The Interactive Effects of Nitrogen Addition and Ozone Pollution on Cathay Poplar-Associated Phyllosphere Bacterial Communities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Treatments
2.2. Leaf Trait Measurements
2.3. Phyllosphere Micorbes Collection
2.4. DNA Extraction and Sequencing
2.5. Statistical Analyses
3. Results
3.1. Changes in Phyllospheric Bacterial Abundance in Response to Elevated O3 and N Levels
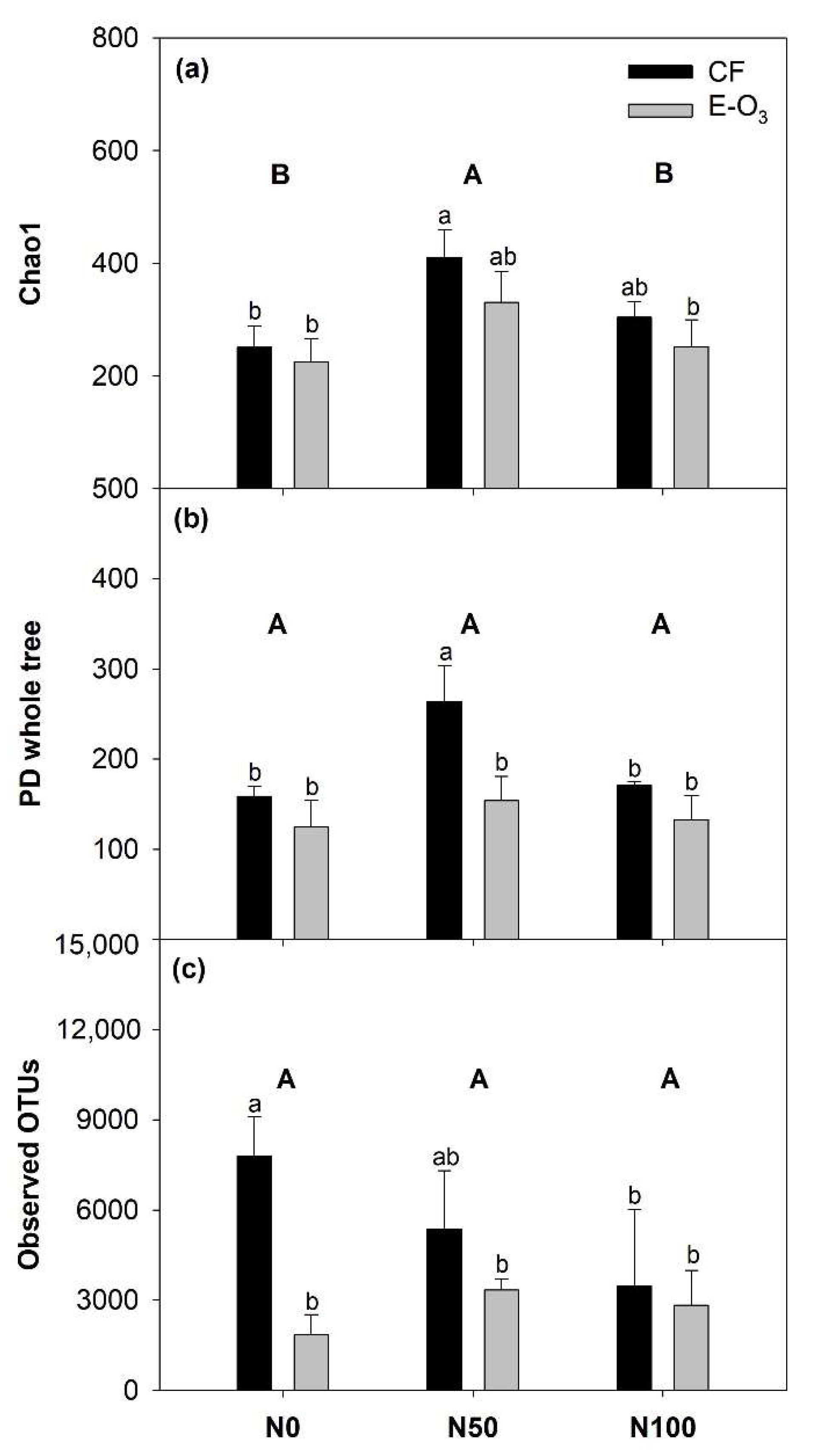
3.2. The Impact of Elevated O3 and N Levels on Phyllosphere Bacterial Community Alpha Diversity
3.3. Changes in Bacterial Community Composition in Response to Elevated O3 and N Levels
3.4. The Relationship between Plant-Specific Variables and the Phyllospheric Bacterial Community
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooper, O.R.; Parrish, D.D.; Ziemke, J.; Balashov, N.V.; Cupeiro, M.; Galbally, I.E.; Gilge, S.; Horowitz, L.; Jensen, N.R.; Lamarque, J.-F.; et al. Global distribution and trends of tropospheric ozone: An observation-based review. Elem.-Sci. Anthrop. 2014, 2, 000029. [Google Scholar] [CrossRef]
- Yu, G.R.; Jia, Y.L.; He, N.P.; Zhu, J.X.; Chen, Z.; Wang, Q.F.; Piao, S.L.; Liu, X.J.; He, H.L.; Guo, X.B.; et al. Stabilization of atmospheric nitrogen deposition in China over the past decades. Nat. Geosci. 2019, 12, 424–429. [Google Scholar] [CrossRef]
- Lu, X.; Hong, J.Y.; Zhang, L.; Cooper, O.W.; Schultz, M.G.; Xu, X.B.; Wang, T.; Gao, M.; Zhao, Y.H.; Zhang, Y.H. Severe surface ozone pollution in China: A global perspective. Environ. Sci. Tech. Lett. 2018, 5, 487–494. [Google Scholar] [CrossRef]
- Wang, T.; Xue, L.; Feng, Z.; Dai, J.; Zhang, Y.; Tan, Y. Ground-level ozone pollution in China: A synthesis of recent findings on influencing factors and impacts. Environ. Res. Lett. 2022, 17, 063003. [Google Scholar] [CrossRef]
- Feng, Z.Z.; De Marco, A.; Anav, A.; Gualtieri, M.; Sicard, P.; Tian, H.; Fornasier, F.; Tao, F.L.; Guo, A.H.; Paoletti, E. Economic losses due to ozone impacts on human health, forest productivity and crop yield across China. Environ. Int. 2019, 131, 104966. [Google Scholar] [CrossRef]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Anav, A.; De Marco, A.; Collalti, A.; Emberson, L.; Feng, Z.; Lombardozzi, D.; Sicard, P.; Verbeke, T.; Viovy, N.; Vitale, M.; et al. Legislative and functional aspects of different metrics used for ozone risk assessment to forests. Environ. Pollut. 2022, 295, 118690. [Google Scholar] [CrossRef]
- De Marco, A.; Garcia-Gomez, H.; Collalti, A.; Khaniabadi, Y.O.; Feng, Z.; Proietti, C.; Sicard, P.; Vitale, M.; Anav, A.; Paoletti, E. Ozone modelling and mapping for risk assessment: An overview of different approaches for human and ecosystems health. Environ. Res. 2022, 211, 113048. [Google Scholar] [CrossRef]
- Xu, C.; Xu, X.; Ju, C.; Chen, H.Y.H.; Wilsey, B.J.; Luo, Y.; Fan, W. Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Glob. Change Biol. 2020, 27, 1170–1180. [Google Scholar] [CrossRef]
- Lu, X.; Hou, E.; Guo, J.; Gilliam, F.S.; Li, J.; Tang, S.; Kuang, Y. Nitrogen addition stimulates soil aggregation and enhances carbon storage in terrestrial ecosystems of China: A meta-analysis. Glob. Change Biol. 2021, 27, 2780–2792. [Google Scholar] [CrossRef]
