Softwood Anatomy: A Review
Abstract
:1. Gymnosperms
2. Geographical Distribution of Conifers
3. Softwood Structure
3.1. Literature
3.2. Softwood Identification
4. Material and Methods
5. Microscopic Features
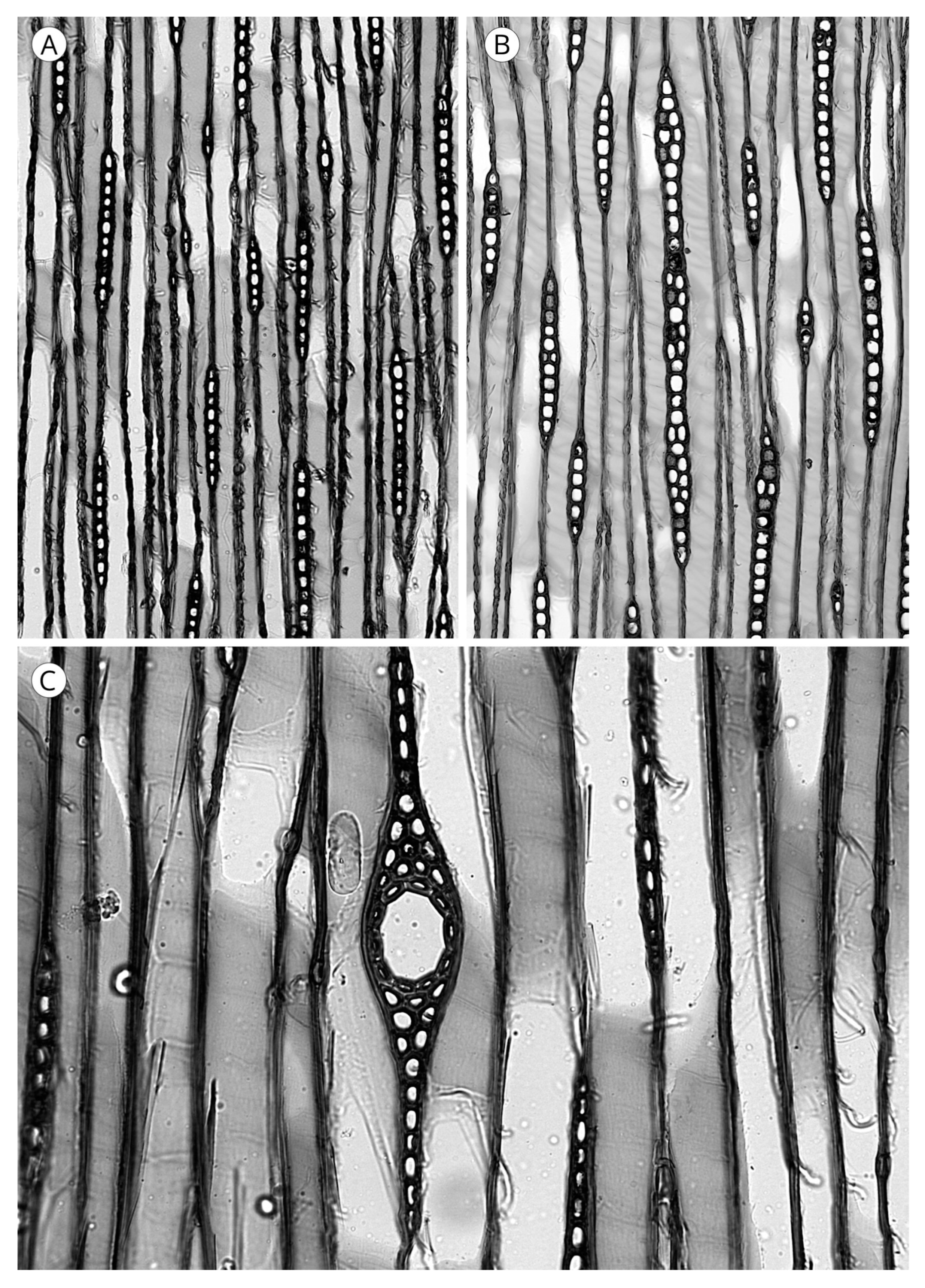
5.1. Axial Tracheids
5.1.1. Tracheid Pits
5.1.2. Warty Layer
5.1.3. Organic Deposits
5.1.4. Helical Thickenings
5.1.5. Callitroid Thickenings
5.1.6. Bars of Sanio
5.1.7. Trabeculae
5.2. Axial Parenchyma
5.3. Rays
5.3.1. Ray Tracheids
5.3.2. Ray Parenchyma
5.3.3. Cross-Field Pitting
5.4. Resin Canals
5.5. Mineral Inclusions
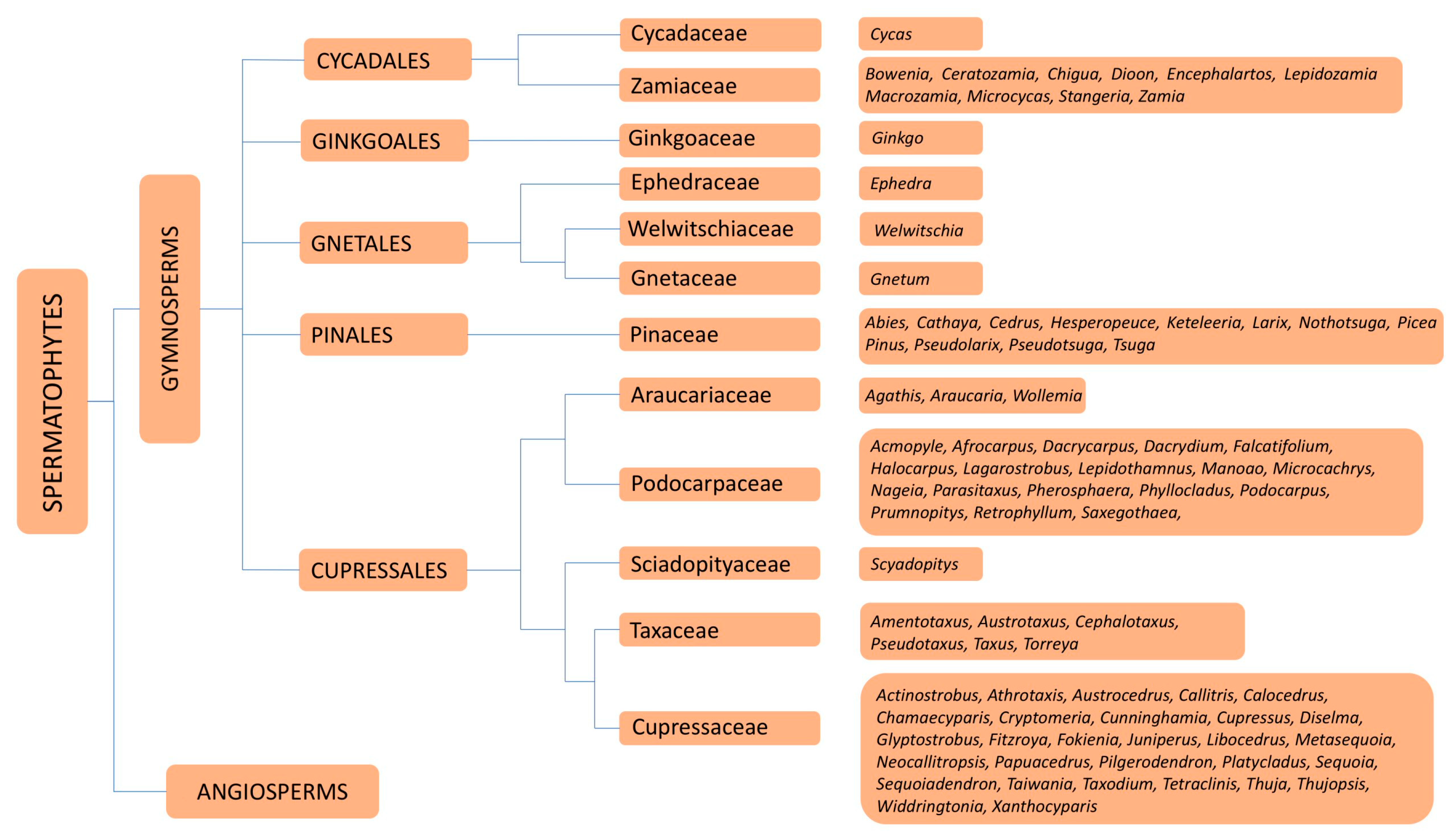
6. Families and Genera
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cole, T.C.H.; Bachelier, J.B.; Hilger, H.H. Tracheophyte Phylogeny Poster—Vascular Plants: Systematics and Characteristics. PeerJ. Prepr. 2019, 7, e2614v3. [Google Scholar] [CrossRef]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2021. Available online: http://www.plantsoftheworldonline.org/ (accessed on 8 December 2021).
- Wang, L.; Wang, D.; Lin, M.M.; Lu, Y.; Jang, X.X.; Jin, B. An embryological study and systematic significance of the primitive gymnosperm Ginkgo biloba. J. Syst. Evol. 2011, 49, 353–361. [Google Scholar] [CrossRef]
- Torelli, N. Dvokrpi ginko (Ginkgo biloba L.) in njegov les [Maidenhair tree (Ginkgo biloba L.) and its wood]. Les 1999, 51, 397–402. [Google Scholar]
- IAWA Committee. IAWA list of microscopic features for softwood identification. IAWA J. 2004, 25, 1–70. [Google Scholar] [CrossRef]
- Terrazas, T. Origin and activity of successive cambia in Cycas (Cycadales). Am. J. Bot. 1991, 78, 1335–1344. [Google Scholar] [CrossRef]
- Carlquist, S. Wood, Bark, and Pith Anatomy of Old World Species of Ephedra and Summary for the Genus. Aliso 1992, 13, 255–295. [Google Scholar] [CrossRef]
- Thompson, W.P. The anatomy and relationships of the Gnetales. I. The genus Ephedra. Ann. Bot. 1912, 26, 1077–1104. [Google Scholar] [CrossRef]
- Bailey, I.W. The development of vessels in angiosperms and its significance in morphological research. Am. J. Bot. 1944, 31, 421–428. [Google Scholar] [CrossRef]
- Muhammad, A.F.; Sattler, R. Vessel structure of Gnetum and origin of angiosperms. Am. J. Bot. 1982, 69, 1004–1021. [Google Scholar] [CrossRef]
- Carlquist, S.; Gowans, D.A. Secondary growth and wood histology of Welwitschia. Bot. J. Linn. Soc. 1995, 118, 107–121. [Google Scholar] [CrossRef]
- Carlquist, S. Wood Anatomy of Gnetales in a Functional, Ecological, and Evolutionary Context. Aliso 2012, 30, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Carlquist, S. Wood, bark, and stem anatomy of gnetales: A summary. Int. J. Plant Sci. 1996, 157, S58–S76. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P. Comparative wood anatomy in Abietoideae (Pinaceae). Bot. J. Linn. Soc. 2009, 160, 184–196. [Google Scholar] [CrossRef]
- Gernandt, D.S.; Magallón, S.; López, G.G.; Flores, O.Z.; Willyard, A.; Liston, A. Use of simultaneous analyses to guide fossil-based calibrations of Pinaceae phylogeny. Int. J. Plant Sci. 2008, 169, 1086–1099. [Google Scholar] [CrossRef]
- Farjon, A.; Hiep, N.T.; Harder, D.K.; Loc, P.K.; Averyanov, L. A new genus and species in Cupressaceae (Coniferales) from northern Vietnam, Xanthocyparis vietnamensis. Novon 2002, 12, 179–189. [Google Scholar] [CrossRef]
- Neale, D.B.; Wheeler, N.C. The Conifers: Genomes, Variation and Evolution; Springer: Cham, Switzerland, 2019; p. 590. [Google Scholar] [CrossRef]
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers. An Analysis of Their Distribution, Biogeography, Diversity and Conservation Status; Ed. Brill.: London, UK, 2013; p. 512. [Google Scholar]
- Farjon, A. World Checklist and Bibliography of Conifers, 2nd ed.; Royal Botanic Gardens at Kew: London, UK, 2001. [Google Scholar]
- Sanio, C. Einige Bemerkungen uber den Bau des Holzes. Bot. Z 1860, 18, 193–198, 201–204, 209–217. [Google Scholar]
