Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Establishment
2.2. Dynamic Simulation
3. Results and Discussion
3.1. Energy
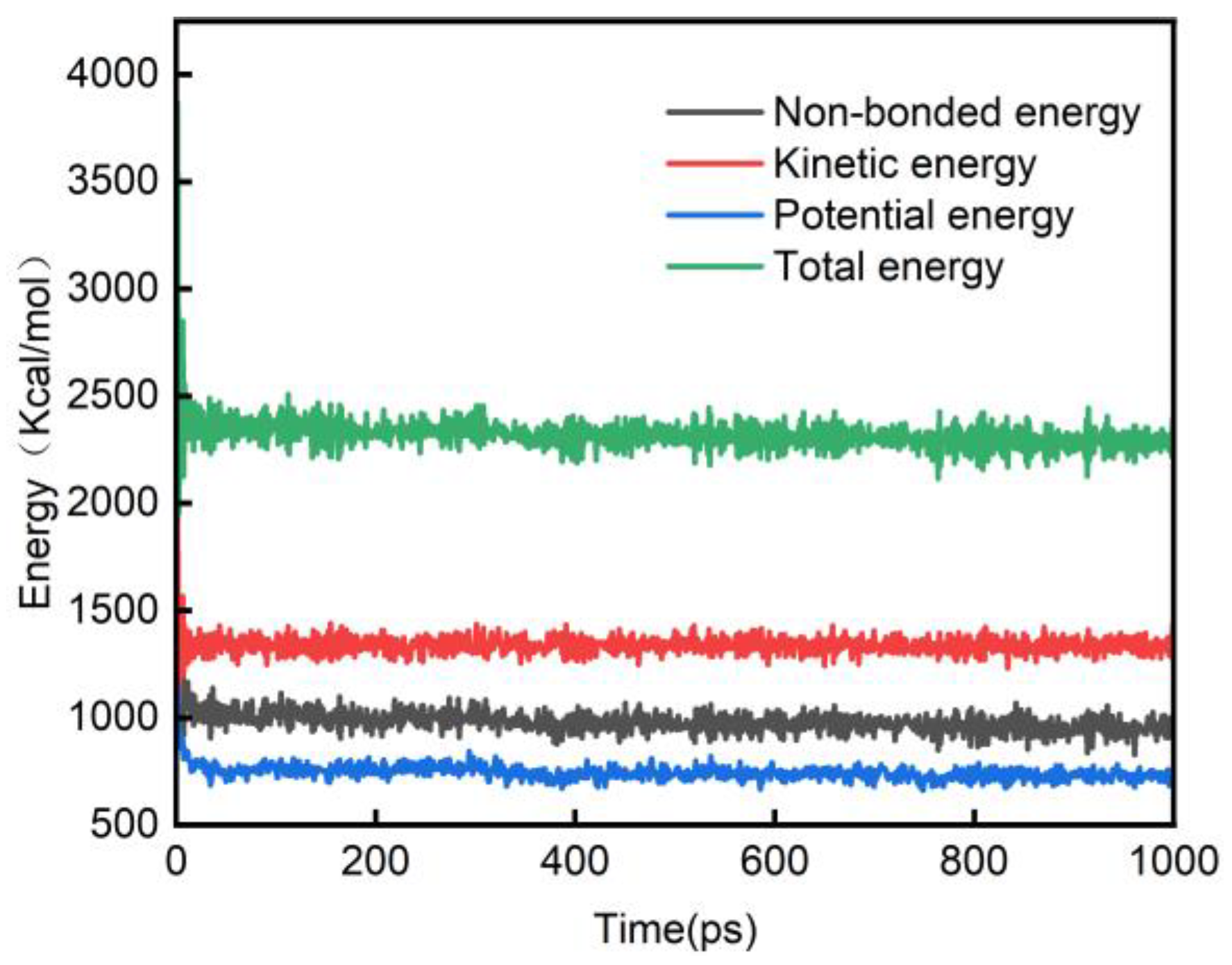
3.1.1. System Energy
3.1.2. Total Potential Energy and Nonbonding Energy
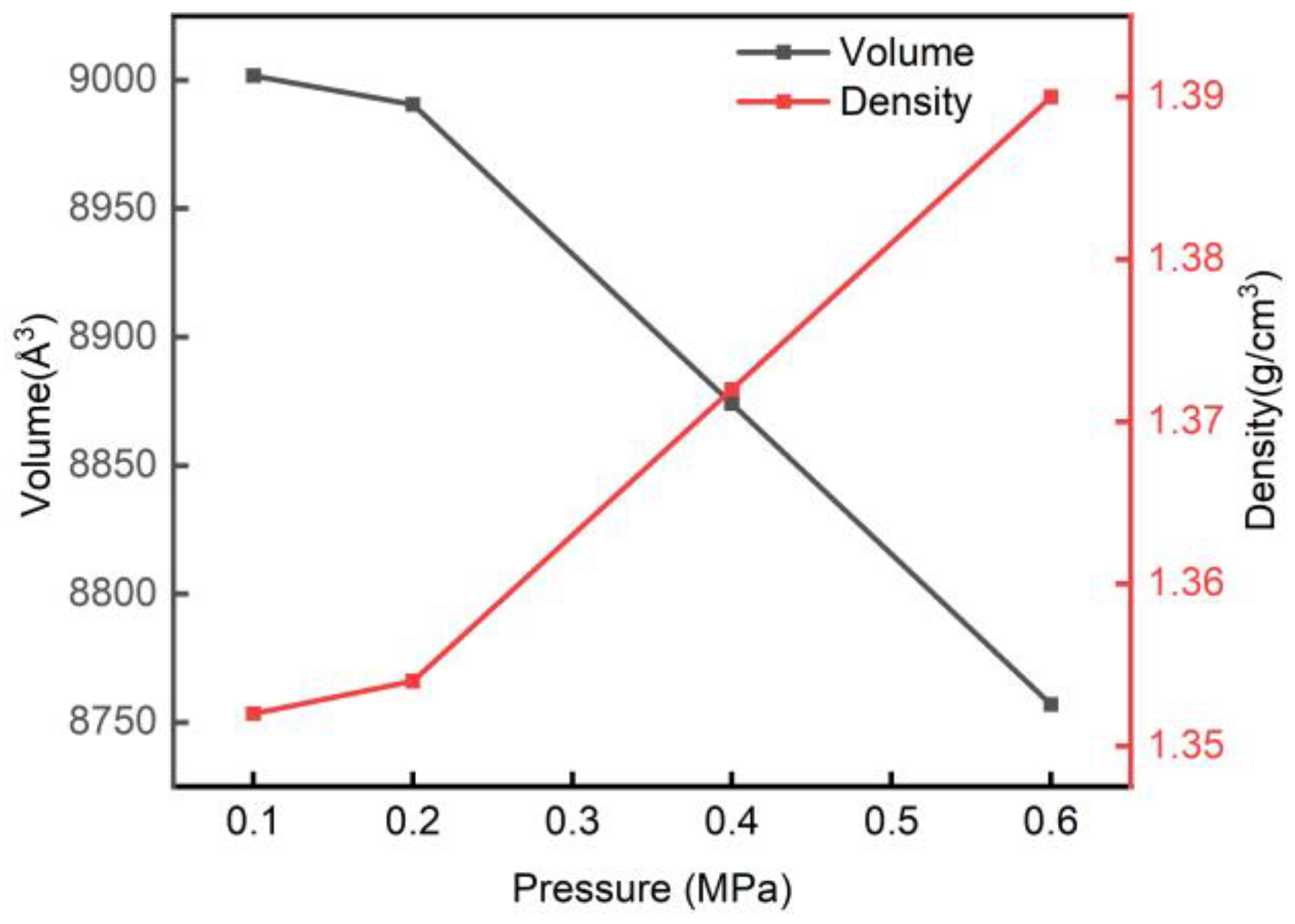
3.2. Lattice Parameters and Density
3.3. Mean Square Displacement of the Cellulose Chains
3.4. Hydrogen Bonding
3.5. Mechanical Properties
4. Conclusions
- With increasing pressure, the difference between the average value of the nonbond energy and the total potential energy gradually decreases, and the interatomic repulsion within the molecule gradually weakens. The bond energy also decreases with increasing pressure, indicating that pressurization has a positive effect on the amorphous region–water model of cellulose and enhances the structural stability of the amorphous cellulose region.
