Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
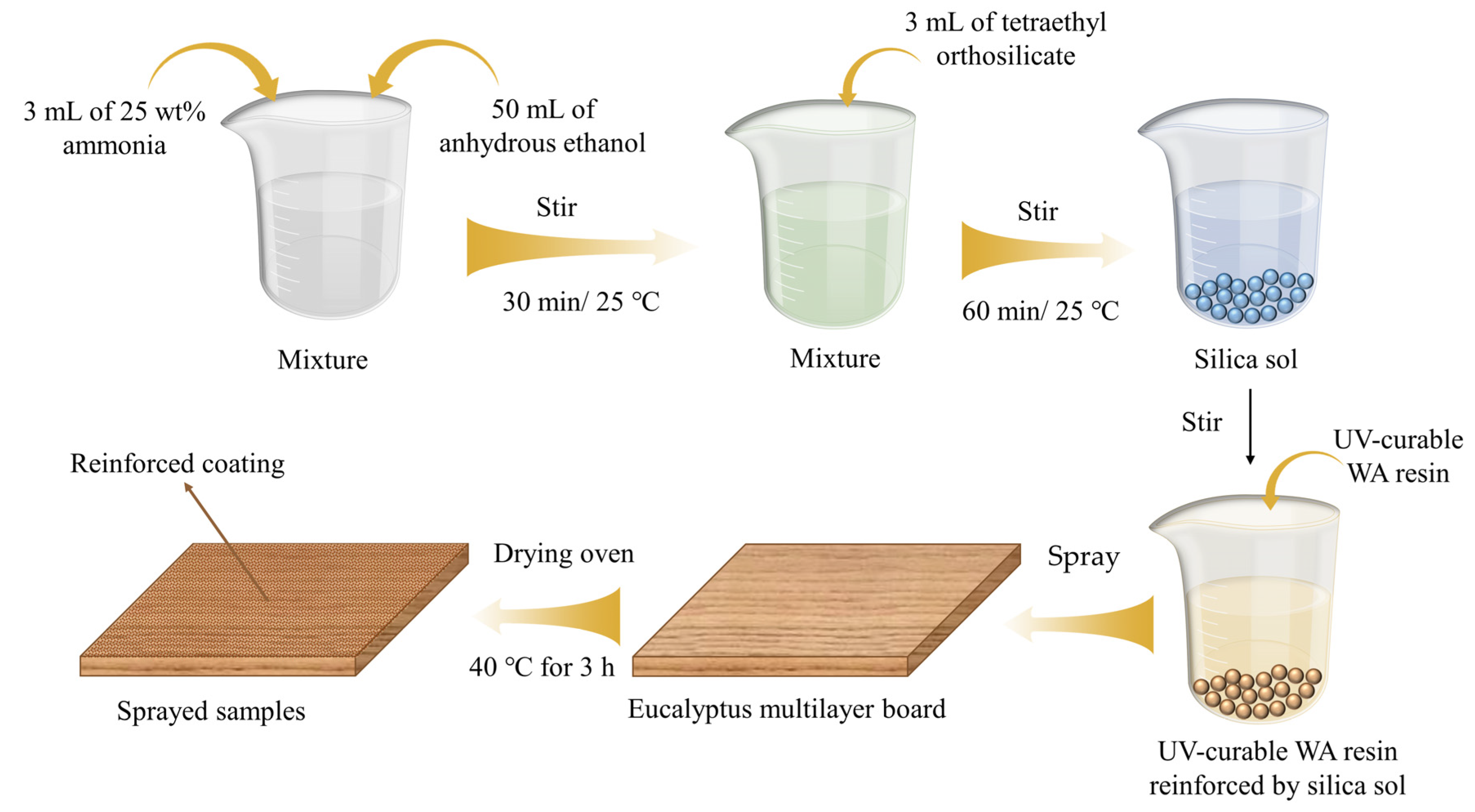
2.2. Preparation of Silica Sol
2.3. Preparation of UV-Curable WA Coating Reinforced by Silica Sol
2.4. Preparation of the Reinforced Coating
2.5. Characterizations
3. Results and Discussion
3.1. Particle Size and SEM Analysis
3.2. FT-IR of Reinforced Coating
3.3. Mechanism Analysis

3.4. Abrasion Resistance Analysis
3.5. Hardness Analysis
3.6. Adhesion Analysis
3.7. Impact Resistance Analysis
3.8. Glossiness Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rowell, R.; Bongers, F. Role of Moisture in the Failure of Coatings on Wood. Coatings 2017, 7, 219. [Google Scholar] [CrossRef]
- Wu, L.; Chen, M.; Xu, J.; Fang, F.; Li, S.; Zhu, W. Nano-SiO2-Modified Waterborne Acrylic Acid Resin Coating for Wood Wallboard. Coatings 2022, 12, 1453. [Google Scholar] [CrossRef]
- Liu, L.; Shan, H.; Jia, X.K.; Duan, S.J.; Gao, Y.J.; Xu, C.Y.; Wei, S.Y. Study on UV curable tung oil based waterborne polyurethane wood coatings. J. For. Eng. 2022, 7, 115–121. [Google Scholar]
- Wang, J.; Wu, X.; Wang, Y.; Zhao, W.; Zhao, Y.; Zhou, M.; Wu, Y.; Ji, G. Green, Sustainable Architectural Bamboo with High Light Transmission and Excellent Electromagnetic Shielding as a Candidate for Energy-Saving Buildings. Nano-Micro Lett. 2023, 15, 11. [Google Scholar] [CrossRef]
- Jia, Z.; Bao, W.; Tao, C.; Song, W. Reversibly photochromic wood constructed by depositing microencapsulated/polydimethylsiloxane composite coating. J. For. Res. 2022, 33, 1409–1418. [Google Scholar] [CrossRef]
- Wu, L.; Zhu, W.; Li, Z.; Li, H.; Xu, J.; Li, S.; Chen, M. Urushiol modified epoxy acrylate as UV spray painting oriental lacquer ink. RSC Adv. 2023, 13, 1106–1114. [Google Scholar] [CrossRef]
- Dai, Y.; Qiu, F.; Xu, J.; Yu, Z.; Yang, P.; Xu, B.; Jiang, Y.; Yang, D. Preparation and properties of UV-curable waterborne graphene oxide/polyurethane-acrylate composites. Plast. Rubber Compos. 2014, 43, 54–62. [Google Scholar] [CrossRef]
- Cai, Z.Y.; Zhu, H.X.; Wang, P.; Wu, C.Y.; Gao, W.C.; Mu, J.Y.; Wei, S.Y. Performance optimization of UV curable waterborne polyurethane acrylate wood coatings modified by castor oil. J. For. Eng. 2020, 5, 89–95. [Google Scholar]
- Herrera, R.; Muszyńska, M.; Krystofiak, T.; Labidi, J. Comparative evaluation of different thermally modified wood samples finishing with UV-curable and waterborne coatings. Appl. Surf. Sci. 2015, 357, 1444–1453. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, B.; Huang, L.; Ma, D.; Jiao, Z.; Xie, Y.; Tan, S.; Cai, X. The utilization of carbon nitride to reinforce the mechanical and thermal properties of UV-curable waterborne polyurethane acrylate coatings. Prog. Org. Coat. 2015, 89, 35–41. [Google Scholar] [CrossRef]