- Sanio, C. Anatomie der gemeinen Kiefer (Pinus silvestris L.). Jahrb. Wiss. Bot. 1873, 9, 50–126. [Google Scholar]
- Castellarnau, J.M. Estudio Micrográfico del Tallo del Pinsapo (Abies pinsapo Boiss.); Anales de la Sociedad Española de Historia Natural: Madrid, Spain, 1880. [Google Scholar]
- Kleeberg, A. Die Markstrahlen der Coniferen. Bot. Zeit. 1885, 43, 673–686. [Google Scholar]
- Penhallow, D.P. The generic characters of the North American Taxaceae and Coniferae. Mémoires et comptes rendus de la Société Royale du Canada. Proc. Trans. R. Soc. Can. 1896, 2, 33–57. [Google Scholar]
- Jeffrey, E.C. The comparative anatomy and phylogeny of the Coniferales. Part 1. The genus Sequoia. Mem. Boston Soc. Nat. Hist. 1903, 5, 441–459. [Google Scholar]
- Bitting, K.G. The histological difference between Pinus taeda and P. palustris. Proc. Ind. Acad. Sci. 1908, 1, 127–132. [Google Scholar]
- Bailey, I.W. The Structure of the Wood in the Pineae. Bot. Gaz. 1909, 48, 47–55. [Google Scholar] [CrossRef]
- Brooks, F.T.; Stiles, W. The Structure of Podocarpus spinulosus, (Smith) R. Br. Ann. Bot.-Lond. 1910, 24, 305–318. [Google Scholar] [CrossRef]
- Baker, R.T.; Smith, H.G. A Research on the Pines of Australia; The Government of the State of New South Wales, William Applegate Gullick, Government Printer: Sydney, Australia, 1910; p. 458. [Google Scholar]
- Thompson, W.P. The origin of the ray tracheids in the Coniferae. Bot. Gaz. 1910, 50, 101–116. [Google Scholar] [CrossRef]
- Thompson, W.P. Ray tracheids in Abies. Bot. Gaz. 1912, 53, 331–338. [Google Scholar] [CrossRef]
- Gordon, M. Ray Tracheids in Sequoia sempervirens. New Phytol. 1912, 11, 1–6. [Google Scholar] [CrossRef]
- Holden, R. Ray tracheids in the Coniferales. Bot. Gaz. 1913, 55, 56–65. [Google Scholar] [CrossRef]
- Chrysler, M.A. The medullary ray of Cedrus. Bot. Gaz. 1915, 59, 387–396. [Google Scholar] [CrossRef]
- Jeffrey, E.C. The Anatomy of Woody Plants; University of Chicago Press: Chicago, CA, USA, 1917. [Google Scholar]
- Kanehira, R. Anatomical Characters and Identification of Formosan Woods with Critical Remarks from the Climatic Point of View; Bureau of Productive Industries, Government of Formosa: Taipei, Taiwan, 1921. [Google Scholar]
- Kanehira, R. Anatomical Characters and Identification of the Important Woods of the Japanese Empire; Bureau of Productive Industries, Government of Formosa: Taipei, Taiwan, 1926. [Google Scholar]
- Saint-Laurent, J. Études sur les caractères anatomiques des bois d’Algérie. I Bull. Sta. Rech For. N. Afr. 1926, 1, 241–255. [Google Scholar]
- Patton, R.T. Anatomy of Australian coniferous timbers. Proc. R. Soc. Vic. 1927, 40, 2–16. [Google Scholar]
- Pool, D.J.W. On the Anatomy of Araucarian Wood. Recl. Des. Trav. Bot. Neerl. 1929, 25, 482–620. [Google Scholar]
- Metcalfe, C.R. The wood structure of Fokienia hodginsii and certain related coniferae. Bull. Misc. Inf. (R. Bot. Gard. Kew) 1931, 8, 420–425. [Google Scholar] [CrossRef]
- Brown, H.P.; Panshin, A.J. Commercial Timbers of the United States: Their Structure, Identification, Properties, and Uses; McGraw-Hill: New York, NY, USA, 1934. [Google Scholar]
- Brem, M. Anatomical method for determining the wood of the spruce and larch. Bull. Intern. Acad. Sei. Lett. Ser B (I) 1934, 8, 103–111. [Google Scholar]
- Bannan, M.W. Vertical resin ducts in the secondary wood of the Abietineae. New Phytol. 1936, 35, 11–46. [Google Scholar] [CrossRef]
- Peirce, A.S. Anatomy of the Xylem of Pseudolarix. Bot. Gaz. 1934, 95, 667–677. [Google Scholar] [CrossRef]
- Peirce, A.S. Anatomy of the xylem of Sciadopitys. Am. J. Bot. 1935, 22, 895–902. [Google Scholar] [CrossRef]
- Peirce, A.S. Anatomical interrelationships of the Taxodiaceae. Trop. Woods 1936, 46, 1–15. [Google Scholar]
- Peirce, A.S. Systematic anatomy of the woods of the Cupressaceae. Trop. Woods 1937, 49, 5–21. [Google Scholar]
