Abstract
A better understanding of responses to water limitation in woody species can help us to cope with the consequences of the progressing climate change. We focused on the putative transgenerational effects of water limitation in the maternal environment during reproduction. Water was restricted for cuttings of Frangula alnus Mill. in a common garden setting, with a Belgian (local), Italian and Swedish provenance, during the growing season of 2020 and mature berries were collected during the whole reproductive phase. Stones that were extracted from the berries were given a cold stratification. In the next spring, the germination percentage of the stones from the water restricted maternal environment was significantly higher than that of the stones from the normal maternal environment, for the three provenances, notwithstanding the fact that stone weight was not different. The timing of seedling emergence was advanced for the water-limited maternal environment, but only for the stones harvested when mature berry production was the highest (9th and 16th of July 2020). Population differentiation was observed for the timing of seedling emergence, which reflected population differentiation for bud burst of the mother plants in the common garden, including a counter-gradient effect for the Swedish provenance, and corroborating the suggestion that the timing of seedling emergence and leaf phenology may have a common genetic basis. In addition, the Swedish provenance displayed a somewhat more stable germination percentage over the whole berry collection period when the stones were harvested. A partitioning of variance analysis suggested that germination percentage is more genetically determined than timing of seedling emergence, probably reflecting the more important need to sense the environment for an adequate timing of emergence.
1. Introduction
Drought is one of the more severe natural disturbances [1]. As climate change progresses, drought will further impede the survival and fitness of plant populations worldwide, and thus affect primary production and biodiversity [2,3,4]. Drought stress creates an adverse effect on plant growth and development [1] and is responsible for an increasing mortality in forests [5,6]. A reduced plant fitness also increases the susceptibility to other (a)biotic stressors [7,8]. Concerns on the future provisioning of forest ecosystem services are being raised [9,10]. Studying plant responses to drought stress may help assessing the putative shifts and changes that climate change may provoke in natural ecosystems. As the velocity of the current climate change is unprecedented [11], a better understanding of the adaptive capacity of tree species to drought events can prove very valuable [12].
Germination, which is the transition from a seed to a seedling, occurs for each plant species at an appropriate time and at an appropriate place, ensuring the best chances for success [13,14]. The timing of germination determines the success rate of establishment of a plant, which is specifically true for areas that are characterized by a strong seasonal variability [15]. When germinating too early, seedlings may be damaged by frost, while germinating too late, seedlings may experience a competitive disadvantage [13]. A combination of environmental cues triggers seed germination, which is called the seed germination niche and which includes the specific conditions for breaking dormancy and the requirements for germination [13,14]. As plant populations adapted to their growth environment and optimized their timing of germination, environmental gradients within the natural distribution range of the plant species lead to population differentiation for this trait [13,14]. In addition, seed germination is not only responsive to the environmental cues experienced by the seed itself but also by the mother plant during seed maturation [13,16].
Studying the way drought stress during reproduction of parental trees affects seed germination traits can augment our knowledge on how climate change will impact forest dynamics. Tree species are characterized by large natural ranges. With the help of natural selection, local populations of tree species adapt to the growing conditions at their home-sites [17]. Common gardens as experimental set-up specifically suit the study of adaptive genetic differentiation between populations originating from variable home-sites within the natural range of the species, as the populations are brought together and therefore share the same environment [18,19]. Population differentiation in a trait as measured or observed in a common garden therefore has a genetic basis. As trees only start sexual reproduction at maturity, studies in common gardens with juvenile plants focus on vegetative traits such as survival, growth and leaf phenology. However, recruitment in forest ecosystems is a prerequisite for the subsequent generation of trees. It controls the structure and the composition of the future forest [20].
Plants, as sessile organisms, have evolved plasticity, which helps them adapting to a changing environment [21]. The environment of a reproductive maternal plant can influence the performance of its offspring [16,22,23,24]. These maternal effects, which can be categorized as transgenerational plasticity, are independent of changes in the DNA code [22,23]. Variable maternal environments may induce epigenetic changes including DNA methylation, histone modification and changes in small non-coding RNAs, that are transferred to the seeds and that can alter gene activity in the progeny [22,23]. For a long time, there was a significant body of evidence that transgenerational plasticity acts in herbaceous plants [25]. For instance, Arabidopsis thaliana plants that were exposed to different types of stresses including salt, cold, heat and flood, resulted in increased methylation of the global genome of the untreated progeny and also in a higher tolerance to the specific stress [26]. The information on trees, especially broadleaves, is comparably scarce. Maternal temperature has been shown to influence leaf phenology in the progeny in Populus nigra [27] and Prunus padus [28]. Transgenerational plasticity in woody species may play an important role in rapid adaptation of forest ecosystems to the predicted climate change [29], as transgenerational plasticity acts in a relatively short timescales [30].
The main objective of this study was to quantify the putative effects that water limitation on reproductive mother plants during the growing season have on the germination traits of the derived seed in the shrub Frangula alnus Mill. (glossy buckthorn). F. alnus is an insect-pollinated and bird-dispersed shrub with a large natural range in Europe [31]. The main advantages of reproductive studies in this species are that the shrub starts flowering and producing berries from an early age onward and that flowering and fruiting occurs on the year’s shoot, so that mature berries are produced during an extended period in the summer and early autumn. The species represents no economic value, but has an important ecological significance in its native range. It often grows in woodlands, hedgerows and along the edges of streams and rivers, and it is an important source of food and cover for wildlife, with its leaves and twigs providing nesting material for birds [31,32]. It can grow in a wide range of soils and light conditions [31,33]. As drought stress may influence fecundity of different woody species in a variable way, it may affect recruitment in forest ecosystems and thus control the structure and composition of future forests. In addition, different provenances within the natural range of a woody species may also respond in a variable way to water limitation. Therefore, our experimental set-up consisted of a common garden including three provenances of different origins. We looked at the germination percentage and at the timing of seedling emergence, which are two important life history traits, representing the transition from the parental plant to the offspring. We hypothesized that water limitation in the maternal environment affects both germination traits and that the responses are provenance-dependent.
