Juvenile Leaf Color Changes and Physiological Characteristics of Acer tutcheri (Aceraceae) during the Spring Season
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Determination of Physiological Indicators
2.3. Preparation of Freehand Sectioning, Paraffin Sectioning, and Staining by Periodic Acid–Schiff (PAS)
2.3.1. Freehand Sectioning Method
2.3.2. Staining Carbohydrates in the Leaf by Periodic Acid–Schiff (PAS) Staining
2.4. Date Statistical and Analysis
3. Results
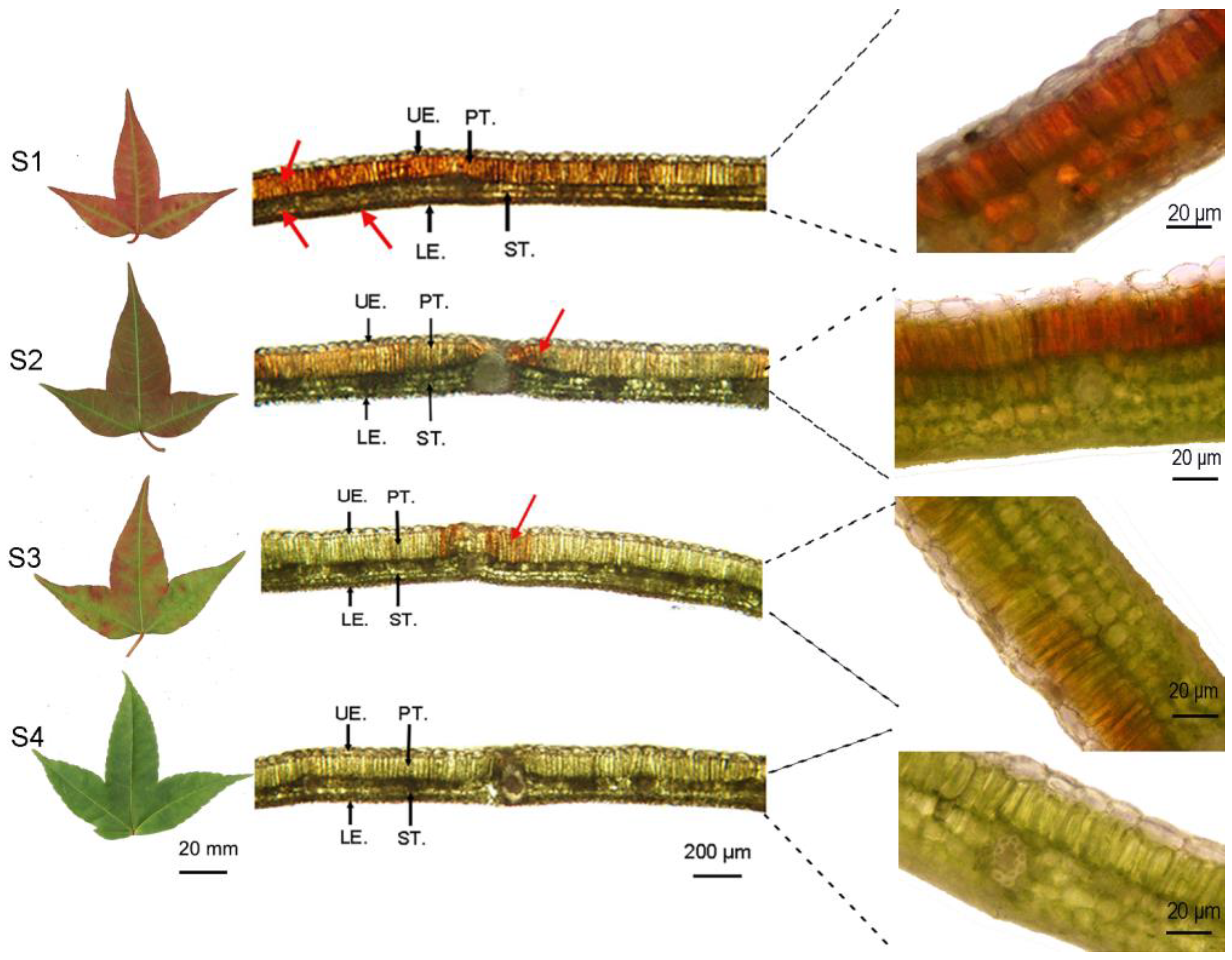
3.1. Characteristics of the Leaf Pigment Content, Ratio, and Distribution
3.2. Changes in PAL Activity, CHI Activity, Vacuole pH and Tannin Content, Soluble Sugar Content, Starch Content, and Soluble Protein Content
3.3. Changes in POD Activity, PPO Activity, Vacuole pH, and Tannin Content
3.4. Correlation Analysis of Anthocyanin with Physiological Factors and Path Analysis
4. Discussion
4.1. Pigment Distribution Characteristics, Content, and the Ratio of Leaves during Different Periods of Color Change
4.2. Analysis of Physiological Factors Related to Anthocyanin Synthesis during Different Leaf Color Periods
4.3. Analysis of Physiological Indicators Related to Anthocyanin Degradation during Different Leaf Color Periods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Sun, B.; Liao, S.B.; Luo, S.X.; Chen, L.; Wang, H.J. Study on early growth performance and scenic beauty values of colored-leaf tree—Acer tutcheri. Guangdong For. Sci. Technol. 2015, 31, 19–22, (In Chinese with English Abstract). [Google Scholar]
- Zhang, K.; Sun, B.; Liao, S.B.; Wu, P.P.; Yan, W.M.; Chen, L. Leaf color expression and color leaf characteristic of Acer tutcheri. Bull. Bot. Res. 2017, 37, 861–869, (In Chinese with English Abstract). [Google Scholar]
- Manetas, Y. Why some leaves are anthocyanic and why most anthocyanic leaves are red? Flora 2006, 201, 163–177. [Google Scholar] [CrossRef]
- Agati, G.; Guidi, L.; Landi, M.; Tattini, M. Anthocyanins in photoprotection: Knowing theactors in play to solve thiscomplex ecophysiological issue. New Phytol. 2021, 232, 2228. [Google Scholar] [CrossRef]
- Hughes, N.M.; Morley, C.B.; Smith, W.K. Coordination of anthocyanin decline and photosynthetic maturation in juvenile leaves of three deciduous tree species. New Phytol. 2007, 175, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, T.J.; Zhang, P.; Peng, C.L. Pigment patterns and photoprotection of anthocyanins in the young leaves of four dominant subtropical forest tree species in two successional stages under contrasting light conditions. Tree Physiol. 2016, 36, 1092–1114. [Google Scholar] [CrossRef]
- Wang, Z.X.; Yu, Y.F.; Chen, L.; Qin, H.Y.; Liu, Y.X.; Ai, J.; Zhao, Y.; Yang, Y.M.; Li, X.Y.; Wang, G.F.; et al. Advances in leaf pigment composition, structure and photosynthetic characteristics of colored-leaf plants. Plant Physiol. J. 2016, 52, 1–7. [Google Scholar]