- Shimakura, M. Anatomy of the wood of Taiwania. Bot. Mag. 1937, 51, 694–700. [Google Scholar] [CrossRef]
- Bernath, E.L. Coniferous forest trees of Chile. Trop. Woods 1937, 52, 19–26. [Google Scholar]
- Covas, G. Las coníferas indígenas de la República Argentina. Rev. Fac. Agron. B Aires 1938, 21, 201–203. [Google Scholar]
- Phillips, E.W.J. The identification of coniferous wood by their microscopic structure. Bot. J. Linn. Soc. 1941, 52, 259–320. [Google Scholar] [CrossRef]
- Greguss, P. Identification of Living Gymnosperms on the Basis of Xylotomy; Akadémiai Kiado: Budapest, Hungary, 1955; p. 263. [Google Scholar]
- Kukachka, F. Identification of coniferous wood. Tappi 1960, 43, 887–896. [Google Scholar]
- Barefoot, A.; Hankins, F.W. Identification of Modern and Tertiary Woods; Oxford University Press: New York, NY, USA, 1982; p. 189. [Google Scholar]
- Esteban, L.G.; de Palacios, P.; Guindeo, A.; García, L.; Lázaro, I.; González, L.; Rodríguez, Y.; García, F.; Bobadilla, I.; Camacho, A. Anatomy and Identification of Conifers Wood as a Species; Fundación Conde del Valle de Salazar—Mundi Prensa: Madrid, Spain, 2002; p. 421. [Google Scholar]
- Heinz, I. Systematische Erfassung und Dokumentation der mikroanatomischen Merkmale der Nadelhölzer aus der Klasse der Pinatae. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2004; p. 209.
- Clarke, S.H. A multiple-entry perforated-card key with special reference to the identification of hardwoods. New Phytol. 1938, 37, 369–374. [Google Scholar] [CrossRef]
- Kennedy, R.W.; Sastry, C.B.R.; Barton, G.M.; Ellis, E.L. Crystals in wood of genus Abies indigenous to Canada and United States. Can. J. Bot. 1968, 46, 1221–1228. [Google Scholar] [CrossRef]
- Sudo, S. Anatomical studies on the wood of species of Picea, with some considerations on their geographical distribution and taxonomy. Bull. Govt. Exp. Stat. 1968, 215, 39–130. [Google Scholar]
- Werker, E.; Fahn, A. Resin ducts of Pinus halepensis MilI.—Their structure, development and pattern of arrangement. Bot. J. Linn. Soc. 1969, 62, 379–411. [Google Scholar] [CrossRef]
- Bauch, J.; Liese, W.; Schultze, R. The morphological variability of the bordered pit membranes in gymnosperms. Wood Sci. Technol. 1972, 6, 165–184. [Google Scholar] [CrossRef]
- Bosshard, H.H. Holzkunde—Mikroskopie und Makroskopie des Holzes, Band 1; Birkhauser, Basel: Stuttgart, Germany, 1974. [Google Scholar]
- Core, H.A.; Cóté, W.A.; Day, A.C. Wood structure and identification; Syracuse University Press: New York, NY, USA, 1979. [Google Scholar]
- Chavchavadze, E.S. Wood of Conifers (Drevesina khvoinykh); Original in Russian; Akad. Nauk SSSR: Moscow-Leningrad, Russia, 1979. [Google Scholar]
- Bartholin, T. The Picea-Larix problem. IAWA Bull. 1979, 1, 7–10. [Google Scholar]
- Panshin, A.J.; De Zeeuw, C. Textbook of Wood Technology, 4th ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Baas, P.; Schmid, R.; van Heuven, B.J. Wood anatomy of Pinus longaeva (bristlecone pine) and the sustained Iength-on-age increase of its tracheids. IAWA Bull. 1986, 7, 221–228. [Google Scholar] [CrossRef]
- Suzuki, M.; Noshiro, S. Wood structure of Himalayan plants. In Himalayan Plants; Ohba, H., Malla, S.B., Eds.; The University Museum, the University of Tokyo, Bulletin Tokyo: Tokyo, Japan, 1988; Volume 31, pp. 341–379. [Google Scholar]
- LaPasha, C.A.; Wheeler, E.A. Resin canals in Pinus taeda: Longitudinal canal lengths and interconnections between longitudinal and radial canals. IAWA Bull. 1990, 11, 227–238. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Anatomy of European Woods; Paul Haupt: Berne, Switzerland, 1990. [Google Scholar]
- Roig, F.A. Comparative wood anatomy of southern South American Cupressaceae. IAWA Bull. 1992, 13, 151–162. [Google Scholar] [CrossRef]
- Vogel, K. Mikroskopische Untersuchung zur Typisierung der Kreuzungsfeldtupfel bei Nadelholzern. Diplomarbeit der Forstwissenschaftlichen. Ph.D. Thesis, Fakultat der Ludwig-Maximilians- Universitat München, Munich, Germany, 1995. [Google Scholar]
