Escape Game: Responses of Northern White Cedar (Thuja occidentalis L.) to an Extreme Reduction in White-Tailed Deer (Odocoileus virginianus Zimmerman) Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Sampling Methods
2.3. Statistical Analyses
3. Results
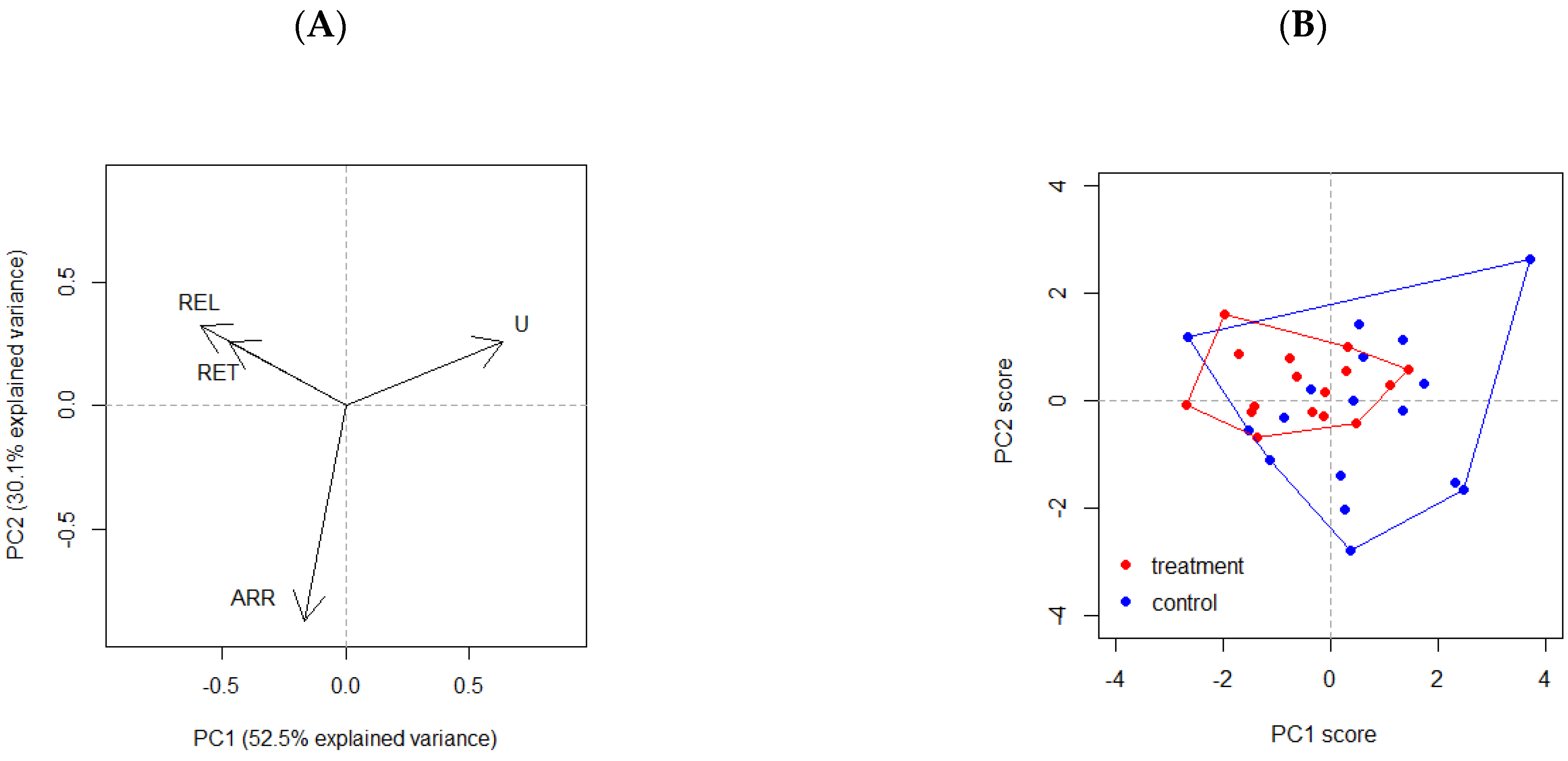
3.1. Characterization of Browsing Pressure
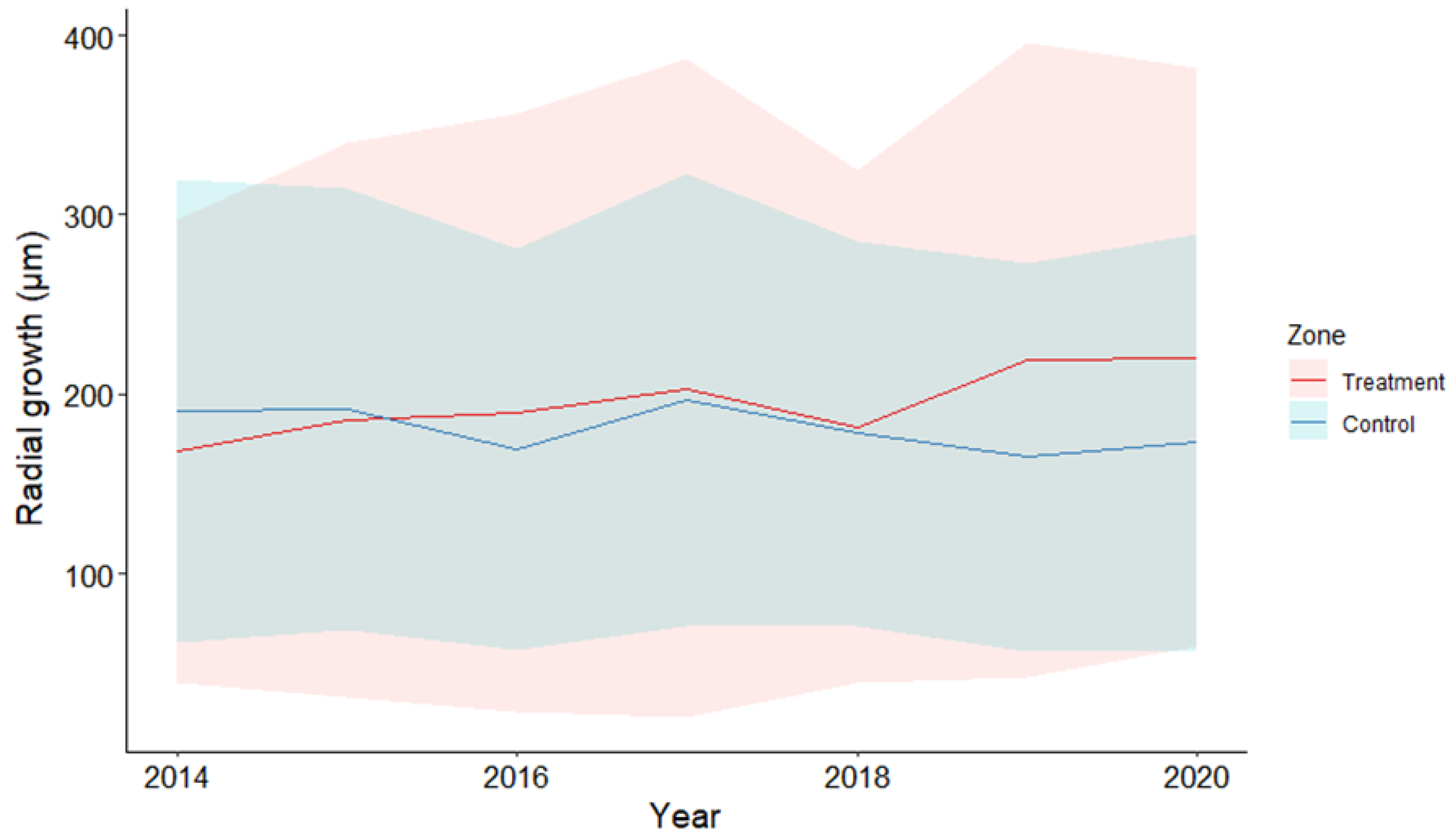
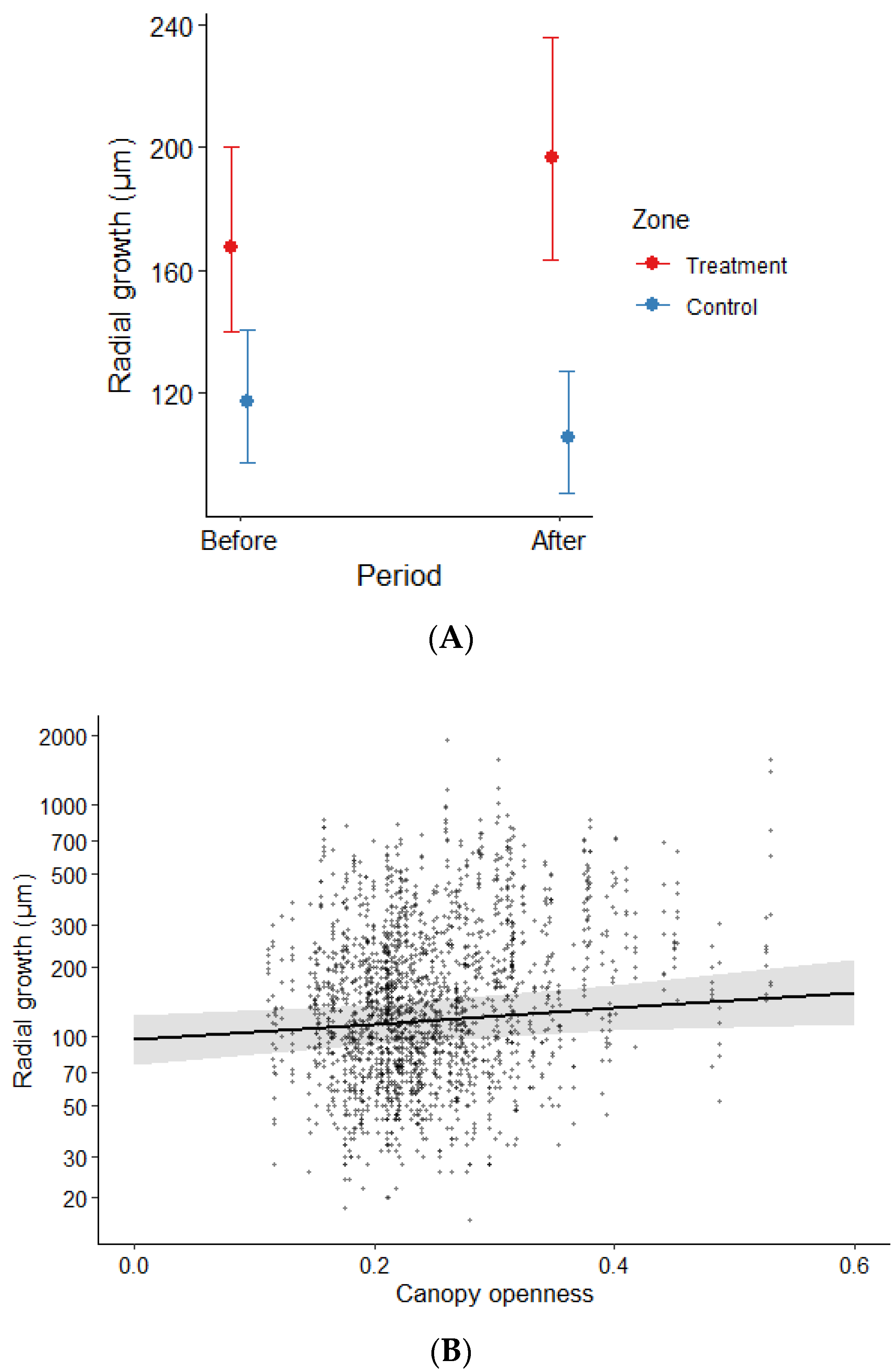
3.2. Radial Growth of Cedar Seedlings
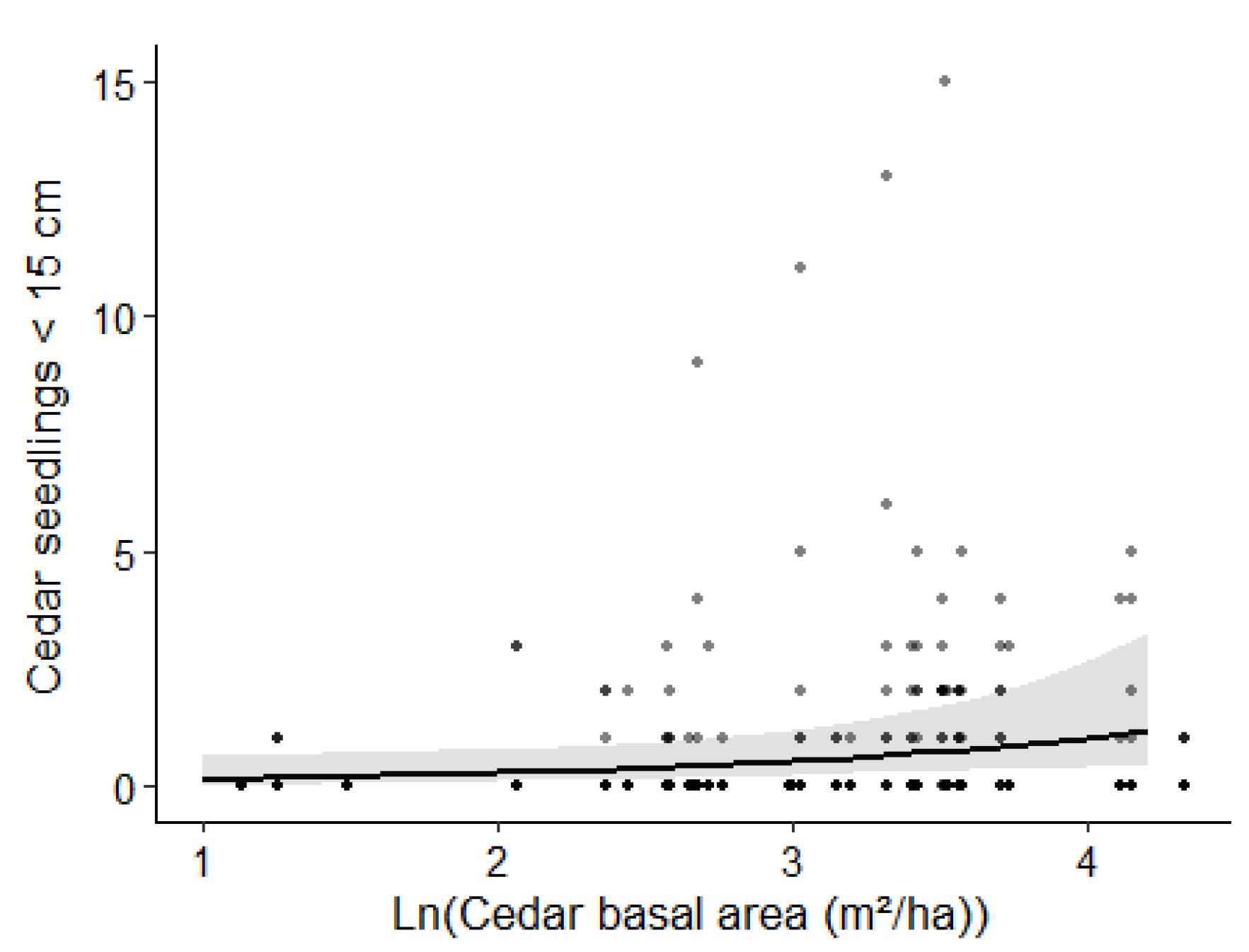
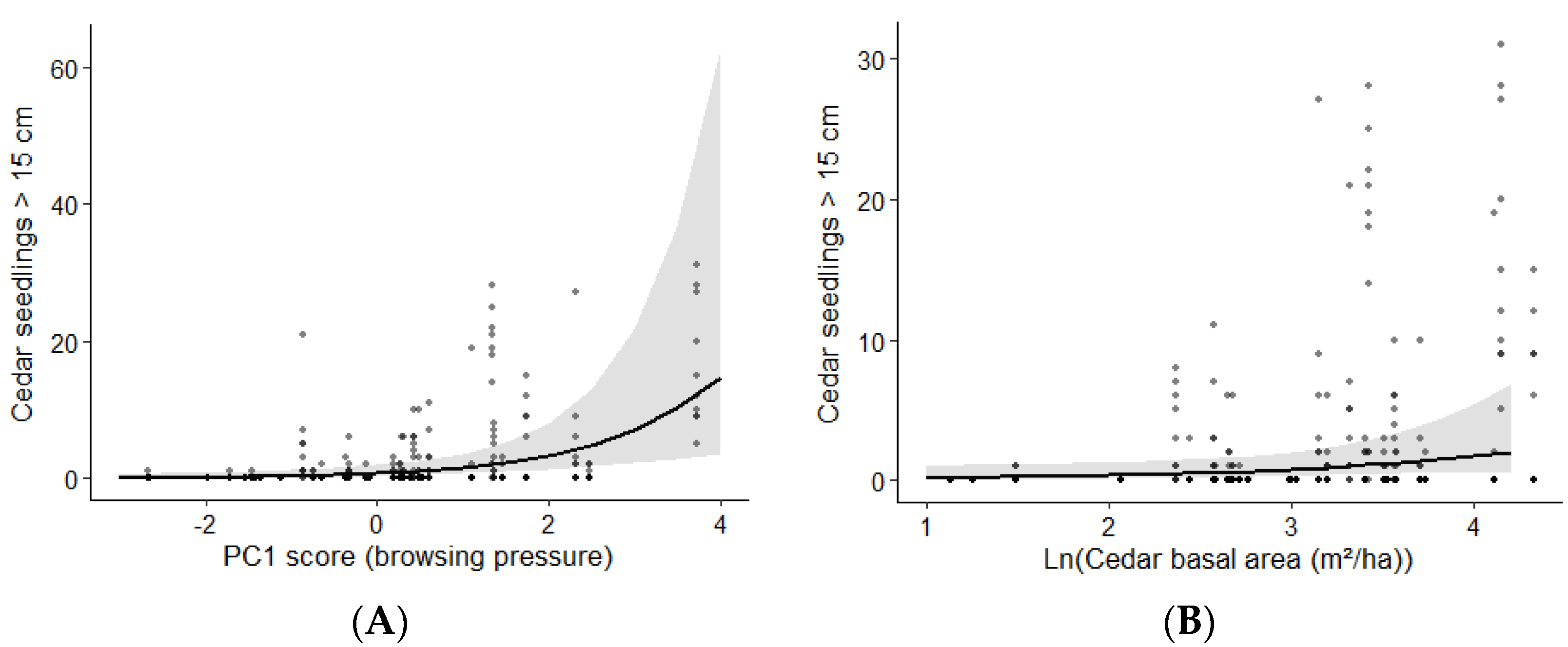
3.3. Abundance of Cedar Regeneration
4. Discussion
4.1. Characterization of Browsing Pressure
4.2. Growth Response of Cedar to a Reduction in Browsing Pressure
4.3. Abundance of Cedar Regeneration
5. Conclusions and Management Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boulfroy, E.; Forget, É.; Hofmeyer, P.V.; Kenefic, L.S.; Larouche, C.; Lessard, G.; Lussier, J.-M.; Pinto, F.; Ruel, J.-C.; Weiskittel, A. Guide Pour la Sylviculture du Thuya Occidental; Ressources Naturelles Canada, Centre Canadien sur la Fibre de Bois: Ottawa, ON, Canada, 2012; p. 84. [Google Scholar]
- Johnston, W.F. Thuja occidentalis L.—Northern white-cedar. In Silvics of North America; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 1990; pp. 580–589. [Google Scholar]