- Yu, Z.C.; Zhang, Q.L.; Zheng, X.T.; Huang, X.D.; Peng, C.L. Anthocyanin accumulation in juvenile Schima superba leaves is a growth trade-off by consuming energy for adaptation to high light during summer. Chin. J. Plant Ecol. 2019, 12, 507–518, (in Chinese with English Abstract). [Google Scholar] [CrossRef]
- Liu, Y.; Tikunov, Y.; Schouten, R.E.; Marcelis, L.; Visser, R.; Bovy, A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous vegetables: A review. Front. Chem. 2018, 6, 52. [Google Scholar] [CrossRef]
- Lee, D.W.; Collins, T.M. Phylogenetic and ontogenetic influences on the distribution of anthocyanins and betacyanins in leaves of tropical plants. Int. J. Plant Sci. 2001, 162, 1141–1153. [Google Scholar] [CrossRef]
- Zhao, D.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; Martin, C. Engineering anthocyanin biosynthesis in plants. Curr. Opin. Plant Biol. 2014, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef]
- Zhao, Y.W.; Wang, C.K.; Huang, X.Y.; Hu, D.G. Anthocyanin stability and degradation in plants. Plant Signal. Behav. 2021, 16, 1987767. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Fukuchi-Mizutani, M.; Miyazaki, K.; Suzuki, K.; Tanaka, Y. RNAi suppression of the anthocyanidin synthase gene in Torenia hybrida yields white flowers with higher frequency and better stability than antisense and sense suppression. Plant Biotechnol. 2006, 23, 13–17. [Google Scholar] [CrossRef]
- Gu, K.D.; Wang, C.K.; Hu, D.G.; Hao, Y.J. How do anthocyanins paint our horticultural products? Sci. Hortic. 2019, 249, 257–262. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2013, 65, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fu, J.; Wang, Y.; Gao, S.; Du, D.; Wu, F.; Guo, J.; Dong, L. Glucose supply improves petal coloration and anthocyanin biosynthesis in Paeonia suffruticosa ‘Luoyang Hong’ cut flowers. Postharvest Biol. Technol. 2015, 101, 73–81. [Google Scholar] [CrossRef]
- Zipor, G.; Duarte, P.; Carqueijeiro, I.; Shahar, L.; Ovadia, R.; Teper-Bamnolker, P.; Eshel, D.; Levin, Y.; Doron-Faigenboim, A.; Sottomayor, M. In planta anthocyanin degradation by a vacuolar class III peroxidase in Brunfelsia calycina flowers. New Phytol. 2015, 205, 653–665. [Google Scholar] [CrossRef]
- Mullineaux, P.; Karpinski, S. Signal transduction in response to excess light: Getting out of the chloroplast. Curr. Opin. Plant Biol. 2002, 5, 43–48. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The grapevine VviPrx31 peroxidase as a candidate gene involved in anthocyanin degradation in ripening berries under high temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef]
- Pang, X.Q.; Huang, X.M.; Yang, X.T. Role of polyphenol oxidase in anthocyanin degradation of lychee pericarp. Sci. Agric. Sin. 2008, 41, 540–545, (In Chinese with English Abstract). [Google Scholar]