- The internal structure of the model is affected by pressure, which reduces the volume and increases the density, creating new interchain hydrogen bonds and enhancing the restraining effect on the arrangement of cellulose molecular chains. At the same time, the increase in weak van der Waals forces that stabilize the stacking of the lamellar structure also reduce the distance between the cellulose chains and slow down the cellulose chain movement. It was shown that the pressurized hydrothermal treatment improves the denseness of the cellulose amorphous region.
- Young’s modulus () and shear modulus () increase with increasing pressure, and Poisson’s ratio () and decrease with increasing pressure during the pressurized hydrothermal treatment of wood. The pressurized hydrothermal treatment increases the stiffness and decreases the toughness of the wood compared with those of the model with atmospheric pressure hydrothermal treatment. In addition, the changes in mechanical properties were corroborated by the changes in the number of hydrogen bonds. The increase in hydrogen bonding also increases the van der Waals forces in the interchain contacts, and the enhanced intermolecular forces lead to a more dense structure of the entire cellulose system. Thus, pressurized hydrothermal treatment of wood can significantly improve the resistance to deformation.
- The effect of pressurized hydrothermal treatment on the properties of the cellulose amorphous region was simulated from a microscopic perspective; model energy changes, cell parameters, mean square displacement, mechanical parameters, and hydrogen bonding were all analyzed to more intuitively explain how pressurized hydrothermal treatment acts on cellulose, the main component of wood.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Esteves, B.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 370–404. [Google Scholar] [CrossRef]
- Pelaez-Samaniego, M.R.; Yadama, V.; Lowell, E.; Espinoza-Herrera, R. A review of wood thermal pretreatments to improve wood composite properties. Wood Sci. Technol. 2013, 47, 1285–1319. [Google Scholar] [CrossRef]
- Stamm, A.J.; Burr, H.K.; Kline, A.A. Staybwood—Heat-stabilized wood. Ind. Eng. Chem. 1946, 38, 630–634. [Google Scholar] [CrossRef]
- Mohebby, B.; Ilbeighi, F.; Kazemi-Najafi, S. Influence of hydrothermal modification of fibers on some physical and mechanical properties of medium density fiberboard (MDF). Holz Als Roh-Und Werkstoff 2008, 66, 213–218. [Google Scholar] [CrossRef]
- Ding, T.; Gu, L.; Cai, J. Effect of heat treatment on moisture absorption properties and dimensional stability of wood. J. Nanjing For. Univ. 2015, 39, 143–147. [Google Scholar]
- Li, Y.; Tang, R.; Bao, B.; Fu, S. Study on mechanical properties and dimensional stability of high temperature heat treated fir wood. J. Beijing For. Univ. 2010, 32, 232–236. [Google Scholar]
- Kamperidou, V. Chemical and structural characterization of poplar and black pine wood exposed to short thermal modification. Drvda Ind. 2021, 72, 155–167. [Google Scholar] [CrossRef]
- Karlsson, O.; Sidorova, E.; Morén, T. Influence of heat transferring media on durability of thermally modified wood. BioResources 2011, 6, 356–372. [Google Scholar] [CrossRef]
- Rahimi, S.; Singh, K.; DeVallance, D. Effect of different hydrothermal treatments (steam and hot compressed water) on physical properties and drying behavior of yellow-poplar (Liriodendron tulipifera). For. Prod. J. 2019, 69, 42–52. [Google Scholar] [CrossRef]