- Ruel, J.-C.; Lussier, J.-M.; Morissette, S.; Ricodeau, N. Growth response of northern white-cedar (Thuja occidentalis) to natural disturbances and partial cuts in mixed wood stands of Quebec, Canada. Forests 2014, 5, 1194–1211. [Google Scholar] [CrossRef]
- Fraver, S.; Kenefic, L.; Cutko, A.; White, A.S. Natural disturbance and stand structure in old-growth Thuja occidentalis forests of northern Maine. For. Ecol. Manag. 2020, 456, 117680. [Google Scholar] [CrossRef]
- Cornett, M.W.; Frelich, L.E.; Puettmann, K.J.; Reich, P.B. Conservation implications of browsing by Odocoileus virginianus in remnant upland Thuja occidentalis forests. Biol. Conserv. 2000, 93, 359–369. [Google Scholar] [CrossRef]
- Villemaire-Côté, O. Dynamiques de Régénération après Perturbation chez une Espèce Sensible au Broutement—Le cas du Thuya occidental. Ph.D. Thesis, Université Laval, Québec, QC, Canada, 2023. [Google Scholar]
- Danneyrolles, V.; Dupuis, S.; Arseneault, D.; Terrail, R.; Leroyer, M.; de Römer, A.; Fortin, G.; Boucher, Y.; Ruel, J.-C. Eastern white cedar long-term dynamics in eastern Canada: Implications for restoration in the context of ecosystem-based management. For. Ecol. Manag. 2017, 400, 502–510. [Google Scholar] [CrossRef]
