmensee, K., Peacock, W.J., Starlinger, P., Ehrlich, S.D., Dagert, M., Romac, S., Michel, B., Levy, S.B., Goebel, W., et al., Eds.; Cambridge University Press: Cambridge, UK, 1984; pp. 161–170. [Google Scholar]
- Brown, A.H.D. Core collections—A practical approach to genetic-resources management. Genome 1989, 31, 818–824. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, J.; Xu, H.M. Methods of constructing core collections by stepwise clustering with three sampling strategies based on the genotypic values of crops. Theor. Appl. Genet. 2000, 101, 264–268. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, W.; Dai, Y.; Fu, C.; Bian, Y. Using SSR markers to evaluate the genetic diversity of Lentinula edodes’ natural germplasm in China. World J. Microbiol. Biotechnol. 2010, 26, 527–536. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, D.; Gu, J.; Hu, X.; Zuo, X.; Wang, H. Development of SSR markers for typing cultivars in the mushroom Auricularia Auricula-Judae. Mycol. Prog. 2012, 11, 587–592. [Google Scholar] [CrossRef]
- Lassois, L.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Hibrand-Saint-Oyant, L.; Poncet, C.; Lasserre-Zuber, P.; Feugey, L.; Durel, C.-E. Genetic diversity, population structure, parentage analysis, and construction of core collections in the French apple germplasm based on SSR markers. Plant Mol. Biol. Report. 2016, 34, 827–844. [Google Scholar] [CrossRef]
- Zane, L.; Bargelloni, L.; Patarnello, T. Strategies for microsatellite isolation: A review. Mol. Ecol. 2002, 11, 1–16. [Google Scholar] [CrossRef]
- Zurn, J.D.; Hummer, K.E.; Bassil, N.V. Exploring the diversity and genetic structure of the U.S. National Cultivated Strawberry Collection. Hortic. Res. 2022, 9, uhac125. [Google Scholar] [CrossRef]
- Díez, C.M.; Imperato, A.; Rallo, L.; Barranco, D.; Trujillo, I. Worldwide core collection of olive cultivars based on simple sequence repeat and morphological markers. Crop Sci. 2012, 52, 211–221. [Google Scholar] [CrossRef]
- Bernard, A.; Barreneche, T.; Donkpegan, A.; Lheureux, F.; Dirlewanger, E. Comparison of structure analyses and core collections for the management of walnut genetic resources. Tree Genet. Genomes 2020, 16, 76. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, R.; Wang, B.; Xu, Z.; Zhou, Z. Construction of core collection of Schima superba based on SSR molecular markers. Sci. Silvae Sin. 2017, 53, 37–46. [Google Scholar]
- Liu, K.; Muse, S.V. PowerMarker: An integrated analysis environment for genetic marker analysis. Bioinformatics 2005, 21, 2128–2129. [Google Scholar] [CrossRef]
- Kim, K.-W.; Chung, H.-K.; Cho, G.-T.; Ma, K.-H.; Chandrabalan, D.; Gwag, J.-G.; Kim, T.-S.; Cho, E.-G.; Park, Y. PowerCore: A program applying the advanced M strategy with a heuristic search for establishing core sets. Bioinformatics 2007, 23, 2155–2162. [Google Scholar] [CrossRef]
- De Beukelaer, H.; Smykal, P.; Davenport, G.F.; Fack, V. Core Hunter II: Fast core subset selection based on multiple genetic diversity measures using Mixed Replica search. BMC Bioinform. 2012, 13, 312. [Google Scholar] [CrossRef]
- De Beukelaer, H.; Davenport, G.F.; Fack, V. Core Hunter 3: Flexible core subset selection. BMC Bioinform. 2018, 19, 203. [Google Scholar] [CrossRef]
