1. Introduction
Pine wilt disease (PWD) is caused by the pinewood nematode (PWN),
Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle, and results in a high rate of pine tree mortality [
1]. This nematode is listed as a quarantinable pest in 52 countries, with Asia being the region with the most serious PWD epidemics. Pine forests in many countries have been severely damaged due to PWN infection and outbreaks, impairing the services and function of these ecosystems and posing a severe general threat to pine forests worldwide [
2,
3]. Despite various methods to control PWD, including forest management practices, physical, biological, and chemical control, in practice, the most effective control method is to cut and burn the infected and dead pine trees in the forest, but this requires a considerable amount of labor and material resources, and its cost is extremely high [
4]. Therefore, current research in forest pest management is focusing on efficient and cost-effective methods for PWD control.
Currently, chemical agents mainly target PWNs and their insect vectors to control PWD [
5]. However, with changing trends in society and the economy, chemical control agents are now expected to have low toxicity, high efficiency, and be environmentally friendly. The traditional, highly toxic organophosphorus nematicides generate unacceptable high levels of residues that can severely harm the environment [
6]. Although some chemical pesticides have been applied effectively via the trunk-injection method in forests, this method lacks systemic activity, coupled with the high cost, making it impractical for application in the forest setting [
7,
8]. Therefore, finding a new high-efficiency and environment-friendly chemical agent capable of having a systemic activity to resolve the problems associated with PWD control is imperative.
Fluopyram is a succinate dehydrogenase inhibitor (SDHI) in the phenyl-benzamide chemical group, which is known to move systemically through foliage acropetally [
9,
10]. Fluopyram was originally developed as a fungicide but could also be used as a nematicide [
11,
12]. Importantly, fluopyram exhibits significant nematicidal activity against
B. xylophilus; therefore, given its robust systemic activity in trees, fluopyram could be a promising candidate trunk-injection agent for controlling PWN [
8]. Nevertheless, reliance on the trunk injection method is still expensive. Considering the systemic activity of fluopyram, the key question becomes, does fluopyram move throughout the tree once absorbed by branches and leaves after aerial spraying it in the pine forest?
To determine whether fluopyram can be absorbed and translocated throughout the tree after spraying, we used a fluopyram suspension concentrate (SC) to control PWD effectively. In this study, we quantified the translocation within trees and duration of the nematicidal effect of fluopyram in Pinus massoniana trees and further evaluated the control effects of fluopyram SC by detecting its residual concentration in branches, needles, soil, and water after spraying it. The diffusibility and persistence of fluopyram SC in the environment were also analyzed as well as the dynamic digestion of fluopyram in P. massoniana. Using the results, we assessed the efficiency of fluopyram SC in controlling PWD in pine forests.
2. Materials and Methods
2.1. Field Study Sites and Nematicide Application
The efficiency of fluopyram SC in the field was conducted in 2019 at four sample plots, including Qingkou, Minhou County, China (25°52′9.00″ N, 119°22′21.03″ E) (sample plot 1), Jin’an District, Fuzhou City, China (26°6′42.46″ N, 119°23′24.47″ E) (sample plot 2), Hongwei, Minhou County, China (26°10′58.60″ N, 119°1′14.04″ E) (sample plot 3), Wuyishan National Park, China (27°38′11.54″ N, 117°55′58.62″ E) (sample plot 4). The tests of the distribution of fluopyram in trees and the degradation of fluopyram in soil and water samples were performed at Hongwei, Minhou, Fuzhou, Fujian (26°9′11.40″ N, 118°57′32.06″ E) (sample plot 5).
P. massoniana forest in all sample plots are mixed stands of natural forest and planted forest, the stand age is about 40 years old, and the canopy density of all sample plots is >0.7. These sampling plots are all located in endemic areas of PWD, which are announced by the government [
13]. The city or county for sample plots 3 and 4 was a PWD endemic area in 2021 only, while sample plots 1 and 2 are located in areas that have been affected by PWD for the past 5 years. Healthy
P. massoniana with 20–30 cm diameter at breast height (DBH) was used for the test in all sample plots (additional information in
Table S1).