- Azuchi, F.; Kinose, Y.; Matsumura, T.; Kanomata, T.; Uehara, Y.; Kobayashi, A.; Yamaguchi, M.; Izuta, T. Modeling stomatal conductance and ozone uptake of Fagus crenata grown under different nitrogen loads. Environ. Pollut. 2014, 184, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Harmens, H.; Wagg, S.; Sharps, K.; Hayes, F.; Fowler, D.; Sutton, M.; Davies, B. Ozone impacts on vegetation in a nitrogen enriched and changing climate. Environ. Pollut. 2016, 208, 898–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.Z.; Shang, B.; Li, Z.Z.; Calatayud, V.; Agathokleous, E. Ozone will remain a threat for plants independently of nitrogen load. Funct. Ecol. 2019, 33, 1854–1870. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Zhou, H.; Yuan, X.; Feng, Z. Soil pH drives poplar rhizosphere soil microbial community responses to ozone pollution and nitrogen addition. Eur. J. Soil Sci. 2022, 73, e13186. [Google Scholar] [CrossRef]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant-microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Delmotte, N.; Knief, C.; Chaffron, S.; Innerebner, G.; Roschitzki, B.; Schlapbach, R.; von Mering, C.; Vorholt, J.A. Community proteogenomics reveals insights into the physiology of phyllosphere bacteria. Proc. Natl. Acad. Sci. USA 2009, 106, 16428–16433. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.G.; Xiong, C.; Wei, Z.; Chen, Q.L.; Ma, B.; Zhou, S.Y.D.; Tan, J.Q.; Zhang, L.M.; Cui, H.L.; Duan, G.L. Impacts of global change on phyllosphere microbiome. New Phytol. 2022, 234, 1977–1986. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microb. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [Green Version]
- Laforest-Lapointe, I.; Paquette, A.; Messier, C.; Kembel, S.W. Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature 2017, 546, 145–147. [Google Scholar] [CrossRef]
- Stone, B.; Weingarten, E.; Jackson, C. The role of the phyllosphere microbiome in plant health and function. In Annual Plant Reviews Online; Roberts, J.A., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar]
- Acuña-Rodríguez, I.S.; Newsham, K.K.; Gundel, P.E.; Torres-Díaz, C.; Montenegro, M.A.M. Functional roles of microbial symbionts in plant cold tolerance. Ecol. Lett. 2020, 23, 1034–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, T.; Nomura, K.; Wang, X.; Sohrabi, R.; Xu, J.; Yao, L.; Paasch, B.C.; Ma, L.; Kremer, J.; Cheng, Y.; et al. A plant genetic network for preventing dysbiosis in the phyllosphere. Nature 2020, 580, 653–657. [Google Scholar] [CrossRef]
- Crombie, A.T.; Larke-Mejia, N.L.; Emery, H.; Dawson, R.; Pratscher, J.; Murphy, G.P.; McGenity, T.J.; Murrell, J.C. Poplar phyllosphere harbors disparate isoprenedegrading bacteria. Proc. Natl. Acad. Sci. USA 2018, 115, 13081–13086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peňuelas, J. Bidirectional interaction between phyllospheric microbiotas and plant volatile emissions. Trends Plant Sci. 2016, 21, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.H.; Harris, R.F. The ecology and biogeography of microorganisms of plant surfaces. Annu. Rev. Phytopathol. 2000, 38, 145–180. [Google Scholar] [CrossRef]