2. Materials and Methods
2.1. Plant Material
An outdoor common garden experiment on a container field using potted plants was established with three provenances of F. alnus, as described before [34]. In summary, berries were collected in three natural populations growing in Italy (lat. 43.12181, lon. 11.17654), Belgium (lat. 51.08424, lon. 4.793124) and Sweden (lat. 62.44210, lon. 17.23451), in 2011. These original growth sites are characterized by an annual mean temperature of 13.7 °C, 10.1 °C and 2.9 °C and an annual precipitation of 706 mm, 785 mm and 682 mm, for the Italian, Belgian and Swedish populations, respectively (data downloaded from WorldClim [35]). The seeds were germinated and the seedlings (all representing different genotypes) were raised in pots under shared conditions in the nursery of the Research Institute of Nature and Forest (Geraardsbergen, Belgium), following standard nursery techniques and using standard potting soil with organic matter 20%, pH 5.0–6.5, electrical conductivity 450 µS/cm, dry matter 25% and 1.5 kg/m³ powdered compound fertilizer NPK 12 + 14 + 24. No additional fertilizer was added. The seedlings were grown in an open greenhouse (a greenhouse without walls) in which an automatic grey shade net operated to protect the young plants from heavy insolation. The plants were watered manually on a regular basis by experienced greenhouse workers. In 2016, cuttings were taken from the raised seedlings (representing 29 different genotypes), using standard nursery techniques (8 from the Italian, 9 from the Belgium and 12 from the Swedish population). For each raised seedling (which means for each genotype), four clones (ramets) were further raised under shared conditions as potted plants, resulting in 116 outdoor potted plants (32 from Italy, 36 from Belgium and 48 from Sweden) on a container field. In the spring of 2020, the four ramets of each genotype were appointed to two groups. The two groups of potted plants (4 L pots) were separated on the container field. Within each group, the plants were randomly individually mingled. Water limitation was applied to one of the two groups as described before [34]. In short, from the 14 May 2020 onwards, the treated group of plants did not receive any additional water above the natural precipitation whereas the control group received additional watering on a regular basis (twice to trice a week) according to the visual needs as judged by experienced workers. There was an exception for the water limitation in the treated group of plants, with one manual watering on the 3rd of June to avoid plant losses. As the weeks before natural precipitation had been absent, several plants of the water-limited group of plants showed wilting symptoms, indicating that drought stress had been imposed.
F. alnus is known to produce berries from an early age onwards, which ripen continuously during the growing season as plants flower on the current year’s growth. Mature berries of F. alnus are characterized by a purple-black color. All mature berries were picked from each plant separately in both the control group and the water-limited group throughout the growing season (collection dates were 26th and 29th of June, 3rd, 9th, 16th and 23rd of July and 6th, 21st and 27th of August 2020) [34]. The stones within the berries were counted.
2.2. Germination of the Stones
Two weeks after the stones were extracted from the picked berries, from each plant and on each collection day, they were stratified according to standard nursery techniques by layering them in trays using a mixture of sand and nursery potting soil in equal volumes (50/50). For this, the trays were first filled with the sand–soil mixture up to one cm from the top of each cell. Then, the batches of stones were placed on the sand–soil mixture and were covered with the mixture up to the top of each cell.
Once filled, the trays were placed in a refrigerator at 4 °C until the end of January 2021 (cold stratification). The soil was kept moist by regularly adding small amounts of water, as judged by experienced greenhouse workers. In total, 404 batches of stones (total amount of stones = 4502) were stratified in this way (Table 1).

Table 1.
Number of stratified batches of stones (nb°) and sum of stones in the batches (ns°), according to the provenance of the mother plant, the treatment of the mother plant in 2020 (control and water-limited) and the berry collection day in 2020.
At the beginning of February 2021, the trays with the stratified stones were placed in a non-heated but frost-free greenhouse. At the beginning of March, the stones started germinating. On a regular basis, all visually germinated stones that emerged above the soil were counted until no more new seedlings appeared. The counting was performed on the 8th, 12th, 15th, 19th, 22nd and 26th of March and on the 2nd, 6th and 8th of April 2021. Two variables were calculated from the data: germination percentage, as an estimate of the viability of the different batches of stones and the timing of the emergence of the seedlings above the soil. Germination percentage was calculated for every stone batch (for every berry collection day in 2020 and for every mother plant separately) as the count of seedlings on the last observation day (8 April 2021) divided by the count of stones in the respective batch.
2.3. Statistical Analysis
Data analysis was carried out in R, a free software environment for statistical computing (R Version 4.2.1, Vienna, Austria [36]).
Linear mixed models and generalized linear mixed models were fitted to the data with the package nlme [37]. In the fixed part of the models, the variable P represents the provenance of the mother shrubs (categorical variable with “Be” for Belgian, “It” for Italian and “Sw” for Swedish), the variable T represents the water limitation treatment of the mother plants in the growing season of 2020 (categorical variable with “control” and “drought”) and the covariate C represents the berry collection day in 2020.
The response variable germination percentage (Gp) was modeled as follows:
Gp = αGp + βPGpP + βTGpT + βCGpC + βC2GpC2 + βPCGpPC + βPC2GpPC2 + βTCGpTC + βTC2GpTC2
As the relationship between the response variable germination percentage and the covariate day of berry collection (C) was not linear but curved, this curvature was represented in the model by means of a quadratic polynomial of this covariate. An interaction term in the model between provenance and day of berry collection allowed the evolution of the germination percentage over time to vary between the provenances. Analogously, an interaction term in the model between treatment of the mother plants in 2020 and day of berry collection in 2020 allowed the evolution of the germination percentage to vary between the treated and the control group of mother plants over time.
The random part of the model contained a unique identifier for every genotype of the mother plants, and a unique identifier for every ramet within each genotype.
The response variable timing of seedling emergence was comprised of counts of emerged seedlings in each batch for every observation day in the spring of 2021 (with a batch for each reproducing mother plant in the treated and in the control group, for each berry collection day in 2020). A binomial generalized linear mixed model was fit to the data, with the total count of germinated seedlings in each batch on the last observation day as the “weight” argument in the model. In this way, the chance (p) was modeled for a seedling to have already emerged on a given day. The model formula:
log(p/(1−p)) = αp + βPpP + βTpT + βDpD
In this formula, the covariate day (D) indicated the day when seedlings were counted in the spring of 2021. The model was run for the stone batches of every berry collection day in 2020 separately, to reduce the complexity and thus to improve the understanding of the models. As none of the stones collected on the last day of berry collection (27 August 2020) germinated (Figure S1), this berry collection day was skipped. In addition, the data of the first two (26 and 29 June 2020) and the second and third last berry collection days (6 and 21 August 2020) were joined, as for these days, germination remained absent in several batches (Figure S1). In this way, six models were run.
The random part of the models contained a unique identifier for every genotype of the mother plants, and a unique identifier for every ramet within each genotype.
To allow an analysis of variance partitioning among the variables in the random part of the mixed models for the timing of seedling emergence, the data were transformed so that a linear model could be fit to the data (a variance partitioning analysis is not possible for generalized linear mixed models). The counts of the emerging seedlings in each batch on every observation day in the spring of 2021 were transformed to percentages by dividing each count by the maximum count for the respective batch on the last observation day, and multiplying it by 100. These percentages were used to fit a linear model for the timing of seedling emergence (E) for every adjusted berry collection day as described above (6 models):
E = αE + βPEP + βTET + βDED
The random part of the models contained a unique identifier for every genotype of the mother plants, and a unique identifier for every ramet within each genotype.
