Multi-Trait Selection and Stability in Norway Spruce (Picea abies) Provenance Trials in Romania
Abstract
:1. Introduction
2. Materials and Methods
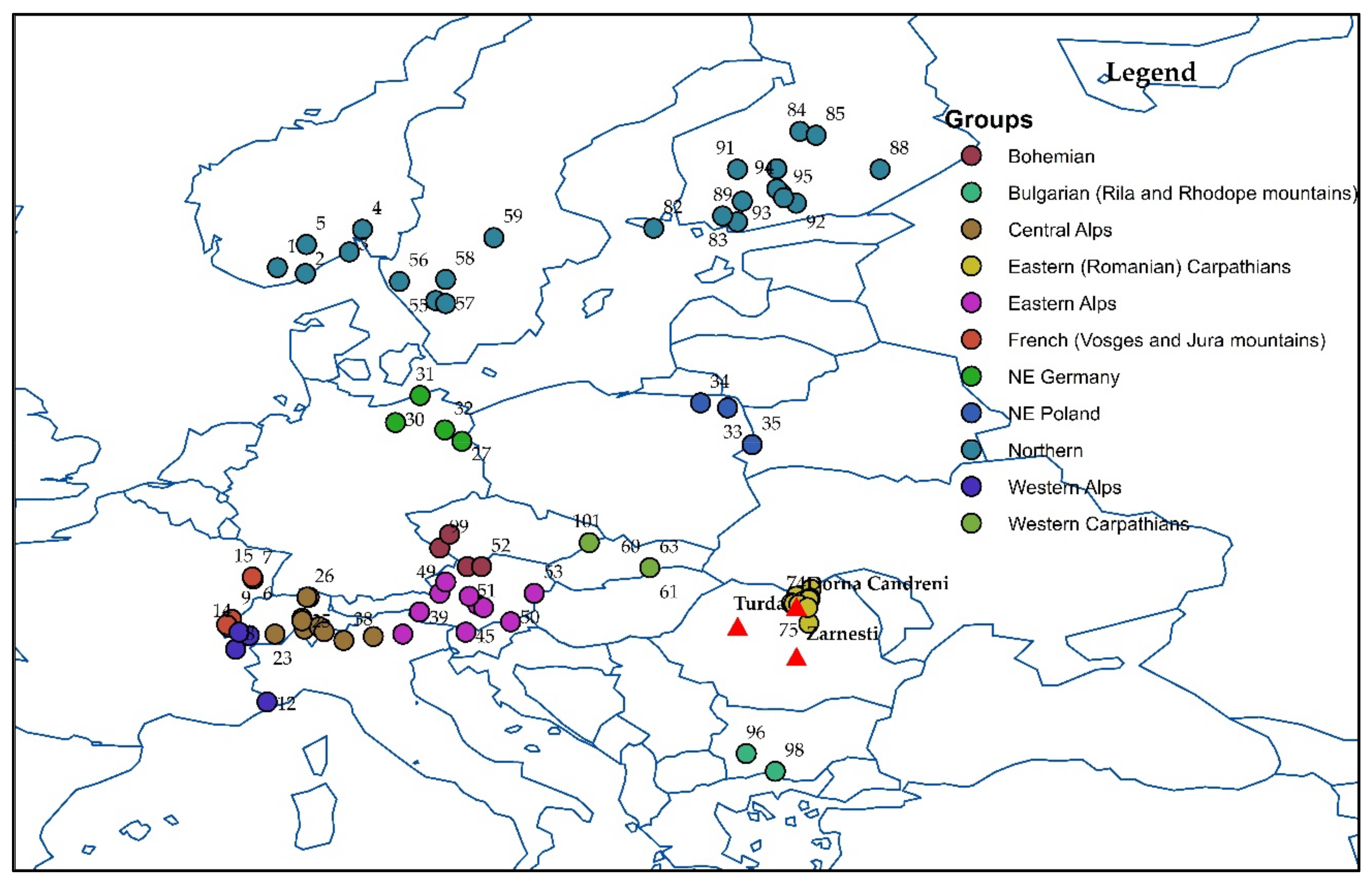
2.1. Genetic Material, Experimental Desing and Measurements
2.2. Statistical Analysis
- (1)
- After the WAASB (Weighted Average of Absolute Scores for quantifying the stability of g genotypes conducted in e environments using linear mixed-effect models) was calculated, it and the performances (values of traits) were rescaled in a 0 to 100 range.
- (2)
- Then, the WAASBY index was computed, which allowed weighting between stability (WAASB) and mean performance (Y) [52]. Different weights can be used for prioritising the performance or the stability of the provenances. In our study, the used weights were 25 for stability and 75 for performance, i.e., performance was put above stability.
- (3)
- After that, the steps were similar to those of MGIDI, the difference being that the ideotype now had the maximum value of 100 for the WAASBY.
- (4)
- Finally, MTSI was estimated according to the equation:where the MTSIi = the multi-trait stability index for the ith genotype; Fij = the jth score of the ith genotype; Fj = the jth score of the ideotype.
3. Results
3.1. Genetic Variability Analysis within Trial Sites
3.2. Genotype by Environment Analysis