- Ali, M.R.; Abdullah, U.H.; Ashaari, Z.; Hamid, N.H.; Hua, L.S. Hydrothermal modification of wood: A review. Polymers 2021, 13, 2612. [Google Scholar] [CrossRef]
- Ding, T.; Gu, L.; Wu, Z. Influence of steam pressure on mechanical properties of heat-treated wood. China For. Prod. Ind. 2010, 37, 32. [Google Scholar]
- Qi, H.; Cheng, W.; Liu, Y. Mechanical characteristics and chemical compositions of superheated steam-treated wood under high temperature and pressure. J. North-East For. Univ. 2005, 33, 44–46. [Google Scholar]
- Ding, T.; Gu, L.; and Liu, X. Comparative on chemical component changes of pressurized-steam-treated Mongolian oak. Sci. Silvae Sin. 2012, 48, 148–152. [Google Scholar]
- Jiang, J.; Lu, J.; Zhou, Y.; Huang, R.; Zhao, Y.; and Jiang, J. Optimization of processing variables during heat treatment of oak (Quercus mongolica) wood. Wood Sci. Technol. 2014, 48, 253–267. [Google Scholar] [CrossRef]
- Rosen H, N. Pressure steam drying of lumber. For. Prod. J. 1983, 33, 17–24. [Google Scholar]
- Khazraji, A.C.; Robert, S. Interaction effects between cellulose and water in nanocrystalline and amorphous regions: A novel approach using molecular modeling. J. Nanomater. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Hou, T.J.; Zhang, W.; Xu, X.J. Binding affinities for a series of selective inhibitors of gelatinase-A using molecular dynamics with a linear interaction energy approach. J. Phys. Chem. B 2005, 105, 5304–5315. [Google Scholar] [CrossRef]
- Wang, W.; Ma, W.; Wu, M.; Sun, L. Effect of Water Molecules at Different Temperatures on Properties of Cellulose Based on Molecular Dynamics Simulation. BioResources 2020, 17, 269–280. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, X.; Yan, Q.; Hu, M. Temperature dependence of mechanical state for nitrocellulose/nitroglycerin blend. J. Propuls. Technol. 2016, 37, 1387–1392. [Google Scholar]
- Zhu, M.Z.; Chen, Y.F.; Chao, K.; Liao, R.J.; Zhu, W.B.; Du, X.M. Molecular dynamics-based simulation of thermodynamic properties of amorphous cellulose. High Volt. Technol. 2015, 41, 432–439. [Google Scholar]
- Theodorou, D.N.; Suter, U.W. Detailed molecular structure of a vinyl polymer glass. Macromolecules 1985, 18, 1467–1478. [Google Scholar] [CrossRef]
- Mazeau, K.; Heux, L. Molecular dynamics simulations of bulk native crystalline and amorphous structures of cellulose. J. Phys. Chem. B 2003, 107, 2394–2403. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Clarendon Press: Oxford, UK, 1987. [Google Scholar]
- Jiang, J.H. Research on the change of properties and mechanism of superheated steam treatment of Quercus serrata. China Acad. For. Sci. 2013. [Google Scholar]
- Ewald, P. Evaluation of optical and electrostatic lattice potentials. Ann. Phys. 1921, 64, 253–287. [Google Scholar] [CrossRef]
- Andersen, H.C. Molecular dynamics simulations at constant pressure and/or temperature. J. Chem. Phys. 1980, 72, 2384–2393. [Google Scholar] [CrossRef]
- Andrea, T.A.; Swope, W.C.; Andersen, H.C. The role of long ranged forces in determining the structure and properties of liquid water. J. Chem. Phys. 1983, 79, 4576–4584. [Google Scholar] [CrossRef]
- Berendsen, H.J.; Postma, J.P.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Wang, W.; Wang, Y.; Li, X. Molecular dynamics study on mechanical properties of cellulose with air/nitrogen diffusion behavior. BioResources 2018, 13, 7900–7910. [Google Scholar] [CrossRef]