3. Results
3.1. Germination Percentage
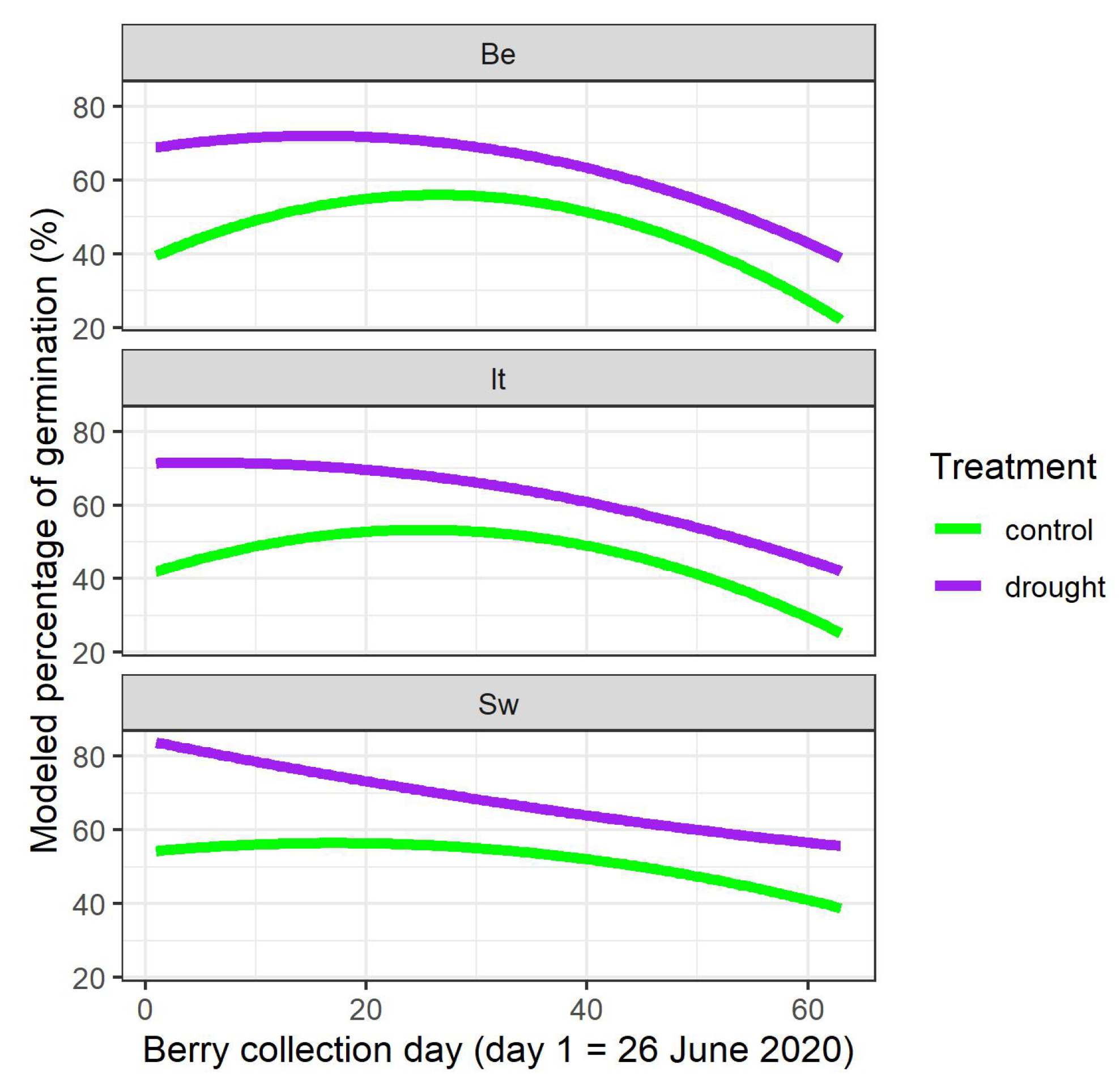
The raw data indicated that the relationship between the germination percentages of the stone batches and the different berry collection days in 2020 when the batches were collected, was not linear (Figure S2). Therefore, the covariate day of berry collection was added in the model as a polynomial of the second degree. The modeling analysis showed a main finding, namely that the water limitation of the mother plants significantly raised the germination percentage of the derived stones (Figure 1, treatment T with p-value < 0.001 and interaction terms between treatment T and day of berry collection C and C2 with p-values = 0.010 and = 0.031, respectively, in Table 2). When looking in detail to the different provenances, the modelling showed that the germination percentage of the Italian provenance did not differ significantly from the Belgian provenance (no significant p-values for It, It:C and It:C2 in Table 2), whereas the Swedish provenance differed significantly from the Belgian provenance for the interaction term Sw:C2 (p-value = 0.005 in Table 2). As visualized in Figure 1, the curvature of the modeled germination percentage of the stones coming from the Swedish mother plants was less concave in comparison to the other provenances.
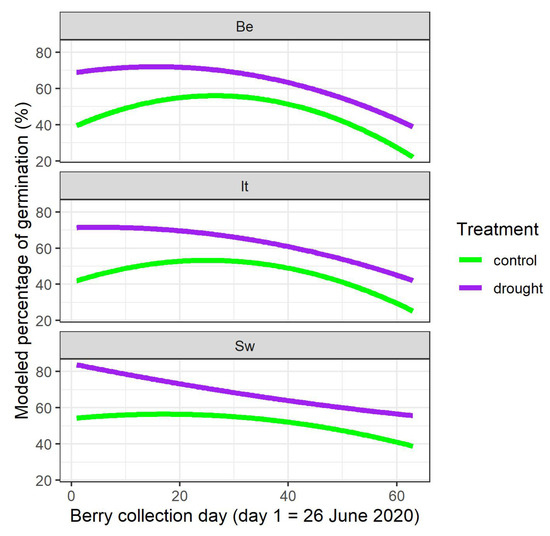
Figure 1.
Modeled germination percentages depending on the berry collection day in 2020, on the provenance and on the treatment of the mother plants in 2020. Be: Belgian; It: Italian; Sw: Swedish.

Table 2.
Test statistics for the modeled germination percentage. C is the day of berry collection in 2020, T is the treatment of the mother plants in 2020 with the water-limited condition being the standard to which the control is compared. The Belgian provenance is the standard to which the Italian (It) and Swedish (Sw) provenances were compared.