- Etheridge, D.A.; Maclean, D.A.; Wagner, R.G.; Wilson, J.S. Changes in landscape composition and stand structure from 1945–2002 on an industrial forest in New Brunswick, Canada. Can. J. For. Res. 2011, 35, 1965–1977. [Google Scholar] [CrossRef]
- Heitzman, E.; Pregitzer, K.S.; Miller, R.O.; Lanasa, M.; Zuidema, M. Establishment and development of northern white-cedar following strip clearcutting. For. Ecol. Manag. 1999, 123, 97–104. [Google Scholar] [CrossRef]
- Dupuis, S.; Arseneault, D.; Sirois, L. Change from pre-settlement to present-day forest composition reconstructed from early land survey records in eastern Québec, Canada: Change from pre-settlement to present-day forest composition. J. Veg. Sci. 2011, 22, 564–575. [Google Scholar] [CrossRef]
- White, M.A. Long-term effects of deer browsing: Composition, structure and productivity in a northeastern Minnesota old-growth forest. For. Ecol. Manag. 2012, 269, 222–228. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Horsley, S.B.; Stout, S.L.; De Calesta, D.S. White-tailed deer impact on the vegetation dynamics of a northern hardwood forest. Ecol. Appl. 2003, 13, 98–118. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can. J. For. Res. 2006, 36, 1345–1362. [Google Scholar] [CrossRef]