- Wang, J.B.; Liu, B.B.; Xiao, Q.; Li, H.L.; Sun, J.H.; Rogerio, M. Cloning and expression analysis of Litchi (Litchi chinensis Sonn.) polyphenol oxidase gene and relationship with postharvest pericarp browning. PLoS ONE 2014, 9, e93982. [Google Scholar] [CrossRef] [PubMed]
- Passeri, V.; Koes, R.; Quattrocchio, F.M. New challenges for the design of high value plant products: Stabilization of anthocyanins in plant vacuoles. Front. Plant Sci. 2016, 7, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Pang, X.; Ji, Z.; Jiang, Y. Role of anthocyanin degradation in litchi pericarp browning. Food Chem. 2001, 75, 217–221. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Color and phenolic content changes during flower development in groundcover rose. J. Am. Soc. Hortic. Sci. 2010, 135, 195–202. [Google Scholar] [CrossRef]
- Luo, H.H.; Deng, S.F.; Fu, W.; Zhang, X.; Zhang, X.L.; Zhang, Z.Q.; Pang, X.Q. Characterization of active anthocyanin degradation in the petals of Rosa chinensis and Brunfelsia calycina reveals the effect of gallated catechins on pigment maintenance. Int. J. Mol. Ences 2017, 18, 699. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.H.; Li, W.J.; Zhang, X.; Deng, S.F.; Xu, Q.C.; Hou, T.; Pang, X.Q.; Zhang, Z.Q.; Zhang, X.L. In planta high levels of hydrolysable tannins inhibit peroxidase mediated anthocyanin degradation and maintain abaxially red leaves of Excoecaria cochinchinensis. BMC Plant Biol. 2019, 19, 315. [Google Scholar] [CrossRef]
- Yin, G.; Wang, Y.; Xiao, Y.; Yang, J.; Wang, R.; Jiang, Y.; Huang, R.; Liu, X.; Jiang, Y. Relationships between leaf color changes, pigment levels, enzyme activity, photosynthetic fluorescence characteristics and chloroplast ultrastructure of Liquidambar formosana Hance. J. For. Res. 2022, 33, 1559–1572. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]
- Li, L. Study on the Formation and Regulation of Leaf Color Form Acer rubrum L. Master’s Thesis, Southwest University, Chongqing, China, 2016. (In Chinese). [Google Scholar]
- Vaknin, H.; Bar-Akiva, A.; Ovadia, R.; Nissim-Levi, A.; Forer, I.; Weiss, D.; Oren-Shamir, M. Active anthocyanin degradation in Brunfelsia calycina (yesterday–today–tomorrow) flowers. Planta 2005, 222, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X. Actinidia kolomikia (Rupr. & Maxim) Maxim Variegated Leaf Formation Mechanism and Photosythetic Function. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2017. (In Chinese). [Google Scholar]
- Dewey, D.R.; Lu, K.H. A correlation and path-coefficient analysis of components of crested wheatgrass seed production. Probl. Tuberk. 1959, 51, 70–74. [Google Scholar] [CrossRef]
- Yuan, Z.f.; Zhou, J.Y.; Guo, M.C.; Lei, X.Q.; Xie, X.L. Decision coefficient—The decision index of path analysis. J. Northwest A F Univ. (Nat. Sci. Ed.) 2001, 29, 131–133, (In Chinese with English Abstract). [Google Scholar]
- Yuan, Z.F. Introduction to path analysis method. J. Triticeae Crops 1981, 3, 42–48. [Google Scholar]