- Ilic, J. Separation of the woods of Callitris glaucophylla (white cypress pine) and C. endlicheri (black cypress pine). Recent Advances in Wood Anatomy. In Proceedings of the IAWA Wood Anatomy Conference, Rotorua, New Zealand, 20–24 November 1994. [Google Scholar]
- Kondo, Y.; Fujii, T.; Hayashi, Y.; Kato, A. Organic crystals in the tracheids of Torreya yunnanensis. IAWA J. 1996, 17, 393–403. [Google Scholar] [CrossRef]
- Heinz, I. Entwicklung von Systemkomponenten für die computerunterstützte Bestimmung von Nadelhölzern in DELTA/INTKEY. Diplomarbeit. Ph.D. Thesis, Universitat Hamburg, Hamburg, Germany, 1997. [Google Scholar]
- Kellogg, R.M.; Rowe, S.; Koeppen, R.C.; Miller, R.B. Identification of the wood of the soft pines of western North America. IAWA Bull. 1982, 3, 95–101. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Itoh, T.; Shimaji, K. Helical thickenings in normal and compression wood of some softwoods. IAWA Bull. 1985, 6, 131–138. [Google Scholar] [CrossRef]
- Esteban, L.G.; Guindeo, A.; de Palacios, P. Maderas de Coníferas. Anatomía de Géneros; Fundación Conde del Valle de Salazar: Madrid, Spain, 1996; p. 336. [Google Scholar]
- Heady, R.D.; Evans, P.D. Callitroid (callitrisoid) thickening in Callitris. IAWA J. 2000, 21, 293–319. [Google Scholar] [CrossRef]
- Heady, R.D.; Banks, J.D.; Evans, P.D. Wood anatomy of Wollemi Pine (Wollemia nobilis, Araucariaceae). IAWA J. 2002, 23, 339–358. [Google Scholar] [CrossRef]
- Wiedenhoeft, A.C.; Miller, R.B. Brief comments on the nomenclature of softwood axial resin canals and their associated cells. IAWA J. 2002, 23, 299–303. [Google Scholar] [CrossRef]
- Visscher, G.E.; Jagels, R. Separation of Metasequoia and Glyptostrobus (Cupressaceae) based on wood anatomy. IAWA J. 2003, 24, 439–451. [Google Scholar] [CrossRef]
- Xiaomei, J. Atlas of Gymnosperms Woods of China; Science Press Ltd.: Beijing, China, 2010; p. 490. [Google Scholar]
- de Palacios, P.; Esteban, L.G.; García Fernández, F.; García-Iruela, A.; Conde, M.; Román-Jordán, E. Comparative wood anatomy of Juniperus from Macaronesia. IAWA J. 2014, 35, 186–198. [Google Scholar] [CrossRef]
- Román-Jordán, E. Anatomía comparada de la madera de Cupressaceae y su correspondencia con los estudios de filogenia. Ph.D. Thesis, Universidad Politécnica de Madrid, Madrid, Spain, 4 April 2016. [Google Scholar]
- Troncoso, O.; Greslebin, A. Trabeculae in Patagonian mountain cypress (Austrocedrus chilensis) associated with Phytophthora austrocedri infection. IAWA J. 2018, 39, 209–220. [Google Scholar] [CrossRef]
- Purusatama, B.D.; Kim, N.H. Cross-field pitting characteristics of compression, lateral, and opposite wood in the stem wood of Ginkgo biloba and Pinus densiflora. IAWA J. 2020, 41, 48–60. [Google Scholar] [CrossRef]
- InsideWood. 2004-Onwards. Available online: http://insidewood.lib.ncsu.edu/search (accessed on 11 October 2021).
- Watson, L.; Dallwitz, M.J. 2008 Onwards. The Families of Gymnosperms. Version: 5 August 2019. Available online: delta-intkey.com (accessed on 11 October 2021).
- Earle, C.J. The Gymnosperm Database, 2001 Owards. Available online: http://www.conifers.org (accessed on 20 November 2022).
- Galtier, J.; Meyer-Berthaud, B. The diversification of early arborescent seed ferns. J. Torrey Bot. Soc. 2006, 133, 7–19. [Google Scholar] [CrossRef]
- Brown, H.P.; Panshin, A.J.; Forsaith, C.C. Textbook of Wood Technology. Vol. I. Structure, Identification, Defects and Uses of the Commercial Woods of the United States; McGraw-Hill Book Company: New York, NY, USA, 1949; 652p. [Google Scholar]
- Esteban, L.G.; Guindeo, A.; Peraza, C.; de Palacios, P. La Madera y su Anatomía; Fundación Conde del Valle de Salazar, Ed.; Mundi-Prensa y AiTiM: Madrid, Spain, 2003; 327p. [Google Scholar]
