Seasonal Dynamics and Influencing Factors of Litterfall Production and Carbon Input in Typical Forest Community Types in Lushan Mountain, China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Area
2.2. Experimental Design and Sample Collection
2.3. Statistical Analyses
3. Results
3.1. Monthly Dynamics and Composition of Total Litterfall in Four Forest Community Types
3.2. Relationship between Litterfall Production and Stand Factors
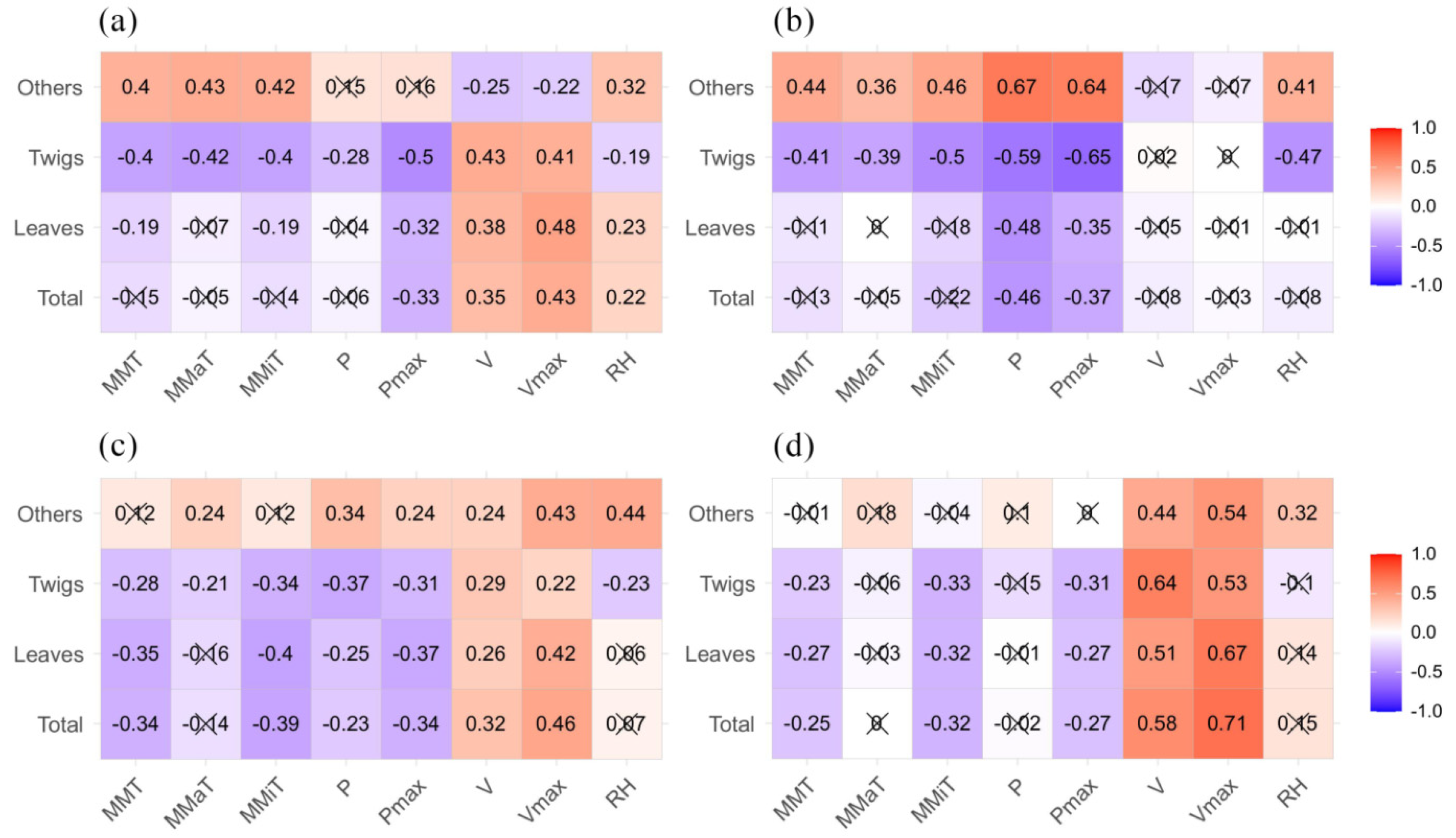
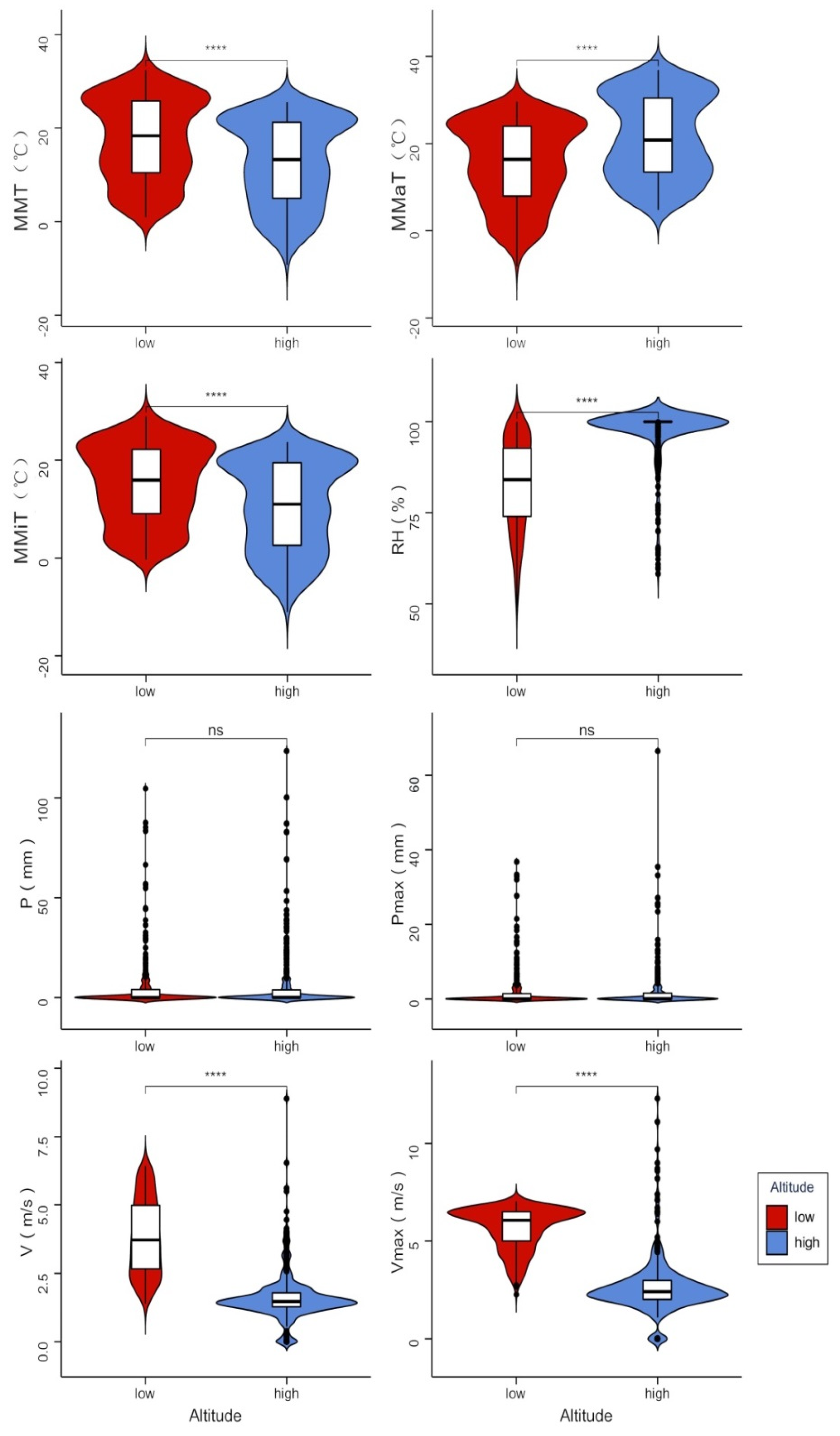
3.3. Relationships between Litterfall Production and Meteorological Factors
3.4. Carbon Production
4. Discussion
4.1. Changes in the Total Amount of Litterfall and Its Components in the Four Forest Communities
4.2. Effects of Stand Factors and Terrain Factors on Litterfall Production of the Four Forest Communities
4.3. Effects of Meteorological Factors on Litterfall Production in Four Forest Communities
4.4. Differences in Carbon Input and Component Characteristics of Litterfall in Four Forest Communities in Different Seasons
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albrich, K.; Rammer, W.; Seidl, R. Climate change causes critical transitions and irreversible alterations of mountain forests. Glob. Chang. Biol. 2020, 26, 4013–4027. [Google Scholar] [CrossRef] [PubMed]
- Marler, T.E.; Cruz, G.N. Temporal Variation of Litterfall and Nutrient Return of Serianthes nelsonii Merr. in a Tropical Karst Forest. Plants 2022, 11, 2310. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.A.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef] [PubMed]
- Morffi-Mestre, H.; Ángeles-Pérez, G.; Powers, J.S.; Andrade, J.L.; Huechacona Ruiz, A.H.; May-Pat, F.; Chi-May, F.; Dupuy, J.M. Multiple factors influence seasonal and interannual litterfall production in a tropical dry forest in mexico. Forests 2020, 11, 1241. [Google Scholar] [CrossRef]
- Liu, X.; Zeng, X.; Zou, X.; González, G.; Wang, C.; Yang, S. Litterfall production prior to and during Hurricanes Irma and Maria in four Puerto Rican forests. Forests 2018, 9, 367. [Google Scholar] [CrossRef]
- Zheng, H.; Ouyang, Z.Y.; Wang, X.K.; Fang, Z.G.; Zhao, T.Q.; Miao, H. Effects of regenerating forest cover on soil microbial communities: A case study in hilly red soil region, Southern China. For. Ecol. Manag. 2005, 217, 244–254. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Osawa, A.; Deshar, R.; Sharma, S.; Mouctar, K. Species composition, biomass, and net primary productivity of mangrove forest in Okukubi River, Okinawa Island, Japan. Reg. Stud. Mar. Sci. 2017, 12, 19–27. [Google Scholar] [CrossRef]
- Williams-Linera, G.; Bonilla-Moheno, M.; López-Barrera, F.; Tolome, J. Litterfall, vegetation structure and tree composition as indicators of functional recovery in passive and active tropical cloud forest restoration. For. Ecol. Manag. 2021, 493, 119260. [Google Scholar] [CrossRef]