- Gong, W.-C.; Liu, Y.-H.; Wang, C.-M.; Chen, Y.-Q.; Martin, K.; Meng, L.-Z. Why are there so many plant species that transiently flush young leaves red in the tropics? Front. Plant Sci. 2020, 11, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nissim-Levi, A.; Kagan, S.; Ovadia, R.; Oren-Shami, M. Effects of temperature, UV-light and magnesium on anthocyanin pigmentation in cocoplum leaves. J. Hortic. Sci. Biotechnol. 2003, 78, 61–64. [Google Scholar] [CrossRef]
- Lo Piccolo, E.; Landi, M.; Pellegrini, E.; Agati, G.; Giordano, C.; Giordani, T.; Lorenzini, G.; Malorgio, F.; Massai, R.; Nali, C. Multiple consequences induced by epidermally-located anthocyanins in young, mature and senescent leaves of Prunus. Front. Plant Sci. 2018, 9, 917. [Google Scholar] [CrossRef]
- Liu, P.; Song, Y.; Lu, X.J.; Cao, B.H.; Liu, S.J.; Wang, Y.T. Research of physiological characteristics in juvenile leaves of Acer×freemanii ‘Autumn Blaze’ and Acer truncatum during leaf color transformation in spring. Ecol. Environ. 2018, 27, 452–458, (In Chinese with English Abstract). [Google Scholar]
- Yu, Z.-C.; Lin, W.; Zheng, X.-T.; Chow, W.S.; Luo, Y.-N.; Cai, M.-L.; Peng, C.-L. The relationship between anthocyanin accumulation and photoprotection in young leaves of two dominant tree species in subtropical forests in different seasons. Photosynth. Res. 2021, 149, 41–55. [Google Scholar] [CrossRef]
- Zhang, T.-J.; Zheng, J.; Yu, Z.-C.; Gu, X.-Q.; Tian, X.-S.; Peng, C.-L.; Chow, W.S. Variations in photoprotective potential along gradients of leaf development and plant succession in subtropical forests under contrasting irradiances. Environ. Exp. Bot. 2018, 154, 23–32. [Google Scholar] [CrossRef]
- Oren-Shamir, M. Does anthocyanin degradation play a significant role in determining pigment concentration in plants? Plant Sci. 2009, 177, 310–316. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, G.; Zhang, W.; Goltsev, V.; Sun, S.; Wang, J.; Li, P.; Ma, F. Anthocyanin concentration depends on the counterbalance between its synthesis and degradation in plum fruit at high temperature. Sci. Rep. 2017, 7, 7684. [Google Scholar] [CrossRef] [PubMed]
- Rehman, R.; You, Y.; Lei, Z.; Goudia, B.D.; Ma, F. High temperature induced anthocyanin inhibition and active degradation in Malus profusion. Front. Plant Sci. 2017, 8, 1401. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Li, R.; Yao, W.; Wang, Y.; Zhang, C.; Li, Y. Genome-wide identification and characterisation of phenylalanine ammonia-lyase gene family in grapevine. J. Hortic. Sci. Biotechnol. 2021, 96, 456–468. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-sspecific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [CrossRef]
- Zheng, Y.; Tian, L.; Liu, H.; Pan, Q.; Zhan, J.; Huang, W. Sugars induce anthocyanin accumulation and flavanone 3-hydroxylase expression in grape berries. Plant Growth Regul. 2009, 58, 251–260. [Google Scholar] [CrossRef]