The formulation of fluopyram SC used in this study was Lufuda® (41.7% fluopyram SC, Bayer CropScience China Co., Ltd., Fuzhou, China). The compound was diluted with water into a solution for spraying, containing 1% fluopyram active ingredient. For its forest application, the T20 plant protection UAV (unmanned aerial vehicle) (Shenzhen DJI Sciences and Technologies Ltd., Shenzhen, China) was used to spray the fluopyram SC on the pine canopy at 1 kg/ha.
2.2. Experiment 1: Fungistatic Effect of Fluopyram
PWN can survive in nature by feeding on
Pestalotiopsis sp. and
Ceratocystis sp. [
14,
15]. These two fungi are also the dominant fungi isolated from dead pine wood infected by PWD. The growth rate method was used to determine the fungistatic effect of fluopyram on
Pestalotiopsis sp. and
Ceratocystis sp. Briefly, fluopyram was diluted with double-distilled water into five different concentrations: 0.041700, 0.010425, 0.002606, 0.000652, and 0.000163 mg/L. Then, 2 mL of the suspension was added to a 20 mL Potato-dextrose agar (PDA) medium. PDA medium with 2 mL of double-distilled water was used as a control. A 0.6 cm diameter fungus cake was added into the center of each PDA plate and then cultured in an incubator at 28 °C. The growth of the fungi in different concentrations was recorded. All concentrations were tested in triplicate. After the mycelium of the control group had grown over the entire plate, the diameter of the mycelia was measured in all treatments by the diameter measured on two perpendicular axes, and the inhibition rate of mycelia growth was calculated as follows:
2.3. Experiment 2: Suppression of Pine Wilt Disease in the Laboratory
The control efficacy of fluopyram SC to PWD was evaluated on pot-grown 3-year-old healthy pine seedlings. The seedlings were planted in pots 18 cm in diameter with soil and placed in a shade house to grow. Then, 41.7% fluopyram SC was diluted 15,000 folds with distilled water before use, and 450 mL of the 15,000-fold dilution was sprayed on each seedling, and 10 seedlings were treated. Ten seedlings were sprayed with water as control. One week after fluopyram SC or water application, PWNs were inoculated by inoculation under the bark [
16]. Briefly, in the central part of the seedling, about 10 cm above the base, a small slit about 0.5 cm in length was cut at an oblique angle of 30° with a scalpel cutting the xylem. Then, a cotton ball was placed in the wound, sealed with parafilm, and slowly injected with PWN suspension through a pipette. Each seedling was inoculated with approximately 3000 nematodes. The incidence of pine seedling disease was observed and recorded until all control seedlings had died (about 60 days after inoculation).
To determine whether PWD caused the death of the inoculated seedlings, the branches of all seedlings were collected, and the PWN in branches was detected by the Baermann funnel method [
17,
18]. Briefly, the Baermann funnel apparatus consisted of a funnel with an inner diameter of 12 cm and a 10 cm piece of silicone hose, which was tightly attached to the funnel end. The end of the silicone hose was closed with a squeezer clip. Three paper towels were in the funnel to prevent samples from entering the nematode solution. Branches samples were cut into small chips and put on the paper; then, the distilled water was added into the funnel just above the paper towel. This apparatus was installed in a horizontal position for 24 h at room temperature. The nematodes were separated from the samples and accumulated at the end of the silicone hose. Then, nematodes were collected into a 10 mL tube and verified using a compound microscope.
2.4. Experiment 3: Distribution of Fluopyram Suspension Concentrate in Trees
At sample plot 5, 1% fluopyram SC was sprayed by the T20 plant protection UAV on the pine canopy at 1 kg/ha in May 2019, and six pine trees in this plot were randomly selected to measure the residual concentration of fluopyram. The fluopyram residue tests were carried out on these trees at 7 and 30 days post-application.