- Lopes, M.C.A.; Araújo, V.F.P.; Vasconcellos, A. The effects of rainfall and vegetation on litterfall production in the semiarid region of northeastern Brazil. Braz. J. Biol. 2015, 75, 703–708. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, P. Study on the diurnal dynamic changes and prediction models of the moisture contents of two litters. Forests 2020, 11, 95. [Google Scholar] [CrossRef] [Green Version]
- Giweta, M. Role of litter production and its decomposition, and factors affecting the processes in a tropical forest ecosystem: A review. J. Ecol. Environ. 2020, 44, 11. [Google Scholar] [CrossRef]
- Erkan, N.; Comez, A.; Aydin, A.C. Litterfall production, carbon and nutrient return to the forest floor in Pinus brutia forests in Turkey. Scand. J. For. Res. 2020, 35, 341–350. [Google Scholar] [CrossRef]
- Du, B.; Liu, C.; Kang, H.; Zhu, P.; Yin, S.; Shen, G.; Hou, J.; Ilvesniemi, H. Climatic control on plant and soil δ13C along an altitudinal transect of Lushan Mountain in Subtropical China: Characteristics and interpretation of soil carbon dynamics. PLoS ONE 2014, 9, e86440. [Google Scholar] [CrossRef]
- Xiao, T.; Wang, C.; Yuan, X.; Tao, L.; Li, P.; Deng, W.; Liu, Y. Effects of Different Forest Gap Ages on Soil Physical Properties and Stoichiometric Characteristics in Cryptomeria japonica plantations (Lf) D. Don, 1839. Forests 2022, 13, 1708. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L. Scientific Survey and Study of Biodiversity on the Lushan Nature Reserve in Jiangxi Province; Science Press: Beijing, China, 2010. [Google Scholar]
- Liu, X.; Nie, Y.; Wen, F. Seasonal dynamics of stem radial increment of Pinus taiwanensis Hayata and its response to environmental factors in the Lushan Mountains, Southeastern China. Forests 2018, 9, 387. [Google Scholar] [CrossRef]
- Liu, W.; Liao, L.; Liu, Y.; Wang, Q.; Murray, P.J.; Jiang, X.; Zou, G.; Cai, J.; Zhao, X. Effects of Phyllostachys Pubescens Expansion on Underground Soil Fauna Community and Soil Food Web in a Cryptomeria Japonica Plantation, Lushan Mountain, Subtropical China. J. Soils Sediments 2021, 21, 2212–2227. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Chen, L.; Wang, J.; Shen, Z. Spatial distribution characteristics of soil organic carbon in subtropical forests of mountain Lushan, China. Environ. Monit. Assess. 2018, 190, 545. [Google Scholar] [CrossRef]
- Koyejo, A.O.; Okpara, D.A.; Onyeonagu, C.C.; Eteng, E.U. Effects of climatic variations on leaf litter production in A. floribunda agroforestry system in South-East Nigeria. Niger. J. Soil Sci. 2020, 30, 42–48. [Google Scholar]
- Huang, Y.; Ma, Y.; Zhao, K.; Niklaus, P.A.; Schmid, B.; He, J.S. Positive effects of tree species diversity on litterfall quantity and quality along a secondary successional chronosequence in a subtropical forest. J. Plant Ecol. 2017, 10, 28–35. [Google Scholar] [CrossRef]