- Neta-Sharir, I.; Shoseyov, O.; Weiss, D. Sugars enhance the expression of gibberellin-induced genes in developing petunia flowers. Physiol. Plant. 2000, 109, 196–202. [Google Scholar] [CrossRef]
- Steyn, W.J.; Wand, S.; Holcroft, D.; Jacobs, G. Anthocyanins in vegetative tissues: A proposed unified function in photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef]
- Turgeon, R. The sink-source transition in leaves. Annu. Rev. Plant Biol. 1989, 40, 119–138. [Google Scholar] [CrossRef]
- Wang, L.; Brouard, E.; Prodhomme, D.; Hilbert, G.; Renaud, C.; Petit, J.-P.; Edwards, E.; Betts, A.; Delrot, S.; Ollat, N. Regulation of anthocyanin and sugar accumulation in grape berry through carbon limitation and exogenous ABA application. Food Res. Int. 2022, 160, 111478. [Google Scholar] [CrossRef] [PubMed]
- Bobeica, N.; Poni, S.; Hilbert, G.; Renaud, C.; Gomès, E.; Delrot, S.; Dai, Z. Differential responses of sugar, organic acids and anthocyanins to source-sink modulation in Cabernet Sauvignon and Sangiovese grapevines. Front. Plant Sci. 2015, 6, 382. [Google Scholar] [CrossRef]
- Hughes, N.; Neufeld, H.; Burkey, K. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Chen, X.; Zhang, Y.; Wang, Y.; Song, Y.; Chen, X.L.; Li, X.; Min, L.; Jin, L.; Wang, Q. Differential expression of proteins in red pear following fruit bagging treatment. Protein J. 2011, 30, 194–200. [Google Scholar] [CrossRef]
- Tang, S.S.; Chen, H.; Qin, Y.K.; Yang, Z.Q.; Wang, T.; Wei, B.L. Physiological basis of Liquidambar formosana during leaf color transformation in autumn. Guihaia 2021, 41, 2061–2068, (In Chinese with English Abstract). [Google Scholar]
- Gould, K.S.; Neill, S.O.; Vogelmann, T.C. A unified explanation for anthocyanins in leaves? Adv. Bot. Res. 2002, 37, 167–192. [Google Scholar]
- Mubarakshina, M.M.; Ivanov, B.N.; Naydov, I.A.; Hillier, W.; Badger, M.R.; Krieger-Liszkay, A. Production and diffusion of chloroplastic H2O2 and its implication to signalling. J. Exp. Bot. 2010, 61, 3577–3587. [Google Scholar] [CrossRef]
- Ferreres, F.; Figueiredo, R.; Bettencourt, S.; Carqueijeiro, I.; Oliveira, J.; Gil-Izquierdo, A.; Pereira, D.M.; Valentão, P.; Andrade, P.B.; Duarte, P.; et al. Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: An H2O2 affair? J. Exp. Bot. 2011, 62, 2841–2854. [Google Scholar] [CrossRef]
- Rehman, R.N.U.; You, Y.H.; Yang, C.Q.; Khan, A.R.; Li, P.M.; Ma, F.W. Characterization of phenolic compounds and active anthocyanin degradation in crabapple (Malus orientalis) flowers. Hortic. Environ. Biotechnol. 2017, 58, 324–333. [Google Scholar] [CrossRef]
- Taranto, F.; Pasqualone, A.; Mangini, G.; Tripodi, P.; Miazzi, M.M.; Pavan, S.; Montemurro, C. Polyphenol oxidases in crops: Biochemical, physiological and genetic aspects. Int. J. Mol. Sci. 2017, 18, 377. [Google Scholar] [CrossRef]