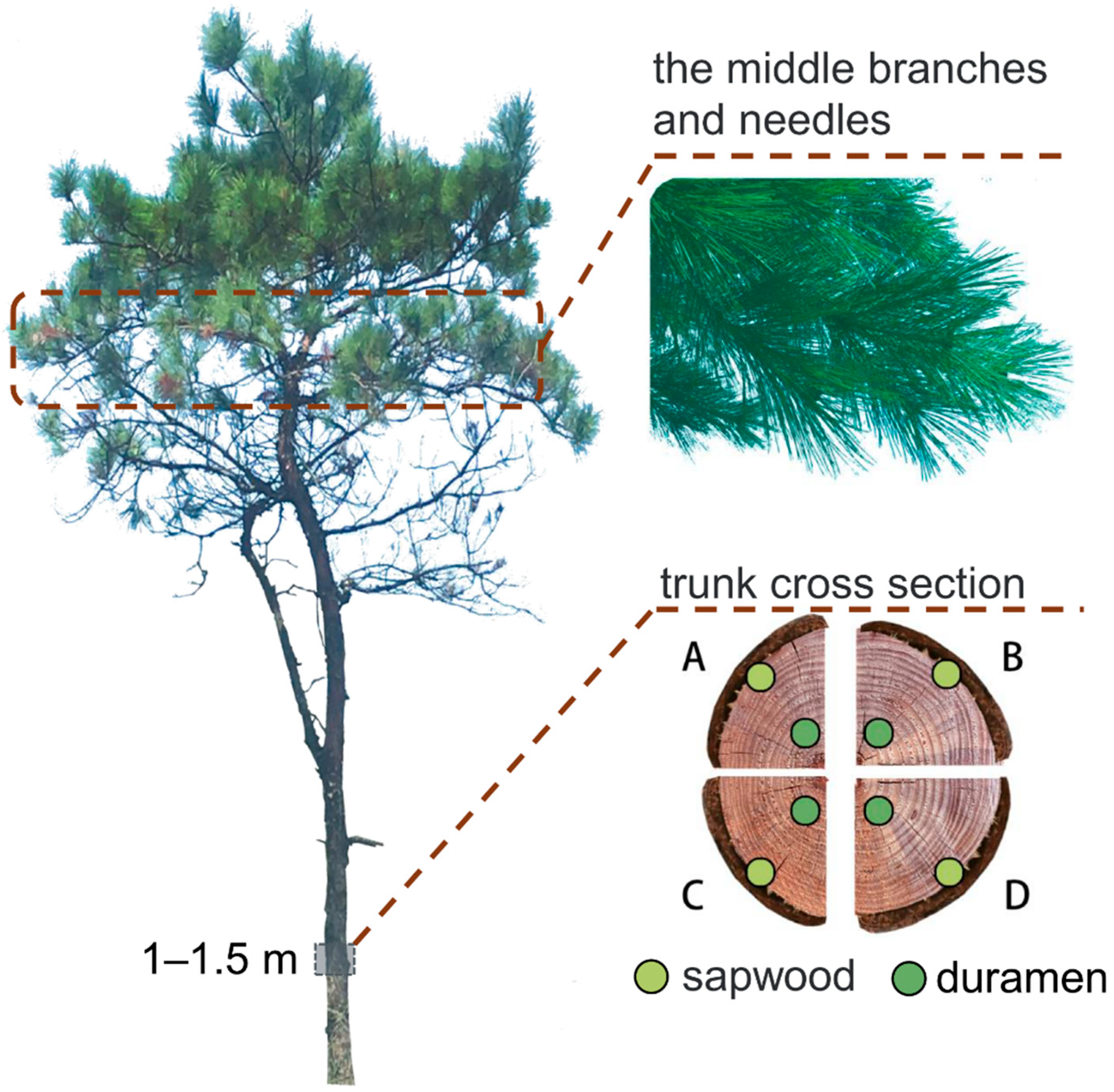
Briefly, three trees were selected for destructive sampling at 7 days post-application, and the remaining three were destructively sampled at 30 days post-application, as shown in
Figure 1. The branches and needles at the approximate mid-point between the base of the crown and the top of the tree were collected and detected for fluopyram residual concentration, respectively. The trunk samples were collected from approximately 1 to 1.5 m above the ground surface, and their sapwood and duramen were detected separately. For each sample position, four samples, one from each cardinal direction, were collected.