- Jia, B.; Xu, Z.; Zhou, G.; Yin, X. Statistical characteristics of forest litterfall in China. Sci. China Life Sci. 2018, 61, 358–360. [Google Scholar] [CrossRef]
- Kamruzzaman, M.D.; Basak, K.; Paul, S.K.; Ahmed, S.; Osawa, A. Litterfall production, decomposition and nutrient accumulation in Sundarbans mangrove forests, Bangladesh. For. Sci. Technol. 2019, 15, 24–32. [Google Scholar] [CrossRef]
- Xing, J.; Wang, K.; Song, Y.; Zhang, Y.; Zhang, Z.; Pan, T. Characteristics of litter return and nutrient dynamic change in four typical forests in the subalpine of central Yunnan province. J. Cent. South Univ. Technol. 2021, 41, 134–144. [Google Scholar]
- Liu, X.; Wang, Z.; Liu, X.; Lu, Z.; Li, D.; Gong, H. Dynamic Change Characteristics of Litter and Nutrient Return in Subtropical Evergreen Broad-Leaved Forest in Different Extreme Weather Disturbance Years in Ailao Mountain, Yunnan Province. Forests 2022, 13, 1660. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Kim, J.S.; Williams, M. Effects of temperature and precipitation on litterfall phenology in four evergreen broad-leaved forests of southern China. Biotropica 2022, 54, 739–753. [Google Scholar] [CrossRef]
- Yoshida, T.; Hijii, N. Spatiotemporal distribution of aboveground litter in a Cryptomeria japonica plantation. J. For. Res.-Jpn. 2006, 11, 419–426. [Google Scholar] [CrossRef]
- Guo, J.; Yu, L.H.; Fang, X.; Xiang, W.H.; Deng, X.W.; Lu, X. Litter production and turnover in four types of subtropical forests in China. Acta Ecol. Sin. 2015, 35, 4668–4677. [Google Scholar]
- Almagro, M.; Maestre, F.T.; Martínez-López, J.; Valencia, E.; Rey, A. Climate change may reduce litter decomposition while enhancing the contribution of photodegradation in dry perennial Mediterranean grasslands. Soil Biol. Biochem. 2015, 90, 214–223. [Google Scholar] [CrossRef]
- Wang, C.G.; Zheng, X.B.; Wang, A.Z.; Dai, G.H.; Zhu, B.K.; Zhao, Y.M.; Dong, S.J.; Zu, W.Z.; Wang, W.; Zheng, Y.G.; et al. Temperature and Precipitation Diversely Control Seasonal and Annual Dynamics of Litterfall in a Temperate Mixed Mature Forest, Revealed by Long-Term Data Analysis. J. Geophys. Res.-Biogeosci. 2021, 126, e2020JG006204. [Google Scholar] [CrossRef]
- Muller-Landau, H.C.; Cushman, K.C.; Arroyo, E.E.; Martinez Cano, I.; Anderson-Teixeira, K.J.; Backiel, B. Patterns and mechanisms of spatial variation in tropical forest productivity, woody residence time, and biomass. New Phytol. 2021, 229, 3065–3087. [Google Scholar] [CrossRef]
- Qun, L.X.C.M.F.; Mei, L.M.H.Y.D. Leaf fall patterns of 12 evergreen woody species in subtropical evergreen forest in Tiantong Mountain of Zhejiang Province, China. Bull. Bot. Res. 2014, 34, 741–750. [Google Scholar]