- Royo, A.A.; Carson, W.P. Stasis in forest regeneration following deer exclusion and understory gap creation: A 10-year experiment. Ecol. Appl. 2022, 32, e2569. [Google Scholar] [CrossRef]
- Morrison, S.F.; Forbes, G.J.; Young, S.J.; Lusk, S. Within-yard habitat use by white-tailed deer at varying winter severity. For. Ecol. Manag. 2003, 172, 173–182. [Google Scholar] [CrossRef]
- Verme, L.J.; Johnston, W.F. Regeneration of northern white cedar deeryards in upper Michigan. J. Wildl. Manag. 1986, 50, 307. [Google Scholar] [CrossRef]
- Dumont, A.; Ouellet, J.-P.; Crête, M.; Huot, J. Winter foraging strategy of white-tailed deer at the northern limit of its range. Écoscience 2005, 12, 476–484. [Google Scholar] [CrossRef]
- Larouche, C.; Kenefic, L.S.; Ruel, J.-C. Northern white-cedar regeneration dynamics on the Penobscot experimental forest in Maine: 40-year results. North. J. Appl. For. 2010, 27, 5–12. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.-C. Development of northern white-cedar regeneration following partial cutting, with and without deer browsing. Forests 2015, 6, 344–359. [Google Scholar] [CrossRef]
- Rooney, T.P.; Solheim, S.L.; Waller, D.M. Factors affecting the regeneration of northern white cedar in lowland forests of the Upper Great Lakes region, USA. For. Ecol. Manag. 2002, 163, 119–130. [Google Scholar] [CrossRef]