- Hong, J.; Cheng, B. Memoir of Chinese fir provenance test. For. Res. 1994, 7, 1–25. (In Chinese) [Google Scholar]
- Duan, H.; Cao, S.; Zheng, H.; Hu, D.; Lin, J.; Cui, B.; Lin, H.; Hu, R.; Wu, B.; Sun, Y.; et al. Genetic characterization of Chinese fir from six provinces in Southern China and construction of a core collection. Sci. Rep. 2017, 7, 13814. [Google Scholar] [CrossRef]
- Wu, H.; Lei, J.; Li, X.; Wang, H.; Duan, A.G.; Zhang, J. Aggregation distributions across stand age in provenances of Cunninghamia lanceolata (Lamb.) Hook. For. Ecol. Manag. 2021, 494, 119317. [Google Scholar] [CrossRef]
- Shi, J. Present situation of genetic improvement of Cunninghamia lanceolata in Fujian Province and its technical countermeasures. J. Fujian For. Sci. Technol. 1994, 21, 28–31. (In Chinese) [Google Scholar]
- Duan, A.; Sun, J.; Deng, Z.; Zhang, J. Studies on genetic variation and evaluation of hybrid offspring of the parents in the third generation seed orchards of Chinese fir. Acta Agric. Univ. Jiangxiensis 2022, 44, 261–270. (In Chinese) [Google Scholar] [CrossRef]
- Duan, A.; Zhang, X.; Zhang, J.; Zhong, J. Growth and genetic evaluation of 21- year -old Chinese fir clonal plantation. For. Res. 2014, 27, 672–676. (In Chinese) [Google Scholar]
- Wen, B. Discussion on selection methods and standards of superior trees of Chinese Fir. Guangxi For. Sci. 1990, 3, 24–28. (In Chinese) [Google Scholar]
- Li, L.; Wu, B.; Hu, D.; Liang, S.; Shen, W.; Huang, Y.; Ruan, Z. Preliminary report on regionlization test of super Chinese fir clone. Guangdong For. Sci. Technol. 2004, 20, 5–8. (In Chinese) [Google Scholar]
- Wen, Y.; Han, W.; Zhou, H.; Xu, G. SSR mining and development of EST-SSR markers for Cunninghamia lanceolata based on transcriptome sequences. Sci. Silvae Sin. 2015, 51, 41–49. (In Chinese) [Google Scholar] [CrossRef]
- Li, Y.; Wang, Z.; Sui, J.; Zeng, Y.; Duan, A.; Zhang, J. Isolation and characterization of microsatellite loci for Cunninghamia lanceolata (Lamb.) Hook. Genet. Mol. Res. 2015, 14, 453–456. [Google Scholar] [CrossRef]
- Perrier, X.; Jacquemoud-Collet, J.P. DARwin Software. 2006. Available online: http://darwin.cirad.fr/ (accessed on 21 October 2022).
- Odong, T.L.; van Heerwaarden, J.; Jansen, J.; van Hintum, T.J.L.; van Eeuwijk, F.A. Statistical techniques for defining reference sets of accessions and microsatellite markers. Crop Sci. 2011, 51, 2401–2411. [Google Scholar] [CrossRef]
- Odong, T.L.; Jansen, J.; van Eeuwijk, F.A.; van Hintum, T.J.L. Quality of core collections for effective utilisation of genetic resources review, discussion and interpretation. Theor. Appl. Genet. 2012, 126, 289–305. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research--an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Wang, J. COANCESTRY: A program for simulating, estimating and analysing relatedness and inbreeding coefficients. Mol. Ecol. Resour. 2011, 11, 141–145. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, S.; Wang, X.; Duan, A.; Zhang, J. Mating pattern and pollen dispersal in an advanced generation seed orchard of Cunninghamia lanceolata (Lamb.) Hook. Front. Plant Sci. 2022, 13, 1042290. [Google Scholar] [CrossRef]