- Dai, Y.; Qiu, F.; Wang, L.; Zhao, J.; Yu, Z.; Yang, P.; Yang, D.; Kong, L. UV-curable electromagnetic shielding composite films produced through waterborne polyurethane-acrylate bonded graphene oxide: Preparation and effect of different diluents on the properties. E-Polymers 2014, 14, 427–440. [Google Scholar] [CrossRef]
- Xu, J.; Qiu, F.; Rong, X.; Dai, Y.; Yang, D. Preparation and Surface Pigment Protection Application of Stone Substrate on UV-Curable Waterborne Polyurethane-acrylate Coating. J. Polym. Mater. 2014, 31, 287–303. [Google Scholar]
- Hwang, H.-D.; Moon, J.-I.; Choi, J.-H.; Kim, H.-J.; Do Kim, S.; Park, J.C. Effect of water drying conditions on the surface property and morphology of waterborne UV-curable coatings for engineered flooring. J. Ind. Eng. Chem. 2009, 15, 381–387. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, T.; Li, L.; Wang, Q.; Guo, C. Facile synthesis and construction of renewable, waterborne and flame-retardant UV-curable coatings in wood surface. Prog. Org. Coat. 2022, 172, 107104. [Google Scholar] [CrossRef]
- He, Y.R.; Wu, Y.Z.; Zhang, J.P.; Qu, W. Preparation of UV-curing flame-retardant coating for parquet using open paint process. J. For. Eng. 2022, 7, 174–180. [Google Scholar]
- Dai, J.; Ma, S.; Wu, Y.; Zhu, J.; Liu, X. High bio-based content waterborne UV-curable coatings with excellent adhesion and flexibility. Prog. Org. Coat. 2015, 87, 197–203. [Google Scholar] [CrossRef]
- Song, S.C.; Kim, S.J.; Park, K.-K.; Oh, J.-G.; Bae, S.-G.; Noh, G.H.; Lee, W.-K. Synthesis and properties of waterborne UV-curable polyurethane acrylates using functional isocyanate. Mol. Cryst. Liq. Cryst. 2017, 659, 40–45. [Google Scholar] [CrossRef]
- Yan, X.X.; Tao, Y.; Qian, X.Y. Preparation of microcapsules for core materials and their effects on properties of waterborne coatings on basswood. J. For. Eng. 2022, 7, 186–192. [Google Scholar]
- Bhavsar, R.A.; Nehete, K.M. Rheological approach to select most suitable associative thickener for water-based polymer dispersions and paints. J. Coat. Technol. Res. 2019, 16, 1089–1098. [Google Scholar] [CrossRef]
- Yan, X.; Peng, W.; Qian, X. Effect of water-based acrylic acid microcapsules on the properties of paint film for furniture surface. Appl. Sci. 2021, 11, 7586. [Google Scholar] [CrossRef]
- Kong, X.; Meng, X. Application of Chemical Technology of Water-Based Acrylic Dipping Paint in Art Painting Creation. J. Chem. 2022, 2022, 7715011. [Google Scholar] [CrossRef]
- Zhu, W.; Ji, M.; Chen, F.; Wang, Z.; Chen, W.; Xue, Y.; Zhang, Y. Formaldehyde-free resin impregnated paper reinforced with cellulose nanocrystal (CNC): Formulation and property analysis. J. Appl. Polym. Sci. 2020, 137, 48931. [Google Scholar] [CrossRef]