- Jaramillo, V.J.; Martínez-Yrízar, A.; Machado, L.I. Hurricane-Induced Massive Nutrient Return via Tropical Dry Forest Litterfall: Has Forest Biogeochemistry Resilience Changed? Ecosystems 2022, 25, 1767–1779. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Wang, L.; Zhou, B.; Cao, Y.; Xiao, W.; Li, M.H. Drought changes litter quantity and quality, and soil microbial activities to affect soil nutrients in moso bamboo forest. Sci. Total. Environ. 2022, 838, 156351. [Google Scholar] [CrossRef]
- Zhou, J.; Lang, X.; Du, B.; Zhang, H.; Liu, H.; Zhang, Y.; Shang, L. Litterfall and nutrient return in moist evergreen broad-leaved primary forest and mixed subtropical secondary deciduous broad-leaved forest in China. Eur. J. For. Res. 2016, 135, 77–86. [Google Scholar] [CrossRef]
- Azad, M.S.; Kamruzzaman, M.; Paul, S.K.; Kanzaki, M. Litterfall release, vegetative, and reproductive phenology investigation of Heritiera fomes Buch-Ham in the Sundarbans mangrove forests, Bangladesh: Relationship with environmental variables. For. Sci. Technol. 2020, 16, 105–115. [Google Scholar] [CrossRef]
- Sun, X.; Liu, F.; Zhang, Q.; Li, Y.; Zhang, L.; Wang, J.; Zhang, H.; Wang, C.; Wang, X. Biotic and climatic controls on the interannual variation in canopy litterfall of a deciduous broad-leaved forest. Agric. For. Meteorol. 2021, 307, 108483. [Google Scholar] [CrossRef]
- De Queiroz, M.G.; da Silva, T.G.F.; Zolnier, S.; de Souza, C.A.A.; de Souza, L.S.B.; Neto, A.J.S.; de Araújo, G.G.L.; Ferreira, W.P.M. Seasonal patterns of deposition litterfall in a seasonal dry tropical forest. Agric. For. Meteorol. 2019, 279, 107712. [Google Scholar] [CrossRef]
- Gaspar-Santos, E.S.; González-Espinosa, M.; Ramírez-Marcial, N.; Álvarez-Solís, J.D. Acumulación y descomposición de hojarasca en bosques secundarios del sur de la Sierra Madre de Chiapas, México. Bosque 2015, 36, 467–480. [Google Scholar] [CrossRef]
- Erkan, N.; Comez, A.; Aydin, A.C.; Denli, O.; Erkan, S. Litterfall in relation to stand parameters and climatic factors in Pinus brutia forests in Turkey. Scand. J. For. Res. 2018, 33, 338–346. [Google Scholar] [CrossRef]
- Kim, C.; Baek, G.; Choi, B.; Baek, G.; Kim, H. Quantifying Litterfall Input from the Stand Parameters of Korean Red Pine (Pinus densiflora S. et Z.) Stands in Gyeongnam Province. J. Korean Soc. For. Sci. 2021, 110, 569–576. [Google Scholar]
- Feng, C.; Wang, Z.; Ma, Y.; Fu, S.; Chen, H.Y. Increased litterfall contributes to carbon and nitrogen accumulation following cessation of anthropogenic disturbances in degraded forests. For. Ecol. Manag. 2019, 432, 832–839. [Google Scholar] [CrossRef]
- Jiménez, M.N.; Navarro, F.B. Thinning effects on litterfall remaining after 8 years and improved stand resilience in Aleppo pine afforestation (SE Spain). J. Environ. Manag. 2016, 169, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Bahru, T.; Ding, Y. Effect of stand density, canopy leaf area index and growth variables on Dendrocalamus brandisii (Munro) Kurz litter production at Simao District of Yunnan Province, southwestern China. Glob. Ecol. Conserv. 2020, 23, e01051. [Google Scholar] [CrossRef]