- Yang, C.H.; Crowley, D.E.; Borneman, J.; Keen, N.T. Microbial phyllosphere populations are more complex than previously realized. Proc. Natl. Acad. Sci. USA 2001, 98, 3889–3894. [Google Scholar] [CrossRef] [Green Version]
- Remus-Emsermann, M.N.P.; Schlechter, R.O. Phyllosphere microbiology: At the interface between microbial individuals and the plant host. New Phytol. 2018, 218, 1327–1333. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Feng, Z.; Oksanen, E.; Sicard, P.; Wang, Q.; Saitanis, C.J.; Araminiene, V.; Blande, J.D.; Hayes, F.; Calatayud, V.; et al. Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 2020, 6, eabc1176. [Google Scholar] [CrossRef]
- Li, P.; Yin, R.; Shang, B.; Agathokleous, E.; Zhou, H.; Feng, Z. Interactive effects of ozone exposure and nitrogen addition on tree root traits and biomass allocation pattern: An experimental case study and a literature meta-analysis. Sci. Total Environ. 2020, 710, 136379. [Google Scholar] [CrossRef]
- Gundel, P.E.; Sorzoli, N.; Ueno, A.C.; Ghersa, C.M.; Seal, C.E.; Bastías, D.A.; Martinez-Ghersa, M.A. Impact of ozone on the viability and antioxidant content of grass seeds is affected by a vertically transmitted symbiotic fungus. Environ. Exp. Bot. 2015, 113, 40–46. [Google Scholar] [CrossRef]
- Ueda, Y.; Frindte, K.; Knief, C.; Ashrafuzzaman, M.; Frei, M. Effects of elevated tropospheric ozone concentration on the bacterial community in the phyllosphere and rhizoplane of rice. PLoS ONE 2016, 11, e0163178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bustos, L.M.B.; Ueno, A.C.; Di Leo, T.D.; Crocco, C.D.; Martinez-Ghersa, M.A.; MolinaMontenegro, M.A.; Gundel, P.E. Maternal exposure to ozone modulates the endophyte-conferred resistance to aphids in Lolium multiflorum. Insects 2020, 11, 548. [Google Scholar] [CrossRef] [PubMed]
- Sun, A.; Jiao, X.Y.; Chen, Q.; Wu, A.L.; Zheng, Y.; Lin, Y.X.; He, J.Z.; Hu, H.W. Microbial communities in crop phyllosphere and root endosphere are more resistant than soil microbiota to fertilization. Soil Biol. Biochem. 2021, 153, 108–113. [Google Scholar] [CrossRef]
- Ueno, A.C.; Gundel, P.E.; Molina-Montenegro, M.A.; Ramos, P.; Ghersa, C.M.; MartínezGhersa, M.A. Getting ready for the ozone battle: Vertically transmitted fungal endophytes have transgenerational positive effects in plants. Plant Cell. Environ. 2021, 44, 2716–2718. [Google Scholar] [CrossRef]
- Tian, D.; Jiang, L.; Ma, S.H.; Fang, W.J.; Schmid, B.; Xu, L.C.; Zhu, J.X.; Li, P.; Losaapio, G.; Jing, X.; et al. Effects of nitrogen deposition on soil microbial communities in temperate and subtropical forests in China. Sci. Total Environ. 2017, 607, 1367–1375. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.K.; Liu, H.W.; Trivedi, P. Eco-holobiont: A new concept to identify drivers of host-associated microorganisms. Environ. Microbiol. 2020, 22, 564–567. [Google Scholar] [CrossRef]
- Bell, J.K.; Helgason, B.; Siciliano, S.D. Brassica napus phyllosphere bacterial composition changes with growth stage. Plant Soil 2021, 464, 501–516. [Google Scholar] [CrossRef]