- Sun, H. Ab initio calculations and force field development for computer simulation of polysilanes. Macromolecules 1995, 28, 701–712. [Google Scholar] [CrossRef]
- Maple, J.R.; Hwang, M.J.; Stockfisch, T.P.; and Hagler, A.T. Derivation of class II force fields. III. Characterization of a quantum force field for alkanes. Isr. J. Chem. 2013, 34, 195–231. [Google Scholar] [CrossRef]
- Wang, X.; Tu, D.; Chen, C.; Zhou, Q.; Huang, H.; Zheng, Z.; Zhu, Z. A thermal modification technique combining bulk densification and heat treatment for poplar wood with low moisture content. Constr. Build. Mater. 2021, 291, 123395. [Google Scholar] [CrossRef]
- David, G.; Orszag, S.A. Numerical Analysis of Spectral Methods: Theory and Applications; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1977. [Google Scholar]
- Zhu, M.Z.; Liao, R.J.; Zhou, X. Molecular dynamics of hydrated hydrogen ion diffusion in oil media mechanics simulation. High Volt. Technol. 2011, 37, 1930–1936. [Google Scholar]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Taghiyari, H.R.; Bayani, S.; Militz, H.; Papadopoulos, A.N. Heat treatment of pine wood: Possible effect of impregnation with silver nanosuspension. Forests 2020, 11, 466. [Google Scholar] [CrossRef]
- Bao, M.; Huang, X.; Jiang, M.; Yu, W.; Yu, Y. Effect of thermo-hydro-mechanical densification on microstructure and properties of poplar wood (Populus tomentosa). J. Wood Sci. 2017, 63, 591–605. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, F.; Zhu, S.; Li, H. Effects of high-pressure treatment on poplar wood: Density profile, mechanical properties, strength potential index, and microstructure. BioResources 2017, 12, 6283–6297. [Google Scholar] [CrossRef]
- Du, D.; Tang, C.; Zhang, J.; Hu, D. Effects of hydrogen sulfide on the mechanical and thermal properties of cellulose insulation paper: A molecular dynamics simulation. Mater. Chem. Phys. 2020, 240, 122153. [Google Scholar] [CrossRef]





| Pressure (MPa) | 0.1 | 0.2 | 0.4 | 0.6 |
|---|---|---|---|---|
| Bond energy (kcal/mol) | 179.847 | 159.015 | 112.822 | 106.209 |
| Pressure (MPa) | Cell Parameters (Å) | ||
|---|---|---|---|
| The Length | The Width | The Height | |
| 0.1 | 20.80 | 20.80 | 20.80 |
| 0.2 | 20.79 | 20.79 | 20.79 |
| 0.4 | 20.70 | 20.70 | 20.70 |
| 0.6 | 20.61 | 20.61 | 20.61 |
| Pressure (MPa) | 0.1 | 0.2 | 0.4 | 0.6 |
|---|---|---|---|---|
| 7.3312 | 10.2289 | 15.2678 | 16.6379 | |
| 5.8452 | 5.0467 | 6.3971 | 5.5905 | |
| 3.9229 | 2.4219 | 4.0591 | 1.3101 | |
| 1.9223 | 2.6248 | 2.338 | 4.2804 |
| Pressure (MPa) | 0.1 | 0.2 | 0.4 | 0.6 |
|---|---|---|---|---|
| 2.1452 | 6.8942 | 11.4899 | 13.8258 | |
| 0.7430 | 2.5911 | 4.4354 | 5.5237 | |
| 0.4436 | 0.3304 | 0.2952 | 0.2515 | |
| 9.0746 | 2.0470 | 1.4762 | 1.0339 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, X.; Wang, W.; Guo, Y.; Dai, M. Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation. Forests 2023, 14, 314. https://doi.org/10.3390/f14020314
Jiang X, Wang W, Guo Y, Dai M. Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation. Forests. 2023; 14(2):314. https://doi.org/10.3390/f14020314
Chicago/Turabian StyleJiang, Xuewei, Wei Wang, Yuanyuan Guo, and Min Dai. 2023. "Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation" Forests 14, no. 2: 314. https://doi.org/10.3390/f14020314
APA StyleJiang, X., Wang, W., Guo, Y., & Dai, M. (2023). Effect of Pressurized Hydrothermal Treatment on the Properties of Cellulose Amorphous Region Based on Molecular Dynamics Simulation. Forests, 14(2), 314. https://doi.org/10.3390/f14020314