- Murphy, B.P.; Prior, L.D.; Cochrane, M.A.; Williamson, G.J.; Bowman, D.M. Biomass consumption by surface fires across Earth’s most fire prone continent. Glob. Chang. Boil. 2019, 25, 254–268. [Google Scholar] [CrossRef] [PubMed]
- Sanaphre-Villanueva, L.; Dupuy, J.M.; Andrade, J.L.; Reyes, C.; Jackson, P.C.; Paz, H. Patterns of plant functional variation and specialization along secondary succession and topography in a tropical dry forest. Environ. Res. Lett. 2017, 12, 55004. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2010, 34, 137–148. [Google Scholar] [CrossRef]
- Liu, C.; Westman, C.J.; Berg, B.; Kutsch, W.; Wang, G.Z.; Man, R.; Ilvesniemi, H. Variation in Litterfall-Climate Relationships between Coniferous and Broadleaf Forests in Eurasia. Glob. Ecol. Biogeogr. 2010, 13, 105–114. [Google Scholar] [CrossRef]
- Gu, X.; Yang, C.; Zhao, H.; Hu, N.; Krauss, K.W.; Deng, C.; Chen, L. Sap flow evidence of chilling injury and recovery in mangroves following a spring cold spell. Trees 2021, 35, 907–917. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, W.; Chen, H.; Deng, Y.; Chen, C.; Zeng, H. Effects of forest transition on litterfall, standing litter and related nutrient returns: Implications for forest management in tropical China. Geoderma 2019, 333, 123–134. [Google Scholar] [CrossRef]
- Kitayama, K.; Ushio, M.; Aiba, S.I. Temperature is a dominant driver of distinct annual seasonality of leaf litter production of equatorial tropical rain forests. J. Ecol. 2021, 109, 727–736. [Google Scholar] [CrossRef]
- Zhou, G.; Guan, L.; Wei, X.; Zhang, D.; Zhang, Q.; Yan, J.; Wen, D.; Liu, J.; Liu, S.; Huang, Z.; et al. Litterfall production along successional and altitudinal gradients of subtropical monsoon evergreen broadleaved forests in Guangdong, China. Plant Ecol. 2007, 188, 77–89. [Google Scholar] [CrossRef]
- Dai, Y.; Gong, F.; Yang, X.; Chen, X.; Su, Y.; Liu, L.; Sun, Q. Litterfall seasonality and adaptive strategies of tropical and subtropical evergreen forests in China. J. Plant Ecol. 2022, 15, 320–334. [Google Scholar] [CrossRef]
- Wan, S.; Fu, S.; Zhang, C.; Liu, J.; Zhang, Y.; Mao, R. Effects of understory removal and litter addition on leaf and twig decomposition in a subtropical Chinese fir plantation. Land Degrad. Dev. 2021, 32, 5004–5011. [Google Scholar] [CrossRef]
- Asigbaase, M.; Dawoe, E.; Lomax, B.H.; Sjogersten, S. Temporal changes in litterfall and potential nutrient return in cocoa agroforestry systems under organic and conventional management, Ghana. Heliyon 2021, 7, e08051. [Google Scholar] [CrossRef]