- Hu, E.; Gao, F.; Xin, Y.; Jia, H.; Li, K.; Hu, J.; Feng, Z. Concentration- and flux-based ozone dose-response relationships for five poplar clones grown in North China. Environ. Pollut. 2015, 207, 21–30. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, X.; Shang, B.; Manning, W.J.; Yang, A.; Wang, Y.; Feng, Z. Moderate drought did not affect the effectiveness of ethylenediurea (EDU) in protecting Populus cathayana from ambient ozone. Sci. Total Environ. 2016, 569, 1536–1544. [Google Scholar] [CrossRef]
- Yuan, X.; Shang, B.; Xu, Y.; Xin, Y.; Tian, Y.; Feng, Z.Z.; Paoletti, E. No significant interactions between nitrogen stimulation and ozone inhibition of isoprene emission in Cathay poplar. Sci. Total Environ. 2017, 601, 222–229. [Google Scholar] [CrossRef]
- Gao, F.; Catalayud, V.; Paoletti, E.; Hoshika, Y.; Feng, Z. Water stress mitigates the negative effects of ozone on photosynthesis and biomass in poplar plants. Environ. Pollut. 2017, 230, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1989, 148, 350–382. [Google Scholar]
- Knief, C.; Delmotte, N.; Chaffron, S.; Stark, M.; Innerebner, G.; Wassmann, R.; Von Mering, C.; Vorholt, J.A. Metaproteogenomic analysis of microbial communities in the phyllosphere and rhizosphere of rice. ISME J. 2012, 6, 1378–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Z.; Wang, J.; Delgado-Baquerizo, M.; Trivedi, P.; Singh, B.K. Plant microbiomes: Do different preservation approaches and primer sets alter our capacity to assess microbial diversity and community composition? Front. Plant Sci. 2020, 11, 993. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chao, A. Nonparametric-estimation of the number of classes in a population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Faith, D.P. Conservation evaluation and phylogenetic diversity. Biol. Conserv. 1992, 61, 1–10. [Google Scholar] [CrossRef]
- McArdle, B.H.; Anderson, M.J. Fitting multivariate models to community data: A comment on distance-based redundancy analysis. Ecology 2001, 82, 290–297. [Google Scholar] [CrossRef]
- Anderson, M.J. DISTLM forward: A FORTRAN Computer Program to Calculate a Distance-Based Multivariate Analysis for a Linear Model Using forward Selection; University of Auckland: Auckland, New Zealand, 2003. [Google Scholar]
- Papen, H.; Gessler, A.; Zumbusch, E.; Rennenberg, H. Chemolithoautotrophic nitrifiers in the phyllosphere of a spruce ecosystem receiving high atmospheric nitrogen input. Curr. Microbiol. 2002, 44, 56–60. [Google Scholar] [CrossRef]
- Yan, K.; Han, W.H.; Zhu, Q.L.; Li, C.R.; Dong, Z.; Wang, Y.P. Leaf surface microtopography shaping the bacterial community in the phyllosphere: Evidence from 11 tree species. Microbiol. Res. 2022, 254, 126897. [Google Scholar] [CrossRef]
- Xiang, Q.; Chen, Q.L.; Zhu, D.; Yang, X.R.; Qiao, M.; Hu, H.W.; Zhu, Y.G. Microbial functional traits in phyllosphere are more sensitive to anthropogenic disturbance than in soil. Environ. Pollut. 2020, 265, 114954. [Google Scholar] [CrossRef] [PubMed]