3.2. Timing of Seedling Emergence
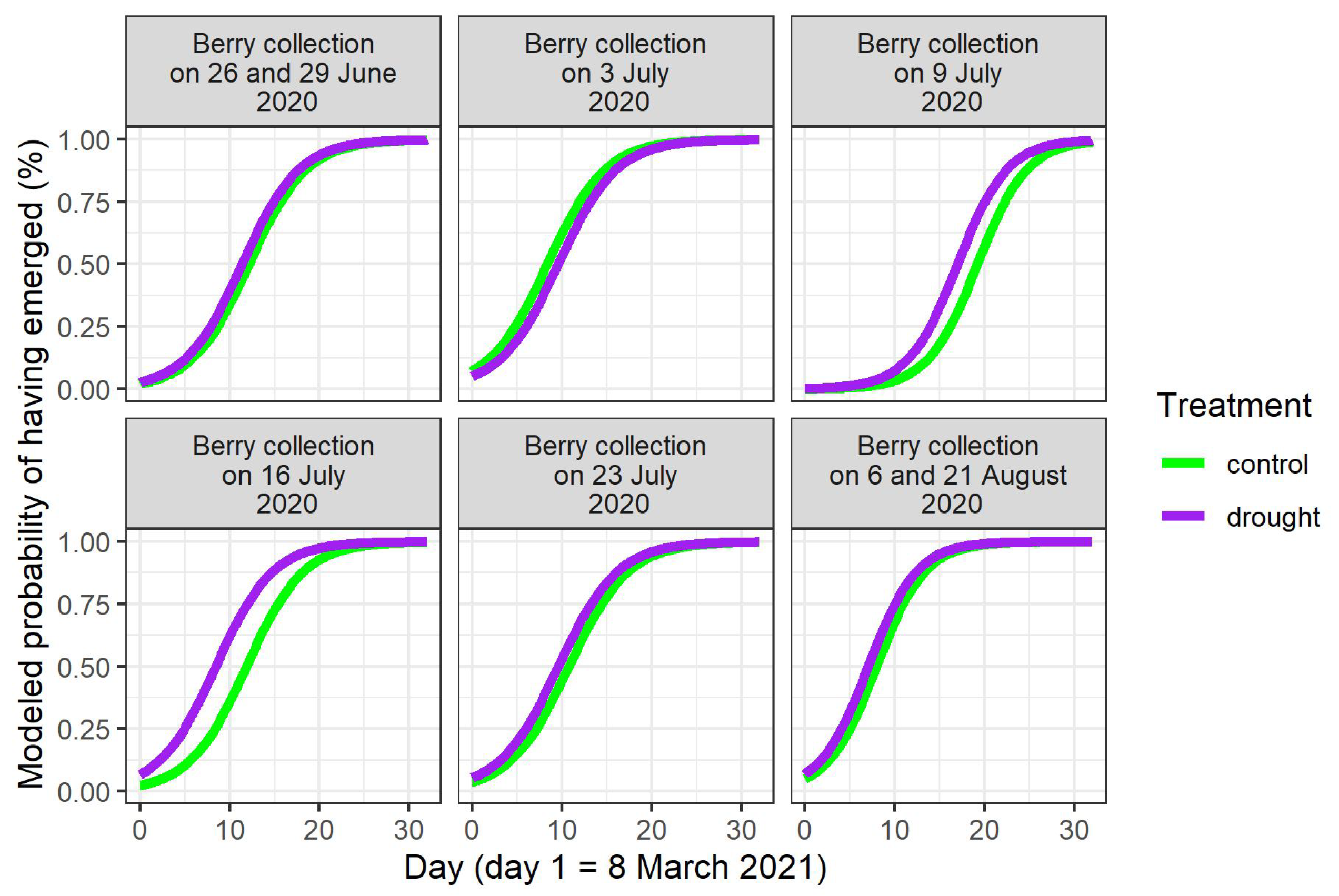
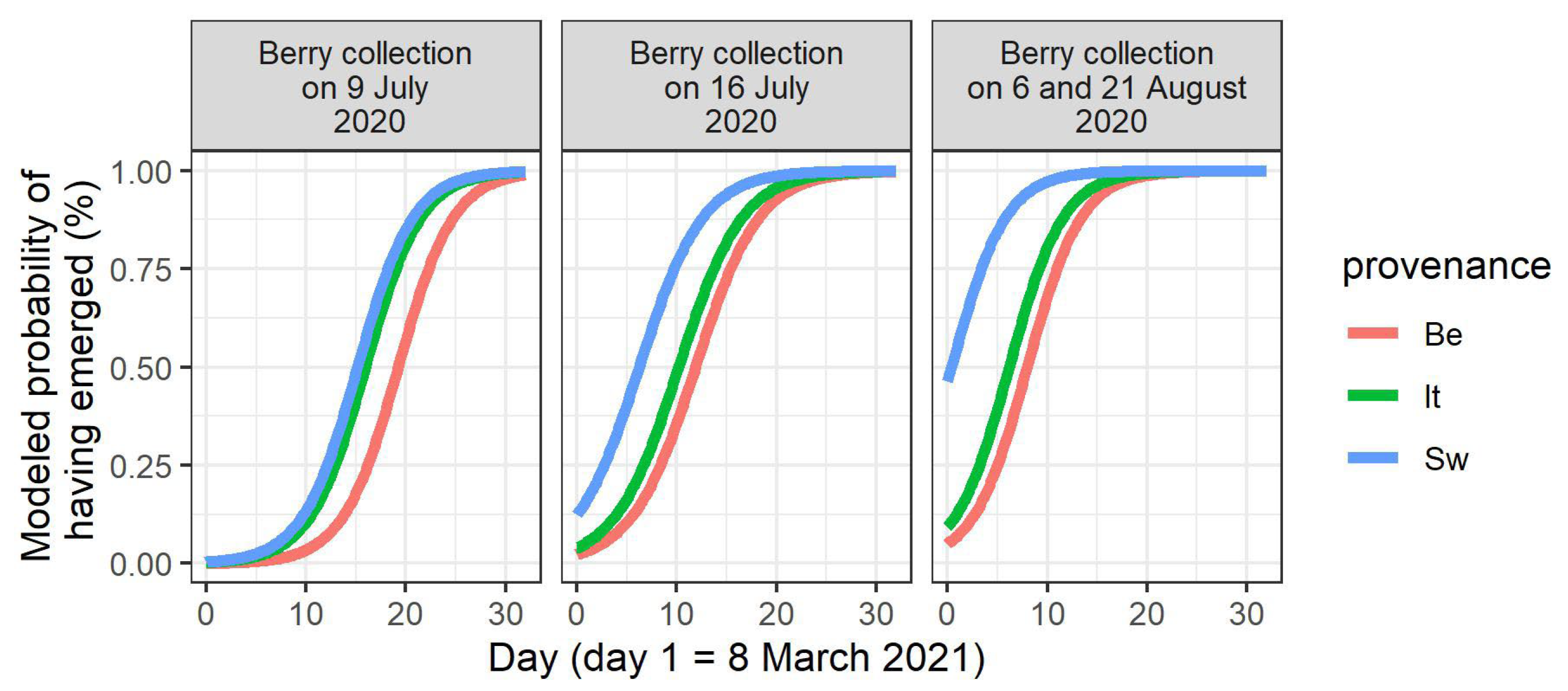
In the spring of 2021, the seedlings did not all emerge at once (Figure S3). The timing of seedling emergence was modeled (Table 3). A main finding was that the water limitation of the mother plants in 2020 significantly advanced the timing of seedling emergence for the stones derived from the two central berry collection days in 2020 (9th and 16th of June; p-value for treatment = 0.003 and < 0.001, respectively, Table 3, Figure 2). On these two berry collection days, the largest numbers of stones were acquired (Table 1). When considering the different provenances in the common garden, a significant provenance effect was displayed for these two central berry collection days, together with the joined collection days of 6 and 21August 2020 (p-value for the Italian provenance = 0.004 for the 9th of July, and p-values for the Swedish provenance < 0.001 for the 9th and the 16th of July, and = 0.005 for the joined 6th and 21st of August in Table 3, Figure 3). In the cases of significant differences, the seedlings of the Italian and Swedish provenances emerged earlier than the Belgian seedlings (Figure 3).