2.5. Experiment 4: Movement and Persistence of Fluopyram in Soil and Water
To test the fluopyram residual concentration in the environmental samples of pine forest, 1% fluopyram SC was applied in May 2021 in the PWD epidemic area of sample plot 5. Soil and water samples were collected at 7 and 30 days post-application of fluopyram SC. Three routes (A–C) were selected around the sample plot, and four sampling points with different distances were set on each route, including from the sample plot (i.e., 0 m) and 10 m, 50 m, and 200 m from its edge. There is no water at the 0 m site, so only soil samples are collected here. Three samples were randomly collected at each sampling point. Water samples were collected from springs in the pine forest and reservoirs outside the treated areas (
Figure S1). The sampling sites are located in a mountain area with an altitude of about 90–120 m (26°8′59.20″ N, 118°57′14.27″ E), and the soil nature is krasnozem. The annual average temperature is about 18–20 °C, and the annual average relative humidity is 75%–80%. Briefly, about 5 g soil samples from 0–5 cm below the surface were collected using collection ziplock bags, and 50 mL water samples from the stream in the plots were collected in 50 mL plastic tubes. The samples from the untreated plots were used as controls. All samples were placed in dry ice immediately after collection, brought back to the laboratory, and stored at −80 °C until use.
2.6. Experiment 5: Efficacy of Aerial-Applied Fluopyram in Controlling PWN Infestations
To evaluate the control efficacy of fluopyram against PWD in the field, fluopyram SC was sprayed in four sampling plots from 2019 to 2021. The crowns of
P. massoniana trees were sprayed with fluopyram SC (1% active ingredient) at an application rate of 1 kg/ha by the T20 plant protection UAV in the morning on a sunny day. The blocks in four sample plots without SC spray were used as controls. The control efficacy was recorded and calculated over three successive years, 2019–2021 (
Figure 2, more detailed information in
Table S1).
In detail, sample plot 1 was sprayed in May 2019, 6 blocks with an area of 0.67 ha were selected, and three blocks each were in the control and sprayed groups. To detect the fluopyram residue in trees, the four main directions’ branches with needles, at the approximate mid-point between the base of the crown and the top of the tree (middle branches), were collected from three to four randomly selected trees at 90, 455, and 820 d after SC application. Likewise, two sprayed blocks and three control blocks in sample plot 2 were sprayed in June 2020; the area of each block was 1.67 ha. The branches’ sampling times were 90 and 455 d after the application. Sample plots 3 and 4 were sprayed in May and July 2021, respectively. In sample plot 3, three blocks with an area of about 3.33 to 13.33 ha were selected for spraying, and four blocks were used as control groups. While sample plot 4 includes two sprayed blocks and two control blocks (about 5.33 to 16.67 ha each). Branch samples from sample plots 3 and 4 were collected at 90 d after SC application.
Before spraying, the initial number of dead woods was counted in each sample plot. After the spray of fluopyram SC, the death of
P. massoniana individuals was registered and counted in the sprayed and control blocks in November of every year. A P4 Multispectral unit (Shenzhen DJI Sciences and Technologies Ltd., Shenzhen, China) was used to take the photographs positioned at 150–200 m of height in the sprayed and control plots, then suspected dead trees were marked according to the regional map that was spliced by the photographs. According to the recognized regional distribution pictures of dead pine trees, a field confirmation was conducted in person in the sample plot and all dead pine trees were counted. The 1 or 2 middle branches of all dead pine trees were collected, and the PWN in branches was determined by the Baermann funnel method in the laboratory to corroborate that those pine trees were killed by PWD. The efficiency of fluopyram SC to control PWD was calculated by using the following formula [
19]:
2.7. Experiment 6: Degradation Kinetics Analysis of Fluopyram in Trees
Combined with the data of the distribution test of fluopyram SC in the trees and the field experiments in sample plot 1, the fluopyram residue in the middle branch samples was determined at 0, 7, 30, 90, 455, and 820 d after fluopyram SC application. Excel 2020 software was used to analyze the fluopyram residual concentration data, and the degradation kinetics equation and degradation half-life of fluopyram in
P. massoniana were calculated as follows [
20]:
where
C0 is the residual concentration of fluopyram at 0 days (mg/kg);
Ct is the residual concentration of fluopyram at day
t (mg/kg); and
k is the degradation kinetic constant.