- El Bakkali, A.; Haouane, H.; Moukhli, A.; Costes, E.; Van Damme, P.; Khadari, B. Construction of core collections suitable for association mapping to optimize use of Mediterranean olive (Olea europaea L.) genetic resources. PLoS ONE 2013, 8, e61265. [Google Scholar] [CrossRef][Green Version]
- van Treuren, R.; Tchoudinova, I.; van Soest, L.J.M.; van Hintum, T.J.L. Marker-assisted acquisition and core collection formation: A case study in barley using AFLPs and pedigree data. Genet. Resour. Crop Evol. 2006, 53, 43–52. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Zhang, Q.L.; Yang, Y.; Luo, Z.R. Development of Japanese persimmon core collection by genetic distance sampling based on SSR narkers. Biotechnol. Biotechnol. Equip. 2009, 23, 1474–1478. [Google Scholar] [CrossRef]
- Marita, J.; Rodriguez, J.; Nienhuis, J. Development of an algorithm identifying maximally diverse core collections. Genet. Resour. Crop Evol. 2000, 47, 515–526. [Google Scholar] [CrossRef]
- Gouesnard, B.; Bataillon, T.; Decoux, G.; Rozale, C.; Schoen, D.; David, J. MSTRAT: An algorithm for building germ plasm core collections by maximizing allelic or phenotypic richness. J. Hered. 2001, 92, 93–104. [Google Scholar] [CrossRef]
- Hu, J.; Wang, P.; Su, Y.; Wang, R.; Li, Q.; Sun, K. Microsatellite diversity, population structure, and core collection formation in melon germplasm. Plant Mol. Biol. Report. 2014, 33, 439–447. [Google Scholar] [CrossRef]
- Liang, W.; Dondini, L.; De Franceschi, P.; Paris, R.; Sansavini, S.; Tartarini, S. Genetic diversity, population structure and construction of a core collection of apple cultivars from Italian germplasm. Plant Mol. Biol. Report. 2014, 33, 458–473. [Google Scholar] [CrossRef]
- Öztürk, S.C.; Balık, H.İ.; Balık, S.K.; Kızılcı, G.; Duyar, Ö.; Doğanlar, S.; Frary, A. Molecular genetic diversity of the Turkish national hazelnut collection and selection of a core set. Tree Genet. Genomes 2017, 13, 113. [Google Scholar] [CrossRef]
- van Hintum, T.J.L.; Brown, A.H.D.; Spillane, C.; Hodgkin, T. Core Collections of Plant Genetic Resources; IPGRI Technical Bulletin No. 3; International Plant Genetic Resources Institute: Rome, Italy, 2000. [Google Scholar]
- Kaur, V.; Aravind, J.; Manju; Jacob, S.R.; Kumari, J.; Panwar, B.S.; Pal, N.; Rana, J.C.; Pandey, A.; Kumar, A. Phenotypic characterization, genetic diversity assessment in 6,778 accessions of barley (Hordeum vulgare L. ssp. vulgare) germplasm conserved in national genebank of India and development of a core set. Front. Plant Sci. 2022, 13, 771920. [Google Scholar] [CrossRef]
- Risliawati, A.; Suwarno, W.; Lestari, P.; Trikoesoemaningtyas, T.; Sobir, S. A strategy to identify representative maize core collections based on kernel properties. Genet. Resour. Crop Evol. 2022, 1–12. [Google Scholar] [CrossRef]
- Zheng, H.; Hu, D.; Wei, R.; Yan, S.; Wang, R. Chinese fir breeding in the high-throughput sequencing era: Insights from SNPs. Forests 2019, 10, 681. [Google Scholar] [CrossRef]
- Gong, F.; Geng, Y.; Zhang, P.; Zhang, F.; Fan, X.; Liu, Y. Genetic diversity and structure of a core collection of Huangqi (Astragalus ssp.) developed using genomic simple sequence repeat markers. Genet. Resour. Crop Evol. 2022. [Google Scholar] [CrossRef]