- Durand, A.; Maillard, F.; Alvarez-Lopez, V.; Guinchard, S.; Bertheau, C.; Valot, B.; Blaudez, D.; Chalot, M. Bacterial diversity associated with poplar trees grown on a Hg-contaminated site: Community characterization and isolation of Hg-resistant plant growth promoting bacteria. Sci. Total Environ. 2018, 622, 1165–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thapa, S.; Prasanna, R. Prospecting the characteristics and significance of the phyllosphere microbiome. Ann. Microbiol. 2018, 68, 229–245. [Google Scholar] [CrossRef]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between phyllosphere bacterial communities and plant functional traits in a neotropical forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperato, V.; Kowalkowski, L.; Portillo-Estrada, M.; Gawronski, S.W.; Vangronsveld, J.; Thijs, S. Characterisation of the Carpinus betulus L. phyllomicrobiome in urban and forest areas. Front. Microbiol. 2019, 10, 1110. [Google Scholar] [CrossRef] [Green Version]
- Stark, M.; Berger, S.A.; Stamatakis, A.; von Mering, C. MLTreeMap-accurate Maximum Likelihood placement of environmental DNA sequences into taxonomic and functional reference phylogenies. BMC Genom. 2010, 11, 461. [Google Scholar] [CrossRef] [Green Version]
- Knief, C.; Ramette, A.; Frances, L.; Alonso-Blanco, C.; Vorholt, J.A. Site and plant species are important determinants of the Methylobacterium community composition in the plant phyllosphere. ISME J. 2010, 4, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The ecology of the phyllosphere: Geographic and phylogenetic variability in the distribution of bacteria on tree leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [Green Version]
- Laforest-Lapointe, I.; Messier, C.; Kembel, S.W. Host species identity, site and time drive temperate tree phyllosphere bacterial community structure. Microbiome 2016, 4, 27. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, J.L.; Carroll, T.L.; Sundin, G.W. The role of pigmentation, ultraviolet radiation tolerance, and leaf colonization strategies in the epiphytic survival of phyllosphere bacteria. Microb. Ecol. 2005, 49, 104–113. [Google Scholar] [CrossRef]
- Gunasekera, T.S.; Sundin, G.W. Role of nucleotide excision repair and photoreactivation in the solar UVB radiation survival of Pseudomonas syringae pv. syringae B728a. J. Appl. Microbiol. 2006, 100, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N.P. Driving factors of epiphytic bacterial communities: A review. J. Adv. Res. 2019, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.; Lindow, S.E. Coexistence among epiphytic bacterial populations mediated through nutritional resource partitioning. Appl. Envrion. Microbiol. 1994, 60, 4468–4477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercier, J.; Lindow, S.E. Role of leaf surface sugars in colonization of plants by bacterial epiphytes. Appl. Environ. Microbiol. 2000, 66, 369–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveau, J.H.J.; Lindow, S.E. Appetite of an epiphyte: Quantitative monitoring of bacterial sugar consumption in the phyllosphere. Proc. Natl. Acad. Sci. USA 2001, 98, 3446–3453. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Saijo, Y.; Nakagami, H.; Takano, Y. Regulation of sugar transporter activity for antibacterial defense in Arabidopsis. Science 2016, 354, 1427–1430. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Atamna-Ismaeel, N.; Finkel, O.; Glaser, F.; von Mering, C.; Vorholt, J.A.; Koblížek, M.; Belkin, S.; Béjà, O. Bacterial anoxygenic photosynthesis on plant leaf surfaces. Environ. Microbiol. Rep. 2012, 4, 209–216. [Google Scholar] [CrossRef]