- Kai-li, L.; Qi-ming, F.; Yan-hui, H.; Fan, L.; Quan-fei, H.; Wei, Z.; Xue-cong, W. Effect of Modified Acrylic Water-Based Paint on the Properties of Paint Film. Spectrosc. Spectr. Anal. 2020, 40, 2133–2137. [Google Scholar]
- Goodarzi, I.M.; Farzam, M.; Shishesaz, M.R.; Zaarei, D. Eco-friendly, acrylic resin-modified potassium silicate as water-based vehicle for anticorrosive zinc-rich primers. J. Appl. Polym. Sci. 2014, 131, 40370. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, Y.; Guo, M.; Peng, Z. Preparation and properties of nano SiO2 modified cellulose acetate aqueous polymer emulsion for leather finishing. Cellulose 2021, 28, 7213–7225. [Google Scholar] [CrossRef]
- Fufa, S.M.; Jelle, B.P.; Hovde, P.J. Weathering performance of spruce coated with water based acrylic paint modified with TiO2 and clay nanoparticles. Prog. Org. Coat. 2013, 76, 1543–1548. [Google Scholar] [CrossRef]
- Deflorian, F.; Fedel, M.; DiGianni, A.; Bongiovanni, R.; Turri, S. Corrosion protection properties of new UV curable waterborne urethane acrylic coatings. Corros. Eng. Sci. Technol. 2008, 43, 81–86. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, C.; Wang, Y. Preparation and colloidal dispersion behaviors of silica sol doped with organic pigment. J. Sol-Gel Sci. Technol. 2012, 62, 266–272. [Google Scholar] [CrossRef]
- Dong, D.; Xiaobo, L.; Wencheng, H. Stability of the Silica Sols Prepared by Acid/Base Two-Step Catalytic Sol-Gel Process. Rare Met. Mater. Eng. 2010, 39, 61–64. [Google Scholar]
- Trupp, L.; Marchi, M.C.; Barja, B.C. Lanthanide–based luminescent hybrid silica materials prepared by sol-gel methodologies: A review. J. Sol-Gel Sci. Technol. 2021, 102, 63–85. [Google Scholar] [CrossRef]
- Morosanova, E.I. Silica and silica–titania sol–gel materials: Synthesis and analytical application. Talanta 2012, 102, 114–122. [Google Scholar] [CrossRef]
- Gonçalves, M.C. Sol-gel silica nanoparticles in medicine: A natural choice. Design, synthesis and products. Molecules 2018, 23, 2021. [Google Scholar] [CrossRef]
- Cristea, M.V.; Riedl, B.; Blanchet, P. Effect of addition of nanosized UV absorbers on the physico-mechanical and thermal properties of an exterior waterborne stain for wood. Prog. Org. Coat. 2011, 72, 755–762. [Google Scholar] [CrossRef]
- Sangermano, M.; Foix, D.; Kortaberria, G.; Messori, M. Multifunctional antistatic and scratch resistant UV-cured acrylic coatings. Prog. Org. Coat. 2013, 76, 1191–1196. [Google Scholar] [CrossRef]
- Bongiovanni, R.; Montefusco, F.; Priola, A.; Macchioni, N.; Lazzeri, S.; Sozzi, L.; Ameduri, B. High performance UV-cured coatings for wood protection. Prog. Org. Coat. 2002, 45, 359–363. [Google Scholar] [CrossRef]