- Thachuk, C.; Crossa, J.; Franco, J.; Dreisigacker, S.; Warburton, M.; Davenport, G.F. Core Hunter: An algorithm for sampling genetic resources based on multiple genetic measures. BMC Bioinform. 2009, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Wen, Y.; Lin, J.; Wu, X.; Yuan, M.; Zhang, Y.; Wang, M. Genetic diversity of of Chinese fir (Cunninghamia lanceolata) breeding population among different generations. Sci. Silvae Sin. 2020, 56, 53–61. (In Chinese) [Google Scholar]

| Method | Sampling Intensity | P | Na | Ne | I | Ho | He | uHe | F |
|---|---|---|---|---|---|---|---|---|---|
| Whole collection | 100% | 0.563 | 13.619 | 4.282 | 1.497 | 0.553 | 0.657 | 0.657 | 0.149 |
| MLS | 10% | 0 | 9.619 * | 4.851 | 1.637 | 0.531 | 0.723 | 0.729 | 0.269 * |
| 20% | 0 | 10.810 | 4.807 | 1.610 | 0.535 | 0.706 | 0.709 | 0.238 | |
| 30% | 0 | 11.476 | 4.677 | 1.584 | 0.536 | 0.693 | 0.695 | 0.222 | |
| 40% | 0.017 | 12.095 | 4.578 | 1.565 | 0.537 | 0.684 | 0.685 | 0.209 | |
| 50% | 0.026 | 12.571 | 4.505 | 1.547 | 0.539 | 0.676 | 0.677 | 0.196 | |
| Power Core | 20% | 0.180 | 13.619 | 4.830 | 1.625 | 0.552 | 0.685 | 0.688 | 0.191 |
| E–NE | 10% | 0 | 9.333 * | 4.483 | 1.558 | 0.470 | 0.694 | 0.700 | 0.320 ** |
| 20% | 0 | 10.857 | 4.639 | 1.576 | 0.507 | 0.691 | 0.694 | 0.259 * | |
| 30% | 0 | 11.476 | 4.545 | 1.564 | 0.520 | 0.686 | 0.688 | 0.234 | |
| 40% | 0.008 | 12.048 | 4.505 | 1.556 | 0.524 | 0.679 | 0.681 | 0.221 | |
| 50% | 0.023 | 12.286 | 4.489 | 1.537 | 0.531 | 0.671 | 0.672 | 0.202 | |
| A–NE | 10% | 0 | 9.333 * | 4.478 | 1.563 | 0.476 | 0.699 | 0.704 | 0.317 * |
| 20% | 0 | 10.857 | 4.676 | 1.584 | 0.510 | 0.693 | 0.696 | 0.260 * | |
| 30% | 0 | 11.429 | 4.525 | 1.566 | 0.522 | 0.687 | 0.689 | 0.233 | |
| 40% | 0 | 12.000 | 4.499 | 1.553 | 0.523 | 0.679 | 0.680 | 0.223 | |
| 50% | 0.03 | 12.381 | 4.476 | 1.539 | 0.530 | 0.672 | 0.673 | 0.205 | |
| O_CV | 10% | 0 | 12.667 | 4.579 | 1.598 | 0.560 | 0.672 | 0.677 | 0.163 |
| 20% | 0.149 | 13.619 | 4.586 | 1.562 | 0.563 | 0.666 | 0.669 | 0.142 | |
| 30% | 0.247 | 13.619 | 4.501 | 1.541 | 0.560 | 0.667 | 0.669 | 0.151 | |
| 40% | 0.314 | 13.619 | 4.270 | 1.509 | 0.556 | 0.658 | 0.660 | 0.146 | |
| 50% | 0.366 | 13.619 | 4.275 | 1.504 | 0.553 | 0.659 | 0.66 | 0.149 | |
| O_He | 10% | 0.067 | 9.571 * | 4.996 | 1.668 | 0.606 | 0.738 | 0.745 | 0.178 |
| 20% | 0.322 | 10.810 | 4.988 | 1.653 | 0.607 | 0.724 | 0.727 | 0.156 | |
| 30% | 0.302 | 11.619 | 4.861 | 1.630 | 0.597 | 0.714 | 0.716 | 0.157 | |
| 40% | 0.393 | 11.905 | 4.776 | 1.609 | 0.596 | 0.706 | 0.708 | 0.150 | |
| 50% | 0.419 | 12.190 | 4.670 | 1.587 | 0.588 | 0.698 | 0.699 | 0.149 | |
| O_Shannon | 10% | 0.067 | 10.857 | 5.382 | 1.704 | 0.597 | 0.723 | 0.729 | 0.175 |
| 20% | 0.132 | 11.952 | 5.191 | 1.678 | 0.587 | 0.714 | 0.717 | 0.174 | |
| 30% | 0.275 | 12.667 | 5.050 | 1.650 | 0.589 | 0.705 | 0.707 | 0.159 | |
| 40% | 0.347 | 12.952 | 4.911 | 1.626 | 0.584 | 0.697 | 0.699 | 0.158 | |
| 50% | 0.393 | 13.048 | 4.816 | 1.603 | 0.58 | 0.691 | 0.692 | 0.152 | |
| SANA | 10% | 0.033 | 10.810 | 4.665 | 1.538 | 0.542 | 0.664 | 0.670 | 0.169 |