2.8. Sample Preparation for GC-MS
Samples were pre-processed before GC-MS detection. The branches, needles, and trunk samples from trees were chopped into more minor chips. Each tissue sample was homogenized with methanol (100 mL) for 10 min (in a homogenizer (Dongguan Fangtai Electric Appliance Co., Ltd., Dongguan, China)). Soil samples were ground into powder, and the same analytical procedures were used for both the plant tissue samples and the soil samples. In detail, 1 g of the homogenized mixture or soil as the powder was added into a 50 mL tube with 10 mL acetonitrile, 2 g of MgSO4, 1 g of NaCl, and 20 mg of activated carbon. After shaking and mixing with a digital Vortex4 (Shenzhen Sanli Chemical Co., Shenzhen, China) for 1 min, the homogenized mixture was sonicated using an Ultrasonic Bath (KH-300DE, Kunshan Ultrasonic Instrument Co., Ltd., Kunshan, Jiangsu, China) for 10 min and centrifuged at 5000× g for 10 min. The supernatant (6 mL in total) was mixed with 400 mg of MgSO4 and 150 mg of N-propyl ethylenediamine (PSA, Welch Materials, Ellocott City, MD, USA), sonicated for 10 min, and centrifuged at 5000× g for 10 min after shaking and mixing for 1 min. Two mL of the resultant supernatant was evaporated to dryness under a nitrogen purge (N-EVAP-45, Organomation, Berlin, MA, USA) at 60 °C, ensuring that no moisture entered the tube. Finally, each sample was resuspended to a 1 mL volume with hexane and filtered through a 0.22 μm organic membrane for its GC-MS analysis.
From each water sample, 10 mL were filtered to remove any solid impurities, and the water was subjected to vortex oscillation for 10 min with 10 mL of n-hexane. This mixture was sonicated for 10 min and centrifuged at 5000× g for 5 min. The supernatant of the n-hexane solution was collected into a 10 mL tube, dried under a nitrogen purge, and finally resuspended with methanol to a volume of 1 mL. Each sample was filtered through a 0.22 μm organic membrane for its GC-MS analysis.
2.9. GC-MS Instrumentation and Chromatographic Conditions
The residual concentration of fluopyram in pine trees, soil, and water samples at 7 and 30 days post-application was statistically analyzed by GC-MS. We used the QP2010 plus gas chromatograph-mass spectrometer (GC-MS, Shimadzu, Shanghai, China) equipped with a GCMS-QP2010 Plus wide-bore sampler (Shimadzu, Shanghai, China). The column used was a DB-5 ms capillary column (30 m × 0.25 mm × 0.25 μm), and the temperature procedure consisted of 80 °C for 1 min + 30 °C/min to 220 °C + 5 °C/min to 240 °C + 10 °C/min to 280 °C, held at 1 min. The mobile phase was nitrogen (purity ≥ 99.999) at a flow rate of 1.0 mL /min; the sample volume was 20 μL at a flow rate of 0.3 mL/min. The electron bombardment (EI) source was 70 eV, the ion source temperature was maintained at 230 °C, and the interface temperature was 290 °C. The solvent delay was 5 min. The SCAN range was 45–550
m/
z. The flow rate of atomized gas was 1.5 L/min, and the drying pressure was 121 kPa. The residual concentration of fluopyram in each sample was calculated with the following formula:
where
C is the concentration of the pesticide in the sample (mg/kg);
C0 is the measured value of the sample (mg/kg);
ρ is the density of n-hexane (kg/L);
V is the volume of n-hexane (L); and
m is the mass of the sample (kg).