- Kiraly, Z.; El-Zahaby, H.M.; Klement, Z. Role of extracellular polysaccharide (EPS) slime in plant pathogenic bacteria in protecting cells to reactive oxygen species. J. Phytopathol. 1997, 145, 59–68. [Google Scholar] [CrossRef]
- Kim, Y.C.; Leveau, J.; Gardener, B.B.M.; Pierson, E.; Pierson, L.S.; Ryu, C.M. The multifactorial basis for plant health promotion by plant-associated bacteria. Appl. Environ. Microbiol. 2011, 77, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melotto, M.; Underwood, W.; Koczan, J.; Nomura, K.; He, S.Y. Plant stomata function in innate immunity against bacterial invasion. Cell 2006, 126, 969–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schellenberg, B.; Ramel, C.; Dudler, R. Pseudomonas syringae virulence factor syringolin A counteracts stomatal immunity by proteasome inhibition. Mol. Plant Microbe Interact. 2010, 23, 1287–1293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirano, S.S.; Upper, C.D. Bacteria in the leaf ecosystem with emphasis on Pseudomonas syringae—A pathogen, ice nucleus, and epiphyte. Microbiol. Mol. Biol. Rev. 2000, 64, 624–653. [Google Scholar] [CrossRef] [Green Version]
- Xiong, C.; Zhu, Y.G.; Wang, J.T.; Singh, B.K.; Han, L.L.; Shen, J.P.; Li, P.P.; Wang, G.B.; Wu, C.F.; Ge, A.H.; et al. Host selection shapes crop microbiome assembly and network complexity. New Phytol. 2021, 229, 1091–1104. [Google Scholar] [CrossRef]




| Variable | (a) Partial Mantal Test | (b) DistLM | |||||
|---|---|---|---|---|---|---|---|
| r | P | SS | F | P | % Var | Cum. (%) | |
| Asat | 0.19 | 0.024 | 4853.4 | 2.66 | 0.039 | 14.27 | 14.27 |
| gs | −0.14 | 0.677 | 647.5 | 0.30 | 0.914 | 1.90 | 16.17 |
| Ci | 0.09 | 0.117 | 586.2 | 0.23 | 0.952 | 1.72 | 17.89 |
| Chl | −0.03 | 0.586 | 495.2 | 0.21 | 0.969 | 1.46 | 19.35 |
| ISO | −0.17 | 0.104 | 1897.3 | 1.04 | 0.360 | 5.58 | 24.93 |
| Fv’/Fm’ | −0.14 | 0.610 | 626.5 | 0.20 | 0.945 | 1.84 | 26.77 |
| qP | −0.10 | 0.838 | 1135.9 | 0.61 | 0.643 | 3.34 | 30.11 |
| Vcmax | −0.02 | 0.817 | 534.3 | 0.26 | 0.936 | 1.57 | 31.68 |
| Jmax | −0.03 | 0.568 | 894.7 | 0.46 | 0.769 | 2.63 | 34.31 |
| Biomass | −0.09 | 0.776 | 500.6 | 0.18 | 0.960 | 1.47 | 35.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ran, K.; Gao, F. The Interactive Effects of Nitrogen Addition and Ozone Pollution on Cathay Poplar-Associated Phyllosphere Bacterial Communities. Forests 2023, 14, 452. https://doi.org/10.3390/f14030452
Li P, Ran K, Gao F. The Interactive Effects of Nitrogen Addition and Ozone Pollution on Cathay Poplar-Associated Phyllosphere Bacterial Communities. Forests. 2023; 14(3):452. https://doi.org/10.3390/f14030452
Chicago/Turabian StyleLi, Pin, Kun Ran, and Feng Gao. 2023. "The Interactive Effects of Nitrogen Addition and Ozone Pollution on Cathay Poplar-Associated Phyllosphere Bacterial Communities" Forests 14, no. 3: 452. https://doi.org/10.3390/f14030452