Table 3.
Test statistics for the modeled timing of seedling emergence. The Belgian provenance is the standard to which the Italian (It) and Swedish (Sw) provenances were compared. T is the treatment of the mother plants in 2020 with the water-limited condition being the standard to which the control is compared. D is the day of seedling emergence in the spring of 2021.
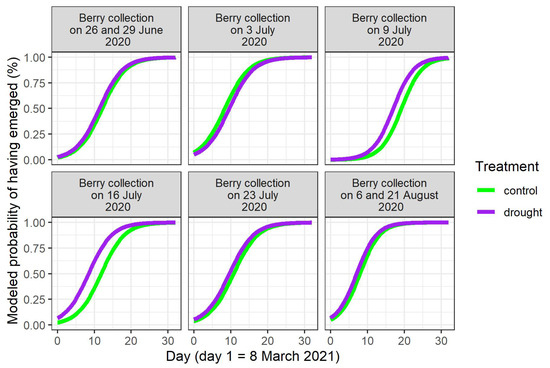
Figure 2.
Modeled timing of seedling emergence in the spring of 2021, according to the day of berry collection in 2020 and to the treatment of the mother plants in 2020.
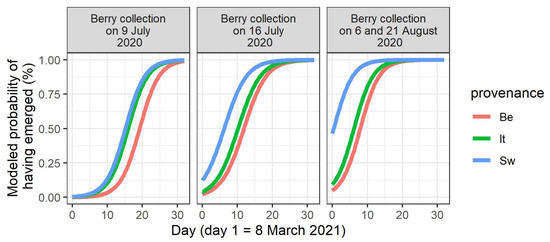
Figure 3.
Modeled timing of seedling emergence in the spring of 2021, according to the day of berry collection in 2020 and to the provenance. Only the berry collection days for which a significant provenance effect is present are shown. Be: Belgian, It: Italian, Sw: Swedish.
3.3. Analysis of the Variance Components
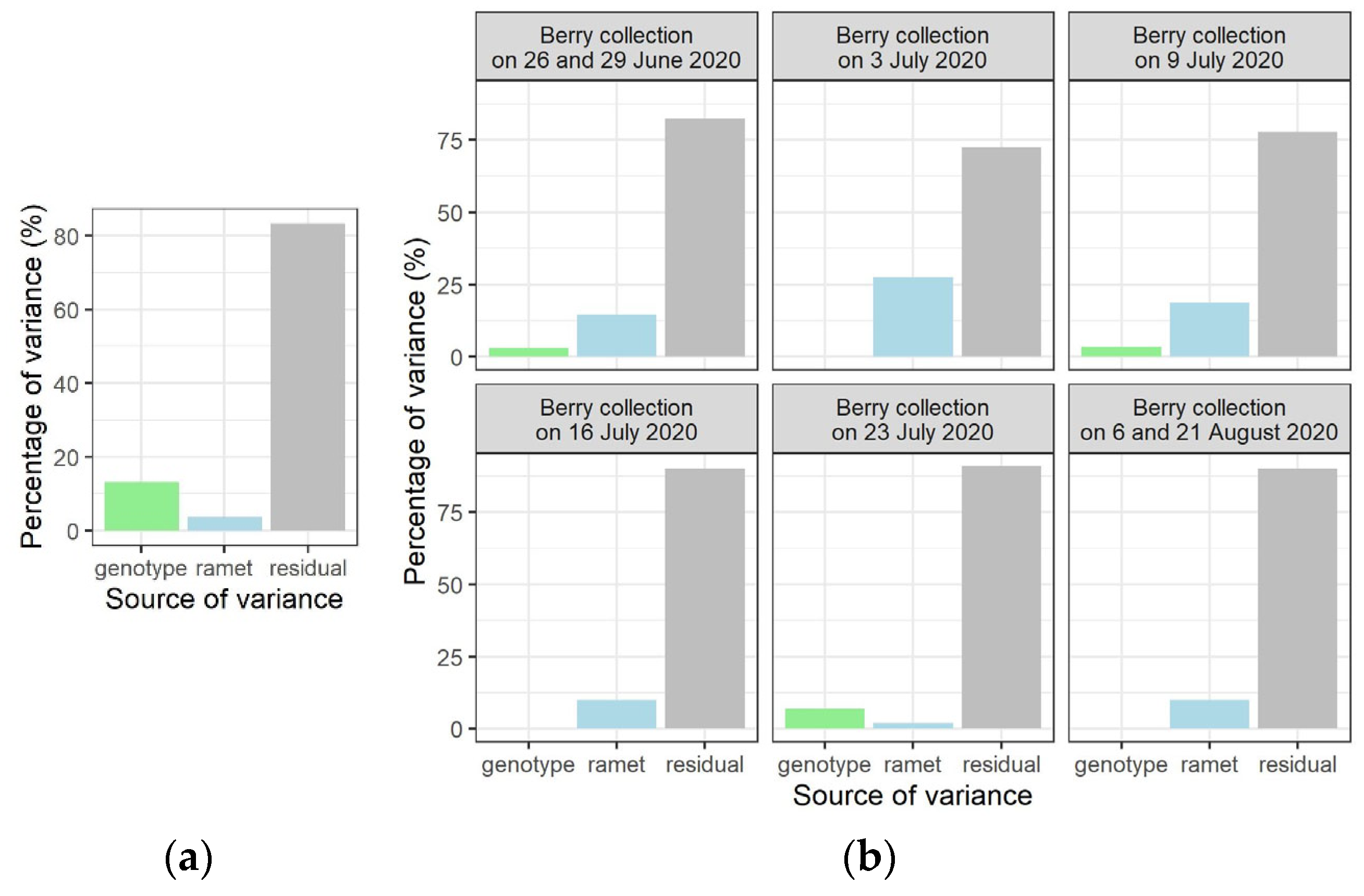
We studied the partitioning of the variance between the two hierarchical levels in the origin of the mother plants (genotype and ramet) which were in the random part of the models for both variables of germination percentage and timing of seedling emergence. For the response of variable germination percentage, the relative contribution to the total variance of the variance component with a genetic basis (variance between genotypes) was larger than the relative contribution of the variance that was attributed to the ramets (Figure 4a). For the variance analysis of the timing of seedling emergence, linear mixed models were run with percentages of seedling emergence on the different observation days as response variable (Table S1). The variance partitioning between genotype and ramet showed that, in most cases, the relative variance that was attributed to the ramet was larger than for genotype (Figure 4b). These results indicate that the germination percentage is relatively more genetically controlled than the timing of seedling emergence.