- Wilson, J.P.; Knoll, A.H. A physiologically explicit morphospace for tracheid-based water transport in modern and extinct seed plants. Paleobiology 2010, 36, 335–355. [Google Scholar] [CrossRef]
- Decombeix, A.-L.; Boura, A.; Tomescu, A.M.F. Plant hydraulic architecture through time: Lessons and questions on the evolution of vascular systems. IAWA J. 2019, 40, 387–420. [Google Scholar] [CrossRef]
- Ladell, J.T. A new method of measuring tracheid length. Forestry 1959, 32, 124–125. [Google Scholar] [CrossRef]
- Wilkins, A.P.; Bamber, R.K. A comparison between Ladell’s wood section method and the macerated wood method for tracheid length determination. IAWA Bull. 1983, 4, 245–247. [Google Scholar] [CrossRef]
- Schweingruber, F.H. Dichteschwankungen in Jahrringen von Nadelhölzern in Beziehung zu klimatisch-ökologischen Faktoren, oder das Problem der Falschen Jahrringe, Bericht Nr. 213; Eidgenössische Anstalt für das forstliche Versuchswesen: Brimensdorf, Switzerland, 1980. [Google Scholar]
- Schweingruber, F.H. Tree Rings and Environment. Dendroecology; Paul Haupt: Berne, Switzerland, 1996; pp. 71–93. [Google Scholar]
- Willebrand, G. Untersuchung von ausgewählten mikroanatomischen Merkmalen zur Bestimmung von Nadelhölzern. Diplomarbeit. Ph.D. Thesis, Fachhochschule Rosenheim, Fachbereich Holztechnik, Rosenheim, Germany, 1995. [Google Scholar]
- Mio, S.; Matsumoto, T. Morphological observation on longitudinal intercellular spaces in normal softwoods. Bull. Kyushu Univ. For. 1979, 51, 13–18, (In Japanese with English abstract). [Google Scholar]
- Nagai, S.; Utsumi, Y. The function of intercellular spaces along the ray parenchyma in sapwood, intermediate wood, and heartwood of Cryptomeria japonica (Cupressaceae). Am. J. Bot. 2012, 99, 1553–1561. [Google Scholar] [CrossRef]
- Beck, C.B.; Wight, D.C. Progymnosperms. In Origin and Evolution of Gymnosperms; Beck, C.B., Ed.; Columbia University Press: New York, NY, USA, 1988; pp. 1–84. [Google Scholar]
- Prestianni, C.; Decombeix, A.-L.; Thorez, J.; Fokand, D.; Gerrienne, P. Famennian charcoal of Belgium. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 291, 60–71. [Google Scholar] [CrossRef]
- Decombeix, A.-L.; Meyer-Berthaud, B.; Galtier, J.; Talent, J.; Mawson, R. Diversity of arborescent lignophytes in the Tournaisian vegetation of Queensland (Australia): Paleoecological and paleogeographical significance. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 301, 39–55. [Google Scholar] [CrossRef]
- Morrow, A.C.; Dute, R.R. Development and structure of pit membranes in the rhizome of the woody fern Botrychium dissectum. IAWA J. 1998, 19, 429–441. [Google Scholar] [CrossRef]
- Bodnar, J.; Ruiz, D.P.; Artabe, A.E.E.; Morel, E.M.; Ganuza, D.G. Voltziales y Pinales (= Coniferales) de la Formación Cortaderita (Triásico Medio), Argentina, y su implicancia en la reconstrucción de las coníferas triásicas. Rev. Bras. Paleontol. 2015, 18, 141–160. [Google Scholar] [CrossRef]
- Jansen, S.; Lamy, J.B.; Burlett, R.; Cochard, H.; Gasson, P.; Delzon, S. Plasmodesmatal pores in the torus of bordered pit membranes affect cavitation resistance of conifer xylem. Plant Cell Env. 2012, 35, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Miyazaki, Y.; Wakashima, T. Electron microscopic investigation on the cell wall structure of wood. Bull. Govt. For. Exp. Stn. 1968, 104, 1–115. [Google Scholar]
- Liese, W. Elektronenmikroskopie des Holzes. In Handbuch der Mikroskopie in der Technik; Freund, H., Ed.; Umschau-Verlag: Frankfurt, Germany, 1970; pp. 109–170. [Google Scholar]
- Sano, Y.; Kawakami, Y.; Ohtani, J. Variation in the structure of intertracheary pit membranes in Abies sachalinensis, as observed by field-emission electron microscopy. IAWA J. 1999, 20, 375–388. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; García Fernández, F.; Moreno, R. Wood anatomy of the genus Abies: A review. IAWA J. 2009, 30, 231–245. [Google Scholar] [CrossRef]