- Villemaire-Côté, O.; Ruel, J.-C.; Tremblay, J.-P. Reduction in local white-tailed deer abundance allows the positive response of northern white cedar regeneration to gap dynamics. Can. J. For. Res. 2022, 52, 1383–1397. [Google Scholar] [CrossRef]
- Larouche, C.; Ruel, J.-C.; Lussier, J.-M. Factors affecting northern white-cedar (Thuja occidentalis) seedling establishment and early growth in mixedwood stands. Can. J. For. Res. 2011, 41, 568–582. [Google Scholar] [CrossRef]
- Kern, C.C.; Reich, P.B.; Montgomery, R.A.; Strong, T.F. Do deer and shrubs override canopy gap size effects on growth and survival of yellow birch, northern red oak, eastern white pine, and eastern hemlock seedlings? For. Ecol. Manag. 2012, 267, 134–143. [Google Scholar] [CrossRef]
- Villemaire-Côté, O.; Ruel, J.-C.; Sirois, L. Development of northern white-cedar (Thuja occidentalis L.) plantations within and outside deer yards. Forests 2017, 8, 326. [Google Scholar] [CrossRef]
- MFFP. Bilan des Opérations de Détection et de Contrôle de la Maladie Débilitante Chronique des Cervidés 2018; Ministère des Forêts, de la Faune et des Parcs: Québec, QC, Canada, 2018. Available online: https://cdn-contenu.quebec.ca/cdn-contenu/faune/documents/BI_MDC-Bilan-2018_mffp_fr.pdf?1620076642 (accessed on 15 February 2022).