- Al-Thobity, A.M.; Gad, M.M. Effect of silicon dioxide nanoparticles on the flexural strength of heat-polymerized acrylic denture base material: A systematic review and meta-analysis. Saudi Dent. J. 2021, 33, 775–783. [Google Scholar] [CrossRef]
- Malakauskaite-Petruleviciene, M.; Stankeviciute, Z.; Niaura, G.; Garskaite, E.; Beganskiene, A.; Kareiva, A. Characterization of sol-gel processing of calcium phosphate thin films on silicon substrate by FTIR spectroscopy. Vib. Spectrosc. 2016, 85, 16–21. [Google Scholar] [CrossRef]
- Zhu, W.; Kim, D.; Han, M.; Jang, J.; Choi, H.; Kwon, G.; Jeon, Y.; Ryu, D.Y.; Lim, S.-H.; You, J.; et al. Fibrous Cellulose Nanoarchitectonics on N-doped Carbon-based Metal-Free Catalytic Nanofilter for Highly Efficient Advanced Oxidation Process. Chem. Eng 2023, 141593. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, S.; Gu, G.; Wu, L. Acrylic-Based Polyurethane/Silica Hybrids Prepared by Acid-Catalyzed Sol–Gel Process: Structure and Mechanical Properties. Macromol. Chem. Phys. 2005, 206, 885–892. [Google Scholar] [CrossRef]
- Zheng, C.X.; Zhu, S.L.; Lu, Y.; Mei, C.T.; Xu, X.W.; Yue, Y.Y.; Han, J.Q. Synthesis and characterization of cellulose nanofibers/polyacrylic acid-polyacrylamide double network electroconductive hydrogel. J. For. Eng. 2020, 5, 93–100. [Google Scholar]
- Nazarabady, M.M.; Farzi, G.A. Tunable morphology for silica/poly (acrylic acid) hybrid nanoparticles via facile one-pot synthesis. Macromol. Res. 2016, 24, 716–724. [Google Scholar] [CrossRef]







| Samples | Addition Amount | |
|---|---|---|
| UV-Cured WA Coating (g) | Silica Sol (g) | |
| 0 | 3 | 0 |
| 1 | 3 | 0.03 |
| 2 | 3 | 0.06 |
| 3 | 3 | 0.09 |
| 4 | 3 | 0.12 |
| 5 | 3 | 0.15 |
| Items | D10/μm | D50/μm | D90/μm | Dav/μm | S:V/(cm2·cm−3) | D [3, 2]/μm | D [4, 3]/μm | Fitting Error |
|---|---|---|---|---|---|---|---|---|
| Data | 11.117 | 29.153 | 62.938 | 34.638 | 2 677.672 | 22.408 | 34.638 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Zhu, W.; Li, Z.; Feng, Y.; Qi, W.; Li, S.; Wang, X.; Chen, M. Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics. Forests 2023, 14, 335. https://doi.org/10.3390/f14020335
Zhu Y, Zhu W, Li Z, Feng Y, Qi W, Li S, Wang X, Chen M. Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics. Forests. 2023; 14(2):335. https://doi.org/10.3390/f14020335
Chicago/Turabian StyleZhu, Yuding, Wenkai Zhu, Zequn Li, Yuan Feng, Wei Qi, Song Li, Xiaoyu Wang, and Meiling Chen. 2023. "Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics" Forests 14, no. 2: 335. https://doi.org/10.3390/f14020335
APA StyleZhu, Y., Zhu, W., Li, Z., Feng, Y., Qi, W., Li, S., Wang, X., & Chen, M. (2023). Enhancement of Wood Coating Properties by Adding Silica Sol to UV-Curable Waterborne Acrylics. Forests, 14(2), 335. https://doi.org/10.3390/f14020335