- Liese, W. The warty layer. In Cellular Infrastructure of Woody Plants; Côté, W.A., Ed.; Syracuse University Press: Syracuse, NY, USA, 1965; pp. 251–269. [Google Scholar]
- Heady, R.D.; Evans, P.D. Wood anatomy of Actinostrobus (Cupressaceae). IAWA J. 2005, 26, 79–92. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; García-Iruela, A.; Román-Jordán, E.; García-Fernández, F.; Díaz Fernández, S.; Conde, M. Wood anatomy of Tetraclinis articulata from its natural distribution area in southeast Spain. IAWA J. 2015, 36, 22–35. [Google Scholar] [CrossRef]
- Jansen, S.; Smets, E.; Baas, P. Vestures in woody plants: A review. IAWA J. 1998, 19, 347–382. [Google Scholar] [CrossRef]
- Zimmerman, M.H. Xylem Structure and the Ascent of Sap; Springer: New York, NY, USA, 1983. [Google Scholar]
- Peraza, C. Estudio de las Maderas de Coníferas Españolas y de la Zona Norte de Marruecos; IFIE: Madrid, Spain, 1964. [Google Scholar]
- Esteban, L.G.; Gasson, P.; Climent, J.M.; de Palacios, P.; Guindeo, A. The wood of Pinus canariensis and its resinous heartwood. IAWA J. 2005, 26, 69–77. [Google Scholar] [CrossRef]
- Hillis, W.E. Heartwood and Tree Exudates; Springer: Berlin, Germany, 1987. [Google Scholar] [CrossRef]
- Funada, R.; Miura, H.; Shibagaki, M.; Furusawa, O.; Miura, T.; Fukatsu, E.; Kitin, P. Involvement of Localized Cortical Microtubules in the Formation of a Modified Structure of Wood. J. Plant Res 2001, 114, 491–497. [Google Scholar] [CrossRef]
- Xie, F.H. Wood Structure and Taxonomical Position of Cathaya (Abstract). Symposium on the Explotation of Tropical Resources in South China; Forestry Press: Beijing, China, 1957. [Google Scholar]
- Yatsenko-Khmelevsky, A.A.; Budkevich, E.V. On the Wood anatomy of Cathaya argyrophylla Chun et Kuang (Pinaceae). Bot. Zurn. 1958, 43, 477–480. [Google Scholar]
- Greguss, P. Similar xylotomy and leaf-epidermis of the Pseudotsuga and the new genus Cathaya. Bot. Kozlem. 1970, 57, 51–55. [Google Scholar]
- Greguss, P. Xylotomy of the Living Conifers; Akadémiai Kiadó: Budapest, Hungary, 1972; p. 329. [Google Scholar]
- Cheng, T.C. Tropical and Subtropical Woods in China (Identifications, Properties and Uses); Beijing Science Press: Beijing, China, 1980. [Google Scholar]
- Hu, Y.S.; Wang, F.H. Anatomical studies of Cathaya (Pinaceae). Am J. Bot. 1984, 71, 727–735. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; García-Iruela, A.; García-Fernández, F.; García-Esteban, L.; González de Vega, D. Comparative anatomy of wood in Pinaceae with reference to its systematic position. Forests 2021, 12, 1706. [Google Scholar] [CrossRef]
- Anagnost, S.E.; Meyer, R.W.; De Zeeuw, C. Confirmation and significance of Bartholin’s method for identification of the wood of Picea and Larix. IAWA J. 1994, 15, 171–184. [Google Scholar] [CrossRef]
- Yatsenko-Khmelevsky, A.A. The Principles and Methods of Anatomical Investigation of Wood (in Russian); Akad. Naúk SSSR: Moscow-Leningrad, Russia, 1954; p. 337. [Google Scholar]
- Phillips, E.W.J. Identification of Softwoods by their Microscopic Structure; Forest Products Research Bull. No. 22; HMSO Department of Scientific and Industrial Research: London, UK, 1948. [Google Scholar]
- Keunecke, D. Elasto-mechanical characterisation of yew and spruce wood with regard to structure-property relationships. Ph.D. Thesis, Eidgenössische Technische Hochschule ETH Zürich, Zürich, Switzerland, 2008. [Google Scholar] [CrossRef]
- Howard, E.T.; Manwiller, F.G. Anatomical characteristics of southern pine stemwood. Wood Sci. 1969, 2, 77–86. [Google Scholar]
- Meylan, B.A.; Butterfield, B.G. The Structure of New Zealand Woods, DSIR Bull. 222; NZ Department of Scientific and Industrial Research: Wellington, New Zealand, 1978. [Google Scholar]
- Carlquist, S. Wood anatomy of Cynareae (Compositae). Aliso 1965, 6, 13–24. [Google Scholar] [CrossRef]
- McElhanney, T.A. Associates in the Forest Products Laboratories of Canada. Canadian Woods: Their Properties and Uses; Department of the Interior: Ottawa, Canada, 1935; p. 345. [Google Scholar]