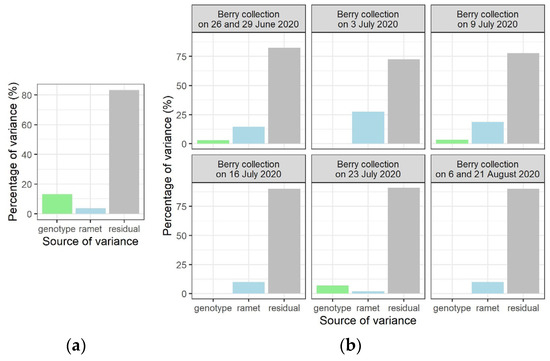
Figure 4.
Partitioning of relative variance for the response variables germination percentage (a) and timing of seedling emergence (b).
4. Discussion
Fine tuning of the germination timing, together with a reasonably high germination percentage, are crucial aspects of establishment success for new generations of trees. Here, we found that drought stress experienced by mother shrubs of F. alnus increased the germination percentage of the non-treated progeny and also advanced seedling emergence for the stones collected during the peak berry production.
4.1. Germination Traits Affected by the Maternal Environment
We observed a significantly higher germination percentage for the stones that were derived from mother plants that experienced water limitation during the growing season of 2020. This transgenerational plasticity was present for all the stone batches obtained from the different berry collection days in 2020, ranging from the end of June to August. As already shown, the water-limited mother plants produced significantly less mature berries in comparison to the control mother plants [34]. Although seed weight is one of the factors that can influence germination traits [38], it did not contribute to the differences in germination percentage we observed between the water-limited and control maternal environments as the weight of the stones that were obtained from treated and non-treated mother plants did not differ [34]. Although the environmental restriction experienced by the water-limited mother plants hampered resource allocation to reproduction in terms of quantities (less berries), it did at the same time raise the germination percentage. As drought stress has a severe economic impact on crop production worldwide, many studies focus on the influence of drought during seed maturation on the germination traits of the derived seed. For several well-known staple food crops, a water-limited maternal environment had no effect on the germination percentage or reduced it [39]. On the other hand, several studies indicate an opposite response with an increased germinability being associated with drought during seed development on the mother plant [25,40,41]. Depending on the strength of the drought stress, germination success may be increased (weak drought) or decreased (severe drought) in the same plant species, as was shown for Eremosparton songoricum (Litv.) Vass., a desert plant [42]. A viable explanation is the acting of an inter-generational memory that is generated by stress-induced adaptations in the epigenome of the seeds. As water limitation during seedling emergence hampers the germination [43], it can be hypothesized that in case of drought stress during seed maturation, which is not too severe, higher quality seeds, in terms of germination success, may be needed to achieve an adequate germination rate in water-limited germination conditions. The germination success dropped drastically for the stones harvested towards the end of the berry production season, both for the water-limited maternal environment and the control, leaving the intriguing question why plants invest resources in berry production with very limited to no germination capacity.
Apart from the higher germination percentage for the stones from the water-limited maternal environment, we also observed a small but significant advancement in the timing of emergence for these stones when collected during maximum berry production in the middle of the growing season of 2020. Early emergence can give a competitive advantage to the growing seedling as it can increase the amount of growth that can be achieved [44] and it can allow consumption of resources to the disadvantage of later emerging competitors [45]. It could be hypothesized that in woody species, a higher germination percentage and an earlier timing of emergence could be linked to a certain extent. Although the literature is scarce, in a study on Quercus ilex subsp. ballota (Desf.), mother trees producing earlier emerging acorns were characterized by a higher germination success in comparison to mother trees with later emerging seedlings [46]. In addition, in the conifer Pinus pinaster, a higher germination percentage co-occurred with an earlier timing of seedling emergence [47].
4.2. Population Differentiation in the Common Garden
Differentiation between the local Belgian provenance and the two non-local provenances in the common garden was detected for both studied germination traits. For germination percentage, the effect was relatively small and only the Swedish provenance differed from the local Belgian one. The modeled curvature over time linking germination percentage with the days of berry collection was less concave for the Swedish provenance, indicating that at the beginning of the berry production season in June, as well as at the end in August, the germination percentage of the derived stones was relatively higher than in the other two provenances. This small effect seems to suggest that germination percentage in the Swedish provenance was a bit more stable over the berry collection time, and slightly less sensitive to less favorable conditions for adequate stone maturation at the beginning and the end of the berry production period. As germination and leaf phenology may have a common genetic basis [48], this observation may be linked to findings of more northern provenances needing less chilling requirements and heat accumulation for bud burst in comparison to more southern provenances [49,50].
An earlier seedling emergence was observed for stones obtained from the mother shrubs originating from the more northernly located (Swedish) provenance at different berry collection dates. Similarly, an earlier seedling emergence was also observed for stones derived from the mother shrubs originating from the more southernly located (Italian) provenance on one of the berry collection days. This population differentiation is likely caused by local adaptation and can probably be linked to the earlier bud burst in the mother shrubs of these two non-local provenances in comparison to the local provenance in the common garden [34]. It should be noted that the Swedish provenance displays a counter-gradient variation for bud burst (a more northerly located provenance flushing earlier than a more southernly located provenance in a common garden setting, without an altitudinal difference at the sites of origin [51]), and that this is also true for seedling emergence timing. Seed and bud dormancy have already been suggested to involve similar processes [48], and evidence has been found for the coevolution of germination and postgermination traits in A. thaliana [13]. In a transcriptome analysis of buds that were bursting in Q. petraea, transcription factors were induced at the onset of the bud burst that are also known to be involved in the control of seed germination in A. thaliana, strongly suggesting that these two processes share a common basis [52].
Finally, the observation that provenance differentiation in germination timing is most evident for stones collected in the middle of the growing season, at the peak of berry production on the mother shrubs, may be related to the finding that under optimal growing conditions differences among provenances in common gardens are more pronounced, whereas under more adverse growing conditions, differences among phenotypes may be diminished [53].