- Zhao, C.L.; Li, Y.; Chen, Z.J.; Li, J.; Liu, F.C. Enzymatic degradation of anthocyanins. J. Trop. Subtrop. Bot. 2011, 19, 576–584, (In Chinese with English Abstract). [Google Scholar]
- Zhang, Y.; Zhang, J.; Song, T.; Li, J.; Tian, J.; Jin, K.; Yao, Y. Low medium pH value enhances anthocyanin accumulation in Malus crabapple leaves. PLoS ONE 2014, 9, e97904. [Google Scholar] [CrossRef] [PubMed]
- Picariello, L.; Gambuti, A.; Picariello, B.; Moio, L. Evolution of pigments, tannins and acetaldehyde during forced oxidation of red wine: Effect of tannins addition. LWT-Food Sci. Technol. 2017, 77, 370–375. [Google Scholar] [CrossRef]






| Number | DHB (cm) | Height (m) | Crown Diameter (m) | Under-Branch Height (m) |
|---|---|---|---|---|
| QN.1 | 3.98 | 2.95 | 2.20 | 1.35 |
| QN.2 | 3.92 | 3.04 | 2.32 | 1.33 |
| QN.3 | 4.00 | 3.10 | 2.40 | 1.50 |
| QN.4 | 4.12 | 2.86 | 2.56 | 0.92 |
| QN.5 | 4.10 | 3.21 | 2.17 | 1.63 |
| QN.6 | 4.05 | 2.96 | 2.65 | 1.13 |
| QN.7 | 4.20 | 2.82 | 2.50 | 1.00 |
| QN.8 | 4.15 | 3.22 | 2.42 | 1.20 |
| QN.9 | 3.98 | 3.12 | 2.55 | 1.35 |
| Factor | Total Effect | Indirect Path Coefficient | Decision-Making Coefficient | |||||
|---|---|---|---|---|---|---|---|---|
| By PAL | By CHI | By POD | By PPO | By pH | By Tan | |||
| PAL | 0.525 | −0.029 | 0.154 | 0.001 | 0.546 | 0.002 | −0.178 | |
| CHI | 0.800 ** | −0.120 | 0.203 | 0.003 | 0.748 | 0.001 | −0.059 | |
| POD | −0.916 ** | 0.098 | 0.031 | −0.003 | −0.809 | −0.001 | 0.372 | |
| PPO | −0.446 | 0.019 | 0.015 | −0.091 | −0.383 | 0.001 | 0.006 | |
| pH | −0.973 ** | 0.093 | 0.031 | −0.215 | −0.003 | −0.002 | 0.937 | |
| Tan | −0.209 | 0.039 | 0.003 | −0.025 | 0.001 | −0.221 | 0.003 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Pei, N.; Hao, Z.; Shi, Z.; Chen, L.; Mai, B.; Liu, Q.; Luo, J.; Luo, M.; Sun, B. Juvenile Leaf Color Changes and Physiological Characteristics of Acer tutcheri (Aceraceae) during the Spring Season. Forests 2023, 14, 328. https://doi.org/10.3390/f14020328
Xie Y, Pei N, Hao Z, Shi Z, Chen L, Mai B, Liu Q, Luo J, Luo M, Sun B. Juvenile Leaf Color Changes and Physiological Characteristics of Acer tutcheri (Aceraceae) during the Spring Season. Forests. 2023; 14(2):328. https://doi.org/10.3390/f14020328
Chicago/Turabian StyleXie, Yating, Nancai Pei, Zezhou Hao, Zhaowan Shi, Lei Chen, Baoying Mai, Qunhui Liu, Jiajie Luo, Mingdao Luo, and Bing Sun. 2023. "Juvenile Leaf Color Changes and Physiological Characteristics of Acer tutcheri (Aceraceae) during the Spring Season" Forests 14, no. 2: 328. https://doi.org/10.3390/f14020328
APA StyleXie, Y., Pei, N., Hao, Z., Shi, Z., Chen, L., Mai, B., Liu, Q., Luo, J., Luo, M., & Sun, B. (2023). Juvenile Leaf Color Changes and Physiological Characteristics of Acer tutcheri (Aceraceae) during the Spring Season. Forests, 14(2), 328. https://doi.org/10.3390/f14020328