- Grosser, D. On the occurrence of trabeculae with special consideration of diseased trees. IAWA Bull. 1986, 7, 319–341. [Google Scholar] [CrossRef]
- Ghimire, B.; Lee, C.; Heo, K. Comparative wood anatomy of Taxaceae. Aust Syst Bot. 2015, 28, 160–172. [Google Scholar] [CrossRef]
- Gasson, P.; Baas, P.; Wheeler, E.A. Wood anatomy of CITES-listed tree species. IAWA J. 2011, 32, 155–198. [Google Scholar] [CrossRef]
- Galtier, J. On the earliest arborescent gymnosperms. Cour. Forsch. Senckenberg 1992, 147, 119–125. [Google Scholar]
- Dunn, M.T. A review of permineralized seed fern stems of the Upper Paleozoic. J. Torrey Bot. Soc. 2006, 133, 20–32. [Google Scholar] [CrossRef]
- Esteban, L.G.; de Palacios, P.; Guindeo, A.; Fernandez, F.G. Comparative anatomy of the wood of Abies pinsapo and its two Moroccan varieties. IAWA J. 2007, 28, 285–299. [Google Scholar] [CrossRef]
- Hudson, R.H. The anatomy of the genus Pinus in relation to its classification. J. Inst. Wood Sci. 1960, 6, 26–46. [Google Scholar]
- Jacquiot, C. Atlas D’anatomie des Bois des Conifères; Institut National du Bois: Paris, France, 1955; p. 197. [Google Scholar]
- Jane, F.W. The structure of Wood, 2nd ed.; A & C Black Publishers Ltd.: London, UK, 1970; p. 478. [Google Scholar]
- Rol, R. Note sur un essai de classification du genre Pinus d’après des caractères tirés de l’anatomie du bois. Rapp Congr Soco Sayo 1932, 65, 333–341. [Google Scholar]
- Kibblewhite, R.P.; Thompson, N.S. The ultrastructure of the middle lamella region in resin canal tissue isolated from slash pine holocellulose. Wood Sci. Technol. 1973, 7, 112–126. [Google Scholar] [CrossRef]
- Esau, K. Anatomy of Seed Plants; Wiley & Sons: New York, NY, USA, 1977. [Google Scholar]
- Fahn, A. Secretory Tissues in Plants; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Ickert-Bond, S.M. Reexamination of wood anatomical features in Pinus krempfii (Pinaceae). IAWA J. 2001, 22, 355–365. [Google Scholar] [CrossRef]
- Takahara, S.; Nobuchi, T.; Harada, H.; Saiki, H. Cell arrangement in the tissue surrounding axial resin canals in the wood of European spruce. Mokuzai Gakkaishi 1982, 28, 197–201. [Google Scholar]
- Sato, K.; Ishida, S. Resin canals in the wood of Larix leptolepis Gord. II. Morphology of vertical resin canals. Res. Bull. Coll. Exp. 1982, 39, 297–326. [Google Scholar]
- Jain, K.K. Evolution of wood structure in Pinaceae. Isr. J. Bot. 1976, 25, 28–33. [Google Scholar]
- Wu, H.; Hu, Z.H. Comparative anatomy of resin ducts of the Pinaceae. Trees 1997, 11, 135–143. [Google Scholar] [CrossRef]
- Lin, J.; Liang, E.; Farjon, A. The occurrence of vertical resin canals in Keteleeria, with reference to its systematic position in Pinaceae. Bot. J. Linn Soc 2000, 134, 567–574. [Google Scholar] [CrossRef]
- Jeffrey, A.H. A cladistic analysis of Conifers: Preliminary results. J. Arnold Arbor. 1987, 68, 269–307. [Google Scholar]
- Lin, J.X.; Hu, Y.S.; Wang, F.H. Wood and bark anatomy of Nothotsuga (Pinaceae). Ann. Mo. Bot. Gard. 1995, 82, 603–609. [Google Scholar]
- Grosser, D. Die Hölzer Mitteleuropas; Springer: New York, NY, USA, 1977. [Google Scholar]
- Schweingruber, F.H. Microscopic Wood Anatomy; Swiss Federal Institute of Forestry Research: Birmensdorf, Switzerland, 1978. [Google Scholar]
- Hoadley, R.B. Wood Identification: Accurate Results with Simple Tools; Taunton Press: Newtown, CT, USA, 1990. [Google Scholar]
- Dickison, W.C. Integrative Plant Anatomy; Academic Press: New York, NY, USA, 2000. [Google Scholar]
- Lotova, L.L. On the Correlation of the Anatomical Features of the Wood and Phloem in the Pinaceae. J. Moscow Univ. 1975, 1, 41–51. [Google Scholar]
- Bailey, I.W.; Faull, A.F. The cambium and its derivative tissues. IX. Structural variability in redwood. Sequoia sempervirens and its significance in the identification of fossil woods. J. Arnold Arbor. 1934, 15, 233–254. [Google Scholar]