4.3. Variance Components
In general, environmental and genetic effects both contribute to phenotypic variation within and among genetic entities. The experimental setup allowed an analysis of the variance components, as the origin of the mother plants reflected a hierarchical structure, with ramets nested into genotypes. For the germination percentage, a larger amount of relative variance had a genetic basis (relative variance between genotypes) than the amount of relative variance between individuals having the same genotype (ramets). For the timing of seedling emergence, mainly the opposite was true, showing that germination success is likely more genetically determined than the timing of seedling emergence, suggesting a stronger dependence on environmental conditions for the latter. As natural selection on germination is able to filter out many genotypes at an early stage of establishment, germination timing will profit from responding appropriately to environmental factors [54], and in the case of F. alnus, as our results suggest, the profit might be larger for timing of emergence than for percentage of germination.
5. Conclusions
We demonstrated that water limitation during seed maturation in F. alnus affects the germination of the derived seedlings. The mechanism behind this observation could be an epigenetic memory, as has been shown in other plant species. Further research is needed to prove the suggested epigenetic mechanism and to characterize in more detail how these transgenerational effects may be helpful in adapting to the climate warming.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/f14020348/s1, Figure S1: Count of mother plants that produced mature berries in 2020, according to the day of berry collection in 2020, and count of mother plants that produced mature berries from which none of the stones germinated in the next spring of 2021. Counts are split according to the different provenances and to the treatment that the mother plants were exposed to in 2020. Be: Belgian, It: Italian, Sw: Swedish. Figure S2: Boxplot of the germination percentages of the stone batches (for every mother plant and for every collection day in 2020) depending on the berry collection day in 2020, on the provenance and on the treatment of the mother plants in 2020. Figure S3: Boxplots of the percentage of seedlings that already emerged on the observation days in the spring of 2021, according to the berry collection day in 2020 and to the treatment of the mother plants in 2020. Table S1: Test statistics for the modeled timing of seedling emergence using as response variable percentages of emerged seedlings on the different observation days and applying a linear mixed model to fit the data. The Belgian provenance is the standard to which the Italian (It) and Swedish (Sw) provenances were compared. T is the treatment of the mother plants in 2020 with the water-limited condition being the standard to which the control was compared. D is the day of seedling emergence in the spring of 2021.
Author Contributions
The experiment was conceptualized by K.V.M. and S.M. In addition, the methodology was defined by K.V.M. and S.M. Plants for the experiment were grown by M.S. and S.M. Data collection and formal analysis were performed by M.S., S.M., S.D.L. and K.V.M. Data processing was performed by S.D.L. and K.V.M. The manuscript was prepared by K.V.M. Review and editing of the manuscript was performed by S.D.L. and K.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are available at https://doi.org/10.5281/zenodo.7419301.
Acknowledgments
We are most grateful to Hanne De Kort for the initial seed collection. We also thank Denis Cattoir for contributing to the data acquisition and Nico De Regge for help in taking care of the plants.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.S.; Running, S.W. Drought-Induced Reduction in Global Terrestrial Net Primary Production from 2000 Through 2009. Science 2010, 329, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The impacts of increasing drought on forest dynamics, structure, and biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef] [PubMed]
- Hampe, A.; Petit, R.J. Conserving biodiversity under climate change: The rear edge matters. Ecol. Lett. 2005, 8, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Choat, B.; Jansen, S.; Brodribb, T.J.; Cochard, H.; Delzon, S.; Bhaskar, R.; Bucci, S.J.; Feild, T.S.; Gleason, S.M.; Hacke, U.G.; et al. Global convergence in the vulnerability of forests to drought. Nature 2012, 491, 752–755. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolstrom, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- McDowell, N.G.; Allen, C.D.; Anderson-Teixeira, K.; Aukema, B.H.; Bond-Lamberty, B.; Chini, L.; Clark, J.S.; Dietze, M.; Grossiord, C.; Hanbury-Brown, A. Pervasive shifts in forest dynamics in a changing world. Science 2020, 368, eaaz9463. [Google Scholar] [CrossRef]
- Seidl, R.; Honkaniemi, J.; Aakala, T.; Aleinikov, A.; Angelstam, P.; Bouchard, M.; Boulanger, Y.; Burton, P.J.; De Grandpré, L.; Gauthier, S. Globally consistent climate sensitivity of natural disturbances across boreal and temperate forest ecosystems. Ecography 2020, 43, 967–978. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.L.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Carta, A.; Fernández-Pascual, E.; Gioria, M.; Müller, J.V.; Rivière, S.; Rosbakh, S.; Saatkamp, A.; Vandelook, F.; Mattana, E. Climate shapes the seed germination niche of temperate flowering plants: A meta-analysis of European seed conservation data. Ann. Bot. 2022, 129, 775–786. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination; Elsevier: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Phil. Trans. R. Soc. B 2009, 364, 1059–1074. [Google Scholar] [CrossRef]
- Sáenz-Romero, C.; Lamy, J.-B.; Ducousso, A.; Musch, B.; Ehrenmann, F.; Delzon, S.; Cavers, S.; Chałupka, W.; Dağdaş, S.; Hansen, J.K.; et al. Adaptive and plastic responses of Quercus petraea populations to climate across Europe. Glob. Change Biol. 2017, 23, 2831–2847. [Google Scholar] [CrossRef]
- Alberto, F.J.; Aitken, S.N.; Alia, R.; Gonzalez-Martinez, S.C.; Hanninen, H.; Kremer, A.; Lefevre, F.; Lenormand, T.; Yeaman, S.; Whetten, R.; et al. Potential for evolutionary responses to climate change—Evidence from tree populations. Glob. Chang. Biol. 2013, 19, 1645–1661. [Google Scholar] [CrossRef] [PubMed]
- Zas, R.; Sampedro, L.; Solla, A.; Vivas, M.; Lombardero, M.J.; Alía, R.; Rozas, V. Dendroecology in common gardens: Population differentiation and plasticity in resistance, recovery and resilience to extreme drought events in Pinus pinaster. Agric. For. Meteorol. 2020, 291, 108060. [Google Scholar] [CrossRef]