2.10. Statistical Analyses
All data were collated and calculated using Microsoft Excel 2020, and all statistics graphs were generated using GraphPad Prism 6 (9.5.0 for Windows). SPSS Statistics (19.0 for Windows) software was used for data analysis. Data from each experiment were tested for homogeneity of variance and one-way analysis of variance (ANOVA, α = 0.05) for comparing the means of the single continuous variable between different groups. Then, data from the fluopyram residue in the tree, soil, and water samples, as well as the inhibition rate of fungal growth, were analyzed by multiple comparisons of the means by Tukey’s test (p = 0.05) and Duncan’s multiple range test were used to make multiple comparisons of the means for the data of forest control efficiency. Values are presented as means ± SE. For the experiment of the fungistatic effect of fluopyram, regression analysis of virulence was performed using concentration and inhibition rate as independent and dependent variables, respectively, and the median effective concentration (EC50) in the maximum effect of inhibition was calculated.
4. Discussion
Currently, commercially available chemical pesticides that can be sprayed on trees for PWD control lack systemic activity, and most of them are insecticides used to control the insect vector
Monochamus spp., so their effect on PWN is limited [
21,
22]. To overcome these challenges, in this study, fluopyram was used as a suspension concentrate to test its efficiency on PWD control due to its systemic activity. The systemic activity of fluopyram may promote the better, more widespread, and stable transport of this chemical agent in trees [
10]. There have been reported other chemicals for nematode control. For example, abamectin is also water-soluble (10 μg/L at 21 °C), but it has a lower water solubility than fluopyram (16 mg/L at 20 °C), which makes its application difficult [
10,
23]. The efficacy of the avermectin derivative against avocado thrips was poor, and this was likely the result of its low water solubility, which restricted the amount of avermectin that could be absorbed into tree tissues [
23]. Emamectin benzoate is another agent used to manage the PWD by trunk injection in Japan, showing a successful distribution and activity against PWN in smaller trees with trunk diameters of less than 15 cm [
24]. In the present study, since the suspension concentrate was sprayed onto the canopy of pine trees, the concentrate likely translocated downward to all the tree tissues via the branches and needles. More extended periods post-application showed that fluopyram could be uniformly distributed in each tissue of pine tree with 20–30 cm DBH. Thirty days post-application, the residual concentration of fluopyram in needles and branches was still significantly higher than the LC
50 of fluopyram to PWN (0.059 mg/L). Therefore, compared with the existing chemical pesticides used for PWN control, fluopyram SC has a robust systemic activity and transport activity, thus providing a new way to control infestations of PWN effectively.
Fluopyram was initially formulated as a fungicide by Bayer Crop Science (RTP, NC). Its activity against various fungi has been critically assessed, including the ability to control
Fusarium virguliforme [
11]. It is reported that conidia germination of
F. virguliforme was determined to be more sensitive to fluopyram (mean EC
50 = 2.28 µg/mL) than the mycelial growth (mean EC
50 = 3.35 µg/mL) [
11]. We found that fluopyram has an obvious fungistatic effect on
Pestalotiopsis sp., on which nematodes feed; the mean EC
50 for fluopyram to
Pestalotiopsis sp. was 63.807 mg/L that lower than the EC
50 of
F. virguliforme. In addition, fluopyram has also been applied as a nematicide against root-knot nematodes, cyst nematodes, and other soil nematodes [
12,
25,
26,
27]. Concerning its effect on nematodes, research has shown that fluopyram provides a good level of control over the root-knot nematode,
Meloidogyne incognita (Kofoid & White) Chitwood, and the reniform nematode,
Rotylenchulus reniformis Linford & Oliveira, for which the 2 h EC
50 values are 5.18 and 12.99 μg/mL fluopyram, respectively [
25]. Crucially, fluopyram also displays activity against the PWN. For example, fluopyram exhibited significant nematicidal activity, with LC
50 values of 0.945, 1.69, and 3.23 mg/L for second-stage juveniles (J
2), third-stage juveniles (J
3), and fourth-stage juveniles/adults (J
4/adults), respectively [
8]. In the present study, the results of spraying pine seedlings with fluopyram SC in the laboratory showed that it had an evident preventive effect on PWD, showing the first case that fluopyram spraying can prevent PWD.