- Benkova, V.E.; Schweingruber, F.H. Anatomy of Russian woods. An Atlas for the Identification of Trees, Shrubs, Dwarf Shrubs and Woody Lianas from Russia; Haupt Verlag: Bern, Switzerland, 2004; p. 456. [Google Scholar]
- Anderson, A.P. Comparative anatomy of the normal and diseased organs of Abies balsamea affected with Aecidium elatinum. Bot. Gaz. 1897, 24, 309–344. [Google Scholar] [CrossRef]
- Jeffrey, E.C. The comparative anatomy and phylogeny of the Coniferales. Part. 2. The Abietineae. Mem. Boston Soc. Nat. Hist. 1905, 6, 38–44. [Google Scholar]
- Chamberlain, C.J. Gymnosperms. Structure and Evolution; The University of Chicago Press: Chicago, IL, USA, 1935; p. 484. [Google Scholar]
- Pearson, R.S.; Brown, H.P. Commercial Timbers of India; Government of India Central Publication Branch: Calcutta, India, 1932; Volume 2. [Google Scholar]
- Penhallow, D.P. North American Gymnosperms; Ginn & Co.: Boston, MA, USA, 1907; p. 374. [Google Scholar]
- Vierhapfer, F. Entwurf eines neuen Systemes der Coniferen; Abhandl der KK Zool botan Gesellschaft in Wien: Jena, Germany, 1910; p. 56. [Google Scholar]
- Record, S. Identification of the Economic Woods of the United States; J. Wiley: New York, NY, USA, 1919; p. 157. [Google Scholar]
- Wiedenhoeft, A.C.; Miller, R.B.; Theim, T.J. Analysis of three microscopic characters for separating the wood of Pinus contorta and P. Ponder. IAWA J. 2003, 24, 257–267. [Google Scholar] [CrossRef]
- Krahmer, R.L.; Hemingway, R.W.; Hillis, W.E. The cellular distribution of lignans in Tsuga heterophylla wood. Wood Sci. Technol. 1970, 4, 122–139. [Google Scholar] [CrossRef]
- Díaz-Vaz, J.E. Anatomía de Maderas; Marisa Cuneo Ediciones: Valdivia, Chile, 2003. [Google Scholar]
- Blanco, M.L.; Carpio, I.M.; Muñoz, F. Fichas Técnicas de Veinte Especies Maderables de Importancia Comercial en Costa Rica, 1st ed.; Editorial Universidad de Costa Rica: San Jose de Costa Rica, Costa Rica, 2005. [Google Scholar]
- Yang, K.C.; Yang, Y.H. Minute Structure of Taiwanese Woods; Hua Shiang Yuan Publishing Co.: Taipei, Taiwan, 1987. [Google Scholar]
- Tang, Y. Timber studies of Chinese trees IV. Anatomical studies and identification of Chinese softwoods I. Bull. Fan Mem. Inst. Biol. 1933, 4, 309–368. [Google Scholar]
- Gamble, J.S. A Manual of INDIAN TIMBERS: An Account of the Growth, Distribution, and Uses of the Trees and Shrubs of India and Ceylon with Descriptions of Their Wood-Structure; Sampson Low, Marston & Company: London, UK, 1902. [Google Scholar]
- Kato, K. A trial to detect optimal pin-pricking timing in evaluating the ability to form traumatic resin canals of Cryptomeria japonica for selecting resistant trees to Semanotus japonicus (Coleoptera: Cerambycidae). J. For. Res.-Jpn. 2008, 13, 386–392. [Google Scholar] [CrossRef]
- Fang, W.; Wu, Y. Anatomical Properties and Colorized Illustrations of Important Commercial Wood Species of Hunan in China; Science Press: Beijing, China, 2010. [Google Scholar]








































Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esteban, L.G.; de Palacios, P.; Heinz, I.; Gasson, P.; García-Iruela, A.; García-Fernández, F. Softwood Anatomy: A Review. Forests 2023, 14, 323. https://doi.org/10.3390/f14020323
Esteban LG, de Palacios P, Heinz I, Gasson P, García-Iruela A, García-Fernández F. Softwood Anatomy: A Review. Forests. 2023; 14(2):323. https://doi.org/10.3390/f14020323
Chicago/Turabian StyleEsteban, Luis G., Paloma de Palacios, Immo Heinz, Peter Gasson, Alberto García-Iruela, and Francisco García-Fernández. 2023. "Softwood Anatomy: A Review" Forests 14, no. 2: 323. https://doi.org/10.3390/f14020323
APA StyleEsteban, L. G., de Palacios, P., Heinz, I., Gasson, P., García-Iruela, A., & García-Fernández, F. (2023). Softwood Anatomy: A Review. Forests, 14(2), 323. https://doi.org/10.3390/f14020323