- MFFP. Données D’abattage pour le cerf de Virginie dans la Zone D’intervention Contrôlée (ZIC) en 2018; Ministère des Forêts, de la Faune et des Parcs, Direction de la Gestion de la Faune de l’Outaouais: Québec, QC, Canada, 2020.
- MFFP. Bilan des Opérations de Détection et de Contrôle de la Maladie Débilitante Chronique des Cervidés 2019; Ministère des Forêts, de la Faune et des Parcs: Québec, QC, Canada, 2019. Available online: https://cdn-contenu.quebec.ca/cdn-contenu/faune/documents/BI_MDC-Bilan-2019_mffp_fr.pdf?1620076639 (accessed on 3 December 2021).
- MFFP. Données D’abattage et de Collision pour le cerf de Virginie de l’Outaouais et des Laurentides (2017 et 2018); Ministère des Forêts, de la Faune et des Parcs, Direction de la Gestion de la Faune de l’Outaouais: Québec, QC, Canada, 2020.
- Gosselin, J. Guide de Reconnaissance des Types Écologiques des Régions Écologiques 3a—Collines de l’Outaouais et du Témiscamingue et 3b—Collines du lac Nominingue; Ministère des Ressources Naturelles du Québec, Direction des Inventaires Forestiers, Division de la Classification Écologique et de la Productivité des Stations: Québec, QC, Canada, 2002; p. 188. [Google Scholar]
- MFFP. Norme de Stratification Écoforestière: Quatrième Inventaire Écoforestier du Québec Méridional; Ministère des Forêts, de la Faune et des Parcs, Secteur des Forêts, Direction des Inventaires Forestiers: Québec, QC, Canada, 2015; p. 111. Available online: http://collections.banq.qc.ca/ark:/52327/2748275 (accessed on 21 November 2021).
- MFFP. Aires de Confinement du cerf de Virginie pour les Régions de l’Outaouais et des Laurentides; Ministère des Forêts, de la Faune et des Parcs, Direction de la Gestion de la Faune de l’Outaouais: Québec, QC, Canada, 2020.
- Keigley, R.B. A growth form method for describing browse condition. Rangelands 1997, 19, 26–29. [Google Scholar]
- Steege, H.T. Hemiphot.R: Free R Scripts to Analyse Hemispherical Photographs for Canopy Openness, Leaf Area Index and Photosynthetic Active Radiation under Forest Canopies; Naturalis Biodiversity Center: Leiden, The Netherlands, 2018. [Google Scholar]
- Centre D’études Nordiques. Dendro2019; Centre D’études Nordiques: Québec, QC, Canada, 2019. [Google Scholar]
- Stevens, S.; Valderas, J.M.; Doran, T.; Perera, R.; Kontopantelis, E. Analysing indicators of performance, satisfaction, or safety using empirical logit transformation. BMJ 2016, 352, i1114. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. How many principal components? Stopping rules for determining the number of non-trivial axes revisited. Comput. Stat. Data Anal. 2005, 49, 974–997. [Google Scholar] [CrossRef]
- Stewart-Oaten, A.; Bence, J.R. Temporal and spatial variation in environmental impact assessment. Ecol. Monogr. 2001, 71, 305–339. [Google Scholar] [CrossRef]
- Underwood, A.J. On beyond BACI: Sampling designs that might reliably detect environmental disturbances. Ecol. Appl. 1994, 4, 3–15. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 14 September 2021).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models; R Package Version 0.4.3; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://cran.r-project.org/package=DHARMa (accessed on 14 September 2021).