- Hacket-Pain, A.; Bogdziewicz, M. Climate change and plant reproduction: Trends and drivers of mast seeding change. Philos. Trans. R. Soc. B: Biol. Sci. 2021, 376, 20200379. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Day, T. Nongenetic Inheritance and Its Evolutionary Implications. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 103–125. [Google Scholar] [CrossRef]
- Herman, J.; Sultan, S. Adaptive Transgenerational Plasticity in Plants: Case Studies, Mechanisms, and Implications for Natural Populations. Front. Plant Sci. 2011, 2, 102. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, K.J.; vonHoldt, B.M.; Sork, V.L. Epigenetics in ecology and evolution: What we know and what we need to know. Mol Ecol 2016, 25, 1631–1638. [Google Scholar] [CrossRef] [PubMed]
- Ono, A.; Kinoshita, T. Epigenetics and plant reproduction: Multiple steps for responsibly handling succession. Curr. Opin. Plant Biol. 2021, 61, 102032. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Boyko, A.; Blevins, T.; Yao, Y.; Golubov, A.; Bilichak, A.; Ilnytskyy, Y.; Hollander, J.; Meins Jr, F.; Kovalchuk, I. Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS ONE 2010, 5, e9514. [Google Scholar] [CrossRef]
- Dewan, S.; Vander Mijnsbrugge, K.; De Frenne, P.; Steenackers, M.; Michiels, B.; Verheyen, K. Maternal temperature during seed maturation affects seed germination and timing of bud set in seedlings of European black poplar. For. Ecol. Manag. 2018, 410, 126–135. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Moreels, S.; Aguas Guerreiro, Y.; Beeckman, S. Temperature during Seed Maturation Influences Timing of Bud Burst in Seedlings and Saplings of Prunus padus. Forests 2022, 13, 1362. [Google Scholar] [CrossRef]
- Skrøppa, T.; Tollefsrud, M.M.; Sperisen, C.; Johnsen, Ø. Rapid change in adaptive performance from one generation to the next in Picea abies—Central European trees in a Nordic environment. Tree Genet. Genomes 2010, 6, 93–99. [Google Scholar] [CrossRef]
- Yakovlev, I.; Fossdal, C.G.; Skrøppa, T.; Olsen, J.E.; Jahren, A.H.; Johnsen, Ø. An adaptive epigenetic memory in conifers with important implications for seed production. Seed Sci. Res. 2012, 22, 63–76. [Google Scholar] [CrossRef]
- Zecchin, B.; Caudullo, G.; de Rigo, D. Frangula alnus in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar]
- Bairlein, F. Habitat Selection and Associations of Species in European Passerine Birds during Southward, Post-Breeding Migrations. Ornis Scand. (Scand. J. Ornithol.) 1983, 14, 239–245. [Google Scholar] [CrossRef]
- Uyttenbroeck, R.; De Vos, B.; Vander Mijnsbrugge, K. Verspreiding en Standplaats van Inheemse Bomen en Struiken in Vlaanderen; Research Institute for Nature and Forest: Brussels, Belgium, 2014; p. 375. [Google Scholar]
- Vander Mijnsbrugge, K.; Schouppe, M.; Moreels, S.; Aguas Guerreiro, Y.; Decorte, L.; Stessens, M. Influence of Water Limitation and Provenance on Reproductive Traits in a Common Garden of Frangula alnus Mill. Forests 2022, 13, 1744. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- R_Core_Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Goszka, A.R.; Snell, R.S. Seed quality and seed quantity in red maple depends on weather and individual tree characteristics. Ecol. Evol. 2020, 10, 13109–13121. [Google Scholar] [CrossRef] [PubMed]
- Alqudah, A.M.; Samarah, N.H.; Mullen, R.E. Drought stress effect on crop pollination, seed set, yield and quality. In Alternative Farming Systems, Biotechnology, Drought Stress and Ecological Fertilisation; Springer: Berlin/Heidelberg, Germany, 2011; pp. 193–213. [Google Scholar]
- Hatzig, S.V.; Nuppenau, J.-N.; Snowdon, R.J.; Schießl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef] [PubMed]
- Karimmojeni, H.; Bazrafshan, A.H.; Majidi, M.M.; Torabian, S.; Rashidi, B. Effect of maternal nitrogen and drought stress on seed dormancy and germinability of Amaranthus retroflexus. Plant Species Biol. 2014, 29, E1–E8. [Google Scholar] [CrossRef]
- Li, H.; Li, X.; Zhang, D.; Liu, H.; Guan, K. Effects of drought stress on the seed germination and early seedling growth of the endemic desert plant Eremosparton songoricum (Fabaceae). EXCLI J. 2013, 12, 89–101. [Google Scholar] [PubMed]
- Hegarty, T.W. The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination: A review. Plant Cell Environ. 1978, 1, 101–119. [Google Scholar] [CrossRef]
- Seiwa, K. Effects of seed size and emergence time on tree seedling establishment: Importance of developmental constraints. Oecologia 2000, 123, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Geber, M.A.; Griffen, L.R. Inheritance and natural selection on functional traits. Int. J. Plant Sci. 2003, 164, S21–S42. [Google Scholar] [CrossRef]
- Leiva, M.J.; Fernández-Alés, R.o. Variability in seedling water status during drought within a Quercus ilex subsp. ballota population, and its relation to seedling morphology. For. Ecol. Manag. 1998, 111, 147–156. [Google Scholar] [CrossRef]
- Cendán, C.; Sampedro, L.; Zas, R. The maternal environment determines the timing of germination in Pinus pinaster. Environ. Exp. Bot. 2013, 94, 66–72. [Google Scholar] [CrossRef]
- Rohde, A.; Howe, G.; Olsen, J.; Moritz, T.; Van Montagu, M.; Junttila, O.; Boerjan, W. Molecular aspects of bud dormancy in trees. Mol. Biol. Woody Plants 2000, 1, 89–134. [Google Scholar]
- Hannerz, M.; Ekberg, I.; Norell, L. Variation in Chilling Requirements for Completing Bud Rest between Provenances of Norway Spruce. Silvae Genet. 2003, 52, 161–168. [Google Scholar]
- Blum, B.M. Variation in the phenology of bud flushing in white and red spruce. Can. J. For. Res. 1988, 18, 315–319. [Google Scholar] [CrossRef]
- Vander Mijnsbrugge, K.; Turcsán, A.; Michiels, B. Population differentiation and phenotypic plasticity in temperature response of bud burst in Frangula alnus provenances of different latitude. Plant Syst. Evol. 2016, 302, 205–213. [Google Scholar] [CrossRef]
- Derory, J.; Léger, P.; Garcia, V.; Schaeffer, J.; Hauser, M.-T.; Salin, F.; Luschnig, C.; Plomion, C.; Glössl, J.; Kremer, A. Transcriptome analysis of bud burst in sessile oak (Quercus petraea). New Phytol. 2006, 170, 723–738. [Google Scholar] [CrossRef]
- Mátyás, C. Modeling climate change effects with provenance test data. Tree Physiol. 1994, 14, 797–804. [Google Scholar] [CrossRef]
- Donohue, K.; Dorn, L.; Griffith, C.; Kim, E.; Aguilera, A.; Polisetty, C.R.; Schmitt, J. The evolutionary ecology of seed germination of Arabidopsis thaliana: Variable natural selection on germination timing. Evol. Int. J. Org. Evol. 2005, 59, 758–770. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).