- Maas, G.C.B.; Sanquetta, C.R.; Marques, R.; Machado, S.D.A.; Sanquetta, M.N.; Corte, A.P.D.; Barberena, I.M. Carbon production from seasonal litterfall in the Brazilian Atlantic Forest. South. For. 2021, 83, 128–134. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, Y.; Yao, X.; Wei, X.; Li, X.; Li, C.; White, J.; Nan, Z. Gene analysis reveals that leaf litter from Epichloë endophyte-infected perennial ryegrass alters diversity and abundance of soil microbes involved in nitrification and denitrification. Soil Biol. Biochem. 2021, 154, 108123. [Google Scholar] [CrossRef]
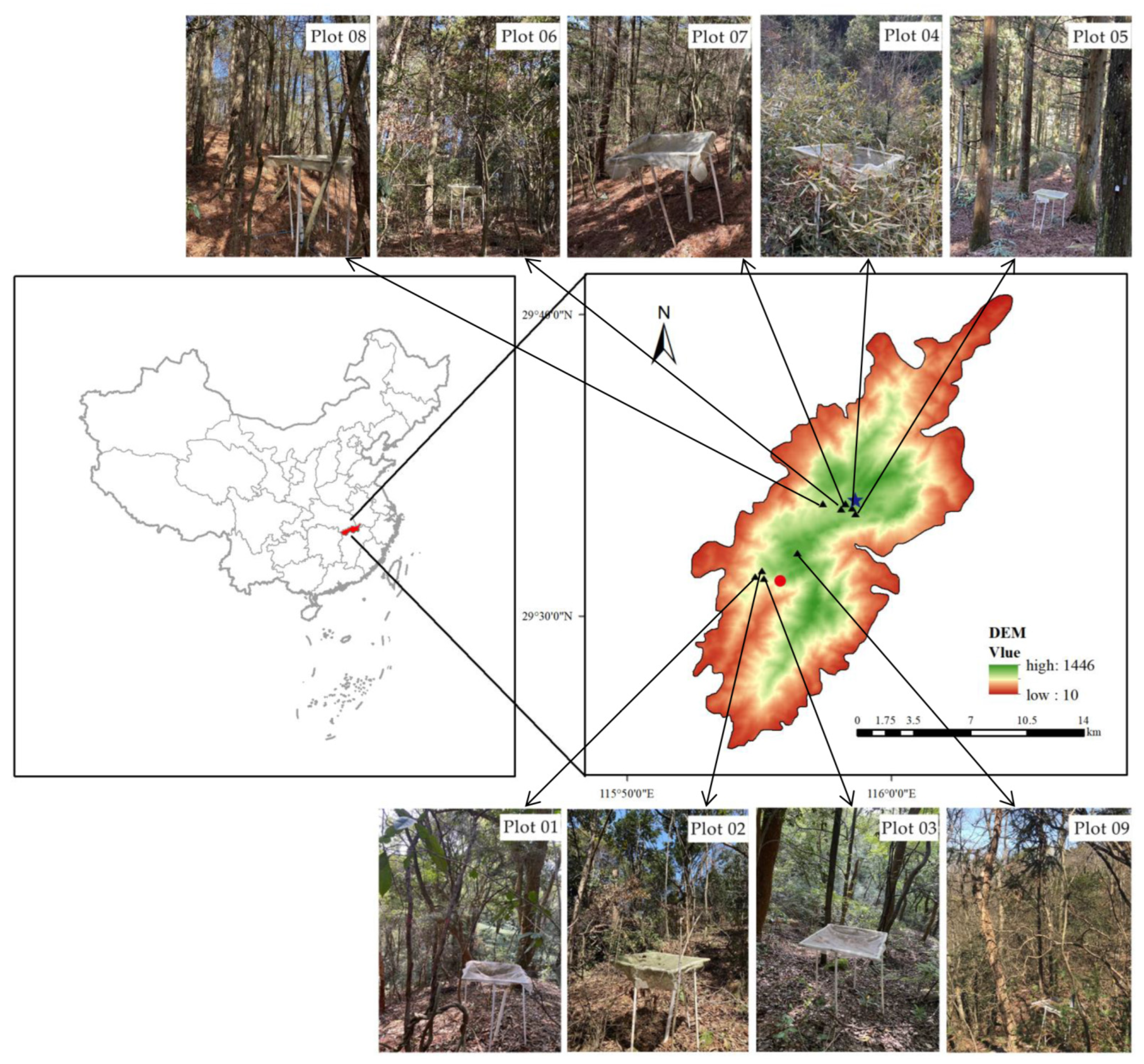

| Plot | Community Types | Sample Size | Altitude (m) | Slope | Slope Gradient | Slope Position | Main Species |
|---|---|---|---|---|---|---|---|
| 01 | Evergreen broad-leaved forest | 30 m × 40 m | 229 | W | 24 | Base of slope | Lithocarpus glaber (Thunb.) Nakai; Loropetalum chinense (R. Br.) Oliver; Castanopsis sclerophylla (Lindl.) Schott.; Cinnamomum Camphora (L.) Presl. |
| 02 | Evergreen deciduous broad-leaved forest | 30 m × 40 m | 319 | NW | 28 | Base of slope | Lithocarpus glaber (Thunb.) Nakai; Clerodendrum cyrtophyllum Turcz.; Castanopsis eyrei (Champ. ex Benth.) Tutch.; Alangium chinense; Liquidambar formosana |
| 03 | Evergreen deciduous broad-leaved forest | 30 m × 40 m | 330 | S | 29 | Base of slope | Photinia beauverdiana C. K. Schneid.; Loropetalum chinense (R. Br.) Oliver; Camellia oleifera Abel.; Styrax japonicus Sieb. et Zucc.; Alniphyllum fortunei (Hemsl.) Makino |
| 04 | Retrofitting regeneration community of Cryptomeria japonica (L. f.) D. Don (2012 years) | 30 m × 30 m | 1084 | SW | 27 | Slope crest | Cryptomeria japonica (L. f.) D. Don; Indocalamus tessellatus (Munro) Keng f.; Lindera reflexa Hemsl.; Symplocos stellaris Brand |
| 05 | Pure forest of Cryptomeria japonica (L. f.) D. Don | 30 m × 30 m | 1080 | SW | 30 | Slope crest | Cryptomeria japonica (L. f.) D. Don |
| 06 | Pure forest of P. taiwanensis | 30 m × 30 m | 1076 | SW | 35 | Slope crest | P. taiwanensis |
| 07 | Retrofitting regeneration community of P. taiwanensis (2012 years) | 30 m × 30 m | 1075 | SW | 20 | Slope crest | P. taiwanensis; Koelreuteria paniculata Laxm.; Pterostyrax corymbosus Sieb. et Zucc.; Quercus glandulifera Bl. |
| 08 | Pure forest of P. taiwanensis | 30 m × 30 m | 972 | W | 45 | Slope crest | P. taiwanensis |
| 09 | Deciduous broad-leaved forest | 200 m × 300 m | 990–1200 | N | 50 | Slope crest | Cerasus serrulata (Lindl.) G. Don ex London; Sorbus folgneri (Schneid.) Rehd.; Cornus kousa subsp. chinensis (Osborn) Q. Y. Xiang; Corylopsis sinensis Hemsl.; Lindera reflexa Hemsl. |
| Forest Types | Component | |||
|---|---|---|---|---|
| Leaves /t·ha−1 | Branches /t·ha−1 | Others /t·ha−1 | Total /t·ha−1 | |
| EBF | 2.45 ± 0.41 a (63.00%) | 0.78 ± 0.15 a (20.00%) | 0.67 ± 0.12 a (17.00%) | 3.90 ± 0.47 a (100.00%) |