- Lüdecke, D.; Ben-Shachar, M.S.; Patil, I.; Waggoner, P.; Makowski, D. Assessment, testing and comparison of statistical models using R. J. Open Source Softw. 2021, 6, 3139. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2011; p. 574. [Google Scholar]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Lenth, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means, R package version 1.5. 5–1; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Briand, C.H.; Posluszny, U.; Larson, D.W. Differential axis architecture in Thuja occidentalis (eastern white cedar). Can. J. Bot. 1992, 70, 340–348. [Google Scholar] [CrossRef]
- Johnson, D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Senft, R.L.; Coughenour, M.B.; Bailey, D.W.; Rittenhouse, L.R.; Sala, O.E.; Swift, D.M. Large herbivore foraging and ecological hierarchies. BioScience 1987, 37, 789–799. [Google Scholar] [CrossRef]
- Johnston, W.F. Balsam fir Dominant Species under Rethinned Northern White-Cedar; Research Note NC-133; U.S. Department of Agriculture, Forest Service, North Central Forest Experiment Station: St. Paul, MN, USA, 1972. [Google Scholar]
- Messier, F.; Crête, M. Moose-wolf dynamics and the natural regulation of moose populations. Oecologia 1985, 65, 503–512. [Google Scholar] [CrossRef]
- Jones, H.; Pekins, P.; Kantar, L.; Sidor, I.; Ellingwood, A.; Lichtenwalner, A.; O’Neal, M. Mortality assessment of moose (Alces alces) calves during successive years of winter tick (Dermacentor albipictus) epizootics in New Hampshire and Maine (USA). Can. J. Zool. 2018, 97, 22–30. [Google Scholar] [CrossRef]
- Hulbert, I.A.; Andersen, R. Food competition between a large ruminant and a small hindgut fermentor: The case of the roe deer and mountain hare. Oecologia 2001, 128, 499–508. [Google Scholar] [CrossRef]
- Alverson, W.S.; Lea, M.V.; Waller, D.M. A 20-year experiment on the effects of deer and hare on eastern hemlock regeneration. Can. J. For. Res. 2019, 49, 1329–1338. [Google Scholar] [CrossRef]
- Logan, K.T. Growth of Tree Seedlings as Affected by Light Intensity. IV Black Spruce, White Spruce, Balsam Fir and Eastern White Cedar; Report No. 1256; Department of Fisheries and Forestry, Canadian Forestry Service: Ottawa, ON, Canada, 1969. [Google Scholar]
- Hofmeyer, P.V.; Kenefic, L.S.; Seymour, R.S. Historical stem development of northern white-cedar (Thuja occidentalis L.) in Maine. North. J. Appl. For. 2010, 27, 92–96. [Google Scholar] [CrossRef]
- Cornett, M.W.; Reich, P.B.; Puettmann, K.J. Canopy feedbacks and microtopography regulate conifer seedling distribution in two Minnesota conifer-deciduous forests. Écoscience 1997, 4, 353–364. [Google Scholar] [CrossRef]
- De la Cretaz, A.L.; Kelty, M.J. Development of tree regeneration in fern-dominated forest understories after reduction of deer browsing. Restor. Ecol. 2002, 10, 416–426. [Google Scholar] [CrossRef]
- Jenkins, L.H.; Murray, B.D.; Jenkins, M.A.; Webster, C.R. Woody regeneration response to over a decade of deer population reductions in Indiana state parks. J. Torrey Bot. Soc. 2015, 142, 205–219. [Google Scholar] [CrossRef]
- Sage, R.W.; Porter, W.F.; Underwood, H.B. Windows of opportunity: White-tailed deer and the dynamics of northern hardwood forests of the northeastern US. J. Nat. Conserv. 2003, 10, 213–220. [Google Scholar] [CrossRef]
- Stout, S.L.; Royo, A.A.; De Calesta, D.S.; McAleese, K.; Finley, J.C. The Kinzua quality deer cooperative: Can adaptive management and local stakeholder engagement sustain reduced impact of ungulate browsers in forest systems? Boreal Environ. Res. 2013, 18 (Suppl. A), 50–64. [Google Scholar]
- Bombardier-Cauffopé, C. Dynamique de Régénération du Thuya occidental (Thuja occidentalis L.) dans les vieilles forêts mixtes sur sol mésique. M.Sc. Thesis, Laval University, Québec, QC, Canada, 2018. [Google Scholar]
- Capolla, B. Contribution du Cerf de Virginie aux Variations Spatio-Temporelles de la Régénération du Thuya Occidental au Québec et au Nouveau-Brunswick. Master’s Thesis, Laval University, Québec, QC, Canada, 2022. [Google Scholar]
- Hurst, J.E.; Porter, W.F. Evaluation of shifts in white-tailed deer winter yards in the Adirondack region of New York. J. Wildl. Manag. 2008, 72, 367–375. [Google Scholar] [CrossRef]