| 20% | 0.116 | 11.952 | 4.508 | 1.530 | 0.549 | 0.660 | 0.663 | 0.154 | |
| 30% | 0.258 | 12.095 | 4.367 | 1.517 | 0.554 | 0.663 | 0.665 | 0.158 | |
| 40% | 0.289 | 12.667 | 4.316 | 1.506 | 0.554 | 0.659 | 0.660 | 0.150 | |
| 50% | 0.373 | 13.048 | 4.326 | 1.503 | 0.551 | 0.656 | 0.657 | 0.149 | |
| SAGD | 10% | 0.131 | 9.476 * | 4.526 | 1.574 | 0.570 | 0.699 | 0.705 | 0.180 |
| 20% | 0.174 | 10.810 | 4.591 | 1.558 | 0.558 | 0.686 | 0.689 | 0.179 | |
| 30% | 0.187 | 11.476 | 4.490 | 1.550 | 0.568 | 0.682 | 0.684 | 0.159 | |
| 40% | 0.368 | 12.000 | 4.478 | 1.544 | 0.565 | 0.679 | 0.680 | 0.161 | |
| 50% | 0.376 | 12.429 | 4.461 | 1.532 | 0.566 | 0.674 | 0.675 | 0.151 | |
| R | 10% | 0.131 | 8.643 * | 4.118 | 1.441 | 0.551 | 0.651 | 0.657 | 0.143 |
| 20% | 0.174 | 10.157 | 4.250 | 1.479 | 0.557 | 0.658 | 0.661 | 0.144 | |
| 30% | 0.242 | 11.043 | 4.237 | 1.480 | 0.554 | 0.655 | 0.657 | 0.144 | |
| 40% | 0.318 | 11.490 | 4.255 | 1.487 | 0.552 | 0.656 | 0.658 | 0.149 | |
| 50% | 0.373 | 12.057 | 4.255 | 1.490 | 0.552 | 0.657 | 0.658 | 0.151 |
| Method (Weight) | A–NE | CV | Method (Weight) | E–NE | CV |
|---|---|---|---|---|---|
| A–NE | 0.3650 | 0.8392 | E–NE | 0.6712 | 0.8426 |
| A–NE&CV 19 | 0.3676 | 1.0000 | E–NE&CV 19 | 0.6545 | 1.0000 |
| A–NE&CV 28 | 0.3676 | 1.0000 | E–NE&CV 28 | 0.6540 | 1.0000 |
| A–NE&CV 37 | 0.3676 | 1.0000 | E–NE&CV 37 | 0.6541 | 1.0000 |
| A–NE&CV 46 | 0.3676 | 1.0000 | E–NE&CV 46 | 0.6542 | 1.0000 |
| A–NE&CV 55 | 0.3678 | 1.0000 | E–NE&CV 55 | 0.6542 | 1.0000 |
| A–NE&CV 64 | 0.3677 | 1.0000 | E–NE&CV 64 | 0.6557 | 0.9967 |
| A–NE&CV 73 | 0.3676 | 1.0000 | E–NE&CV 73 | 0.6580 | 0.9902 |
| A–NE&CV 82 | 0.3670 | 0.9902 | E–NE&CV 82 | 0.6627 | 0.9707 |
| A–NE&CV 91 | 0.3660 | 0.9577 | E–NE&CV 91 | 0.6682 | 0.9186 |
| Population | N | P | Na | Ne | I | Ho | He | uHe | F |
|---|---|---|---|---|---|---|---|---|---|
| Whole collection | 607 | 0.563 | 13.619 | 4.282 | 1.497 | 0.553 | 0.657 | 0.657 | 0.149 |
| A–NE&CV73 | 182 | 0 | 13.619 | 4.395 | 1.534 | 0.562 | 0.666 | 0.668 | 0.147 |
| E–NE&CV55 | 182 | 0 | 13.619 | 4.601 | 1.583 | 0.527 | 0.683 | 0.685 | 0.224 |
| A–NE&CV73-R | 425 | 0.348 | 11.048 | 4.212 | 1.471 | 0.549 | 0.652 | 0.653 | 0.149 |
| E–NE&CV55-R | 425 | 0.541 | 10.952 | 4.122 | 1.446 | 0.565 | 0.644 | 0.645 | 0.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Duan, A.; Wang, X.; Chen, Z.; Zhang, X.; He, G.; Zhang, J. Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards. Forests 2023, 14, 305. https://doi.org/10.3390/f14020305
Wu H, Duan A, Wang X, Chen Z, Zhang X, He G, Zhang J. Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards. Forests. 2023; 14(2):305. https://doi.org/10.3390/f14020305
Chicago/Turabian StyleWu, Hanbin, Aiguo Duan, Xihan Wang, Zhiyun Chen, Xie Zhang, Guiping He, and Jianguo Zhang. 2023. "Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards" Forests 14, no. 2: 305. https://doi.org/10.3390/f14020305
APA StyleWu, H., Duan, A., Wang, X., Chen, Z., Zhang, X., He, G., & Zhang, J. (2023). Construction of a Core Collection of Germplasms from Chinese Fir Seed Orchards. Forests, 14(2), 305. https://doi.org/10.3390/f14020305