It has been suggested that fluopyram might be an alternative agent for use in trunk injections [
8]. However, when managing forests with complex terrain across a large area, applying trunk injections is difficult and costly in terms of both the workforce and material resources required. Therefore, compared with trunk injection, the aerial application of fluopyram SC is more feasible and affordable in forests. In general, SC is a widely used dosage form with many advantages. The solid is non-dusty, disperses well in water with water-soluble adjuvants, is easier to apply, is safe for the operator and environment, and costs relatively less [
28]. Applications of SCs have been studied for their prevention and control of animal parasites, diseases, and pests of crops [
29]. In China, Japan, South Korea, and Portugal, where PWD is more severe, UAVs or ground spraying have been used to control this disease by spraying chemical SCs that are effective against vector beetles [
30,
31]. Previous studies showed that the efficiency of the suspension agent sprayed in the forest against vector beetles was about 72.4% [
32]. However, there are few reports of applying aerial spraying chemical SCs against PWN. In the present study, the fluopyram SC showed a substantial preventative effect on PWN in the laboratory experiment. After spraying in the field, fluopyram SC showed a significant suppressive effect against PWD within 3 years of application, with a persistence of 3 years and average control efficiency reaching 90.48%.
Moreover, there was an incomplete cleaning of dead wood in sample plot 3, so the efficiency of fluopyram SC to PWD in sample plot 3 was lower than in sample 1 or sample 2, indicating that the integrity of dead wood cleaning could affect the control effect of fluopyram SC. In addition, we also found that the prevention effect of PWD was different when spraying in different months, that application of fluopyram SC later than June can significantly reduce the control effect, whereas early applications of fluopyram SC can achieve a better control efficiency. This is related to the life cycle of the PWN transmitted by vector beetles, whereby the wide spread of PWN occurs from June to September [
33]. Therefore, to deploy the efficacy of the fluopyram SC in the field, the best time for application is 1 month before the emergence of beetle adults every year.
In addition, when chemical agents are applied in agriculture and forestry across a large area, their transformation in environmental samples is also the focus of much investigation. For example, several studies have assessed the degradation and metabolism of abamectin and thiacloprid in soil, water, light, and other conditions [
34,
35,
36], as well as the toxicity and safety of imidacloprid for environmental organisms [
37,
38]. In the present study, after applying fluopyram SC over a large area, fluopyram residues were detected in soil and water in the non-forest habitat areas between sampling plots. However, we found that these fluopyram residues at 0–200 m, going from the inside to outside of the sampling plot, were almost non-existent 30 days after the application. Even with the small amount of residue detected, the fluopyram residual concentration for soil samples was still significantly lower than the LC
50 for fluopyram in earthworm (14 d) > 1.00 mg/kg (in dry soil). The residual concentration of water samples 10 m away (0.099 mg/kg) was significantly degraded, which was lower than the LC
50 for fluopyram in carp (96 h) > 0.98 mg/L. It is reported that fluopyram was degraded chiefly by microorganisms in soil and water and is indeed an easily degraded pesticide [
20]. Fluopyram was stable to hydrolysis, with a hydrolysis half-life of more than 1 year at 25 °C [
18]. Microbial degradation is the main mode by which fluopyram degrades in soil [
18]. According to the Chinese government’s standards for the registration and classification of degradation of pesticides in soil, fluopyram should degrade relatively easily [
18]. Therefore, applying fluopyram SC in a pine forest is an environmentally friendly practice for pest control.