- Lesage, L.; Crête, M.; Huot, J.; Dumont, A.; Ouellet, J.-P. Seasonal home range size and philopatry in two northern white-tailed deer populations. Can. J. Zool. 2000, 78, 1930–1940. [Google Scholar] [CrossRef]
- Fryxell, J.M.; Hussell, D.J.T.; Lambert, A.B.; Smith, P.C. Time lags and population fluctuations in white-tailed deer. J. Wildl. Manag. 1991, 55, 377. [Google Scholar] [CrossRef]
- Beguin, J.; Tremblay, J.; Thiffault, N.; Pothier, D.; Côté, S.D. Management of forest regeneration in boreal and temperate deer–forest systems: Challenges, guidelines, and research gaps. Ecosphere 2016, 7, e01488. [Google Scholar] [CrossRef]


| Type | Description | Browse Intensity |
|---|---|---|
| Uninterrupted | The terminal leader has never been browsed and grows each year from the terminal leader of the previous year | Light to moderate |
| Arrested | The terminal leader and lateral shoots are repeatedly browsed, and the tree never exceeds the snowpack height (arrest height) | Intense |
| Retrogressed | The terminal leader and lateral shoots had grown taller than arrest height but an increase in browsing intensity caused a retrogression of the living height | Change from light–moderate to intense |
| Released | Tree showing past signs of arrest or retrogression of which one lateral shoot has become a new terminal leader that grows each year from the terminal leader of the previous year | Change from intense to light–moderate |
| PC1 | PC2 | PC3 | PC4 | |
|---|---|---|---|---|
| Uninterrupted (U) | 0.63 | 0.26 | 0.25 | 0.68 |
| Released (REL) | −0.59 | 0.33 | −0.46 | 0.59 |
| Retrogressed (RET) | −0.47 | 0.26 | 0.84 | -- |
| Arrested (ARR) | −0.17 | −0.87 | 0.15 | 0.43 |
| Proportion of explained variance | 0.53 | 0.30 | 0.16 | 0.02 |
| Cumulative explained variance | 0.53 | 0.83 | 0.98 | 1.00 |
| Parameter | Sum Sq | NumDF | DenDF | F Value | Pr (>F) |
|---|---|---|---|---|---|
| PC1 score | 0.01 | 1 | 33.77 | 0.04 | 0.84 |
| Period * Zone | 7.46 | 1 | 1812.12 | 40.93 | 0.00 |
| Canopy openness | 0.91 | 1 | 286.45 | 5.00 | 0.03 |
| Ln (root collar diameter) | 38.39 | 1 | 301.35 | 210.59 | 0.00 |
| Parameters | Estimate | 2.5% Confidence Interval | 97.5% Confidence Interval | |
|---|---|---|---|---|
| Seedlings 5–15 cm | Intercept | −2.50 | −4.46 | −0.50 |
| PC1 score | 0.07 | −0.28 | 0.41 | |
| Ln(white cedar basal area) | 0.64 | 0.06 | 1.22 | |
| Zone | −0.14 | −0.97 | 0.70 | |
| Deeryard | −0.40 | −1.29 | 0.48 | |
| Seedlings > 15 cm | Intercept | −2.6 | −5.1 | −0.1 |
| PC1 score | 0.7 | 0.3 | 1.1 | |
| Ln(white cedar basal area) | 0.8 | 0.0 | 1.5 | |
| Zone | −0.6 | −1.6 | 0.5 | |
| Deeryard | −0.6 | −1.6 | 0.5 | |
| Seedlings > 50 cm | Intercept | −4.5 | −9.8 | 0.8 |
| PC1 score | 0.9 | 0.0 | 1.7 | |
| Ln(white cedar basal area) | 0.9 | −0.6 | 2.5 | |
| Zone | −2.7 | −5.0 | −0.4 | |
| Deeryard | −1.8 | −4.0 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poirier, F.; Tremblay, J.-P.; Villemaire-Côté, O.; Ruel, J.-C. Escape Game: Responses of Northern White Cedar (Thuja occidentalis L.) to an Extreme Reduction in White-Tailed Deer (Odocoileus virginianus Zimmerman) Population. Forests 2023, 14, 455. https://doi.org/10.3390/f14030455
Poirier F, Tremblay J-P, Villemaire-Côté O, Ruel J-C. Escape Game: Responses of Northern White Cedar (Thuja occidentalis L.) to an Extreme Reduction in White-Tailed Deer (Odocoileus virginianus Zimmerman) Population. Forests. 2023; 14(3):455. https://doi.org/10.3390/f14030455
Chicago/Turabian StylePoirier, Frédéric, Jean-Pierre Tremblay, Olivier Villemaire-Côté, and Jean-Claude Ruel. 2023. "Escape Game: Responses of Northern White Cedar (Thuja occidentalis L.) to an Extreme Reduction in White-Tailed Deer (Odocoileus virginianus Zimmerman) Population" Forests 14, no. 3: 455. https://doi.org/10.3390/f14030455
APA StylePoirier, F., Tremblay, J.-P., Villemaire-Côté, O., & Ruel, J.-C. (2023). Escape Game: Responses of Northern White Cedar (Thuja occidentalis L.) to an Extreme Reduction in White-Tailed Deer (Odocoileus virginianus Zimmerman) Population. Forests, 14(3), 455. https://doi.org/10.3390/f14030455