| DBF | 1.88 ± 0.32 c (64.00%) | 0.51 ± 0.09 c (18.00%) | 0.53 ± 0.12 b (18.00%) | 2.92 ± 0.38 b (100.00%) |
| ACF | 2.03 ± 0.37 b (68.00%) | 0.51 ± 0.17 c (17.00%) | 0.43 ± 0.09 c (15.00%) | 2.98 ± 0.47 b (100.00%) |
| RYF | 1.74 ± 0.22 d (68.00%) | 0.45 ± 0.09 b (18.00%) | 0.37 ± 0.08 d (15.00%) | 2.56 ± 0.27 c (100.00%) |
| Forest Types | Component (y) | Regression Equation | R2 | F | p |
|---|---|---|---|---|---|
| EBF | Total | Y = −0.02 MSD − 3.72 Ht + 152.62 | 0.087 | 25.49 | 0.012 |
| Leaves | Y = −0.02 MSD − 3.91 Ht + 140.89 | 0.127 | 5.033 | 0.001 | |
| Branches | Y = −0.68 DBH + 17.644 | 0.160 | 6.625 | 0.000 | |
| Others | Y = 0.69 DBH − 5.92 | 0.140 | 5.648 | 0.000 | |
| DBF | Total | Y = 38.83 CD − 20.10 DBH + 217.77 | 0.155 | 3.065 | 0.022 |
| Leaves | Y = 29.94 CD − 16.86 DBH + 165.46 | 0.140 | 2.716 | 0.037 | |
| Branches | Y = −5.77 DBH − 2.17 Ht + 85.24 | 0.281 | 6.537 | 0.000 | |
| Others | Y = 1.56 Ht − 32.93 | 0.262 | 5.962 | 0.000 | |
| ACF | Total | Y = −26.78 CD − 0.01 MSD + 79.60 | 0.232 | 10.507 | 0.000 |
| Leaves | Y = −22.19 CD − 0.01 MSD − 1.55 Ht + 75.87 | 0.288 | 14.024 | 0.000 | |
| Branches | Y = −6.31 CD − 0.01 MSD + 0.45 Ht − 3.44 | 0.138 | 5.554 | 0.000 | |
| RYF | Total | Y = −64.95 CD + 0.01 MSD − 1.71 DBH + 38.69 | 0.157 | 4.244 | 0.003 |
| Leaves | Y = −51.53 CD + 0.01 MSD − 1.70 DBH + 38.69 | 0.220 | 6.414 | 0.000 | |
| Branches | Y = −9.80 CD + 3.69 | 0.120 | 2.153 | 0.050 |
| Component (y) | Regression Equation | R2 | F | p | |
|---|---|---|---|---|---|
| Low altitude | Total | Y = −15.29 MMT + 17.27 MMiT + 107.43 V − 46.74 Vmax + 5.61 RH − 479.06 | 0.643 | 33.25 | 0.000 *** |
| Leaves | Y = −13.846 MMT + 14.74 MMiT − 0.10 P + 0.49 Pmax + 89.21 V −29.29 Vmax + 5.13 RH − 509.17 | 0.678 | 38.57 | 0.000 *** | |
| Branches | Y = −1.20 MMaT + 0.88 MMiT + 0.05 P − 0.29 Pmax + 10 V − 11.17 Vmax + 62.19 | 0.510 | 19.61 | 0.000 *** | |
| Others | Y = −1.70 MMT + 1.70 MMiT + 8.44 V − 6.33 Vmax + 0.57 RH − 33.48 | 0.229 | 6.31 | 0.000 *** | |
| High altitude | Total | Y = 1.6 MMaT − 1.62 MMiT − 0.02 P − 10.47 V + 3.53 Vmax + 1.34 RH − 117.47 | 0.386 | 25.49 | 0.000 *** |
| Leaves | Y = 2.34 MMaT − 11.75 V + 3.04 Vmax + 0.80 RH − 67.28 | 0.383 | 25.11 | 0.000 *** | |
| Branches | Y = 1.28 MMT − 0.78 MMaT − 0.76 MMiT + 3.44 V − 0.54 Vmax + 0.34 RH − 30.91 | 0.311 | 18.56 | 0.000 *** | |
| Others | Y = 0.12 Pmax + 2.16 V − 1.03 Vmax + 0.21 RH − 19.34 | 0.323 | 19.54 | 0.000 *** |
| Forest Types | Component | |||
|---|---|---|---|---|
| Leaves /g·kg−1 | Branches /g·kg−1 | Others /g·kg−1 | Total /g·kg−1 | |
| EBF | 448.18 ± 55.51 c (33.55%) | 436.53 ± 47.93 c (32.68%) | 451.11 ± 66.34 b (33.77 %) | 445.36 ± 44.67 c (100.00%) |
| DBF | 469.72 ± 61.44 b (33.94%) | 475.61 ± 68.70 ab (34.37%) | 438.56 ± 62.03 b (31.69%) | 461.87 ± 49.65 b (100.00%) |
| ACF | 479.54 ± 64.16 ab (33.26%) | 483.43 ± 62.25 a (33.53%) | 478.99 ± 48.32 a (33.22%) | 480.71 ± 42.00 a (100.00%) |
| RYF | 492.26 ± 39.39 a (34.49%) | 459.05 ± 47.36 b (32.16%) | 476.14 ± 48.70 a (33.36%) | 476.25 ± 28.98 ab (100.00%) |
| Season | Component | |||
|---|---|---|---|---|
| Leaves /g·kg−1 | Branches /g·kg−1 | Others /g·kg−1 | Total /g·kg−1 | |
| Spring | 479.85 ± 61.02 ab (33.55%) | 454.74 ± 48.66 a (32.68%) | 459.11 ± 55.68 a (33.77%) | 464.64 ± 44.93 ab (100.00%) |
| Summer | 450.75 ± 66.89 c (33.94%) | 469.700 ± 74.74 a (34.37%) | 471.54 ± 52.17 a (31.69%) | 463.85 ± 35.90 ab (100.00%) |
| Autumn | 464.04 ± 38.82 cb (33.26%) | 453.17 ± 47.23 a (33.53%) | 451.48 ± 40.73 a (33.22%) | 456.26 ± 29.64 b (100.00%) |
| Winter | 488.23 ± 58.72 a (34.49%) | 474.06 ± 62.92 a (32.16%) | 465.75 ± 80.35 a (33.36%) | 476.22 ± 60.54 a (100.00%) |
| Forest Types | Component | |||
|---|---|---|---|---|
| Leaves /kg·ha−1 | Branches /kg·ha−1 | Others /kg·ha−1 | Total /kg·ha−1 | |
| EBF | 18.89 ± 14.62 a (64.00%) | 5.66 ± 2.87 a (19.00%) | 5.19 ± 3.77 a (17.00%) | 29.74 ± 21.26 a (100.00%) |
| DBF | 7.46 ± 3.48 c (65.00%) | 1.95 ± 1.35 c (17.00%) | 1.99 ± 1.01 c (18.00%) | 11.40 ± 5.84 c (100.00%) |
| ACF | 16.33 ± 9.34 ab (68.00%) | 4.14 ± 2.16 b (17.00%) | 3.46 ± 1.63 b (15.00%) | 23.93 ± 13.13 b (100.00%) |
| RYF | 14.33 ± 9.24 b (69.00%) | 3.49 ± 1.76 b (17.00%) | 2.95 ± 1.85 b (14.00%) | 20.77 ± 12.85 b (100.00%) |
| Season | Component | |||
|---|---|---|---|---|
| Leaves /kg·ha−1 | Branches /kg·ha−1 | Others /kg·ha−1 | Total /kg·ha−1 | |
| Spring | 21.56 ± 10.73 a (73.00%) | 3.45 ± 1.86 c (12.00%) | 4.59 ± 3.43 a (15.00%) | 29.60 ± 16.02 a (100.00%) |
| Summer | 7.21 ± 3.80 c (55.00%) | 2.40 ± 1.06 d (18.00%) | 3.51 ± 1.12 b (27.00%) | 13.12 ± 5.98 c (100.00%) |
| Autumn | 18.74 ± 7.69 a (66.00%) | 5.57 ± 2.71 a (20.00%) | 3.85 ± 1.50 ab (14.00%) | 28.16 ± 11.90 a (100.00%) |
| Winter | 12.19 ± 5.06 b (63.00%) | 4.69 ± 1.71 b (25.00%) | 2.37 ± 1.03 c (12.00%) | 19.25 ± 7.80 b (100.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, L.; Xiao, T.; Bai, T.; Mo, X.; Huang, J.; Deng, W.; Liu, Y. Seasonal Dynamics and Influencing Factors of Litterfall Production and Carbon Input in Typical Forest Community Types in Lushan Mountain, China. Forests 2023, 14, 341. https://doi.org/10.3390/f14020341
Qiu L, Xiao T, Bai T, Mo X, Huang J, Deng W, Liu Y. Seasonal Dynamics and Influencing Factors of Litterfall Production and Carbon Input in Typical Forest Community Types in Lushan Mountain, China. Forests. 2023; 14(2):341. https://doi.org/10.3390/f14020341
Chicago/Turabian StyleQiu, Lingbo, Tingqi Xiao, Tianjun Bai, Xingyue Mo, Jiahui Huang, Wenping Deng, and Yuanqiu Liu. 2023. "Seasonal Dynamics and Influencing Factors of Litterfall Production and Carbon Input in Typical Forest Community Types in Lushan Mountain, China" Forests 14, no. 2: 341. https://doi.org/10.3390/f14020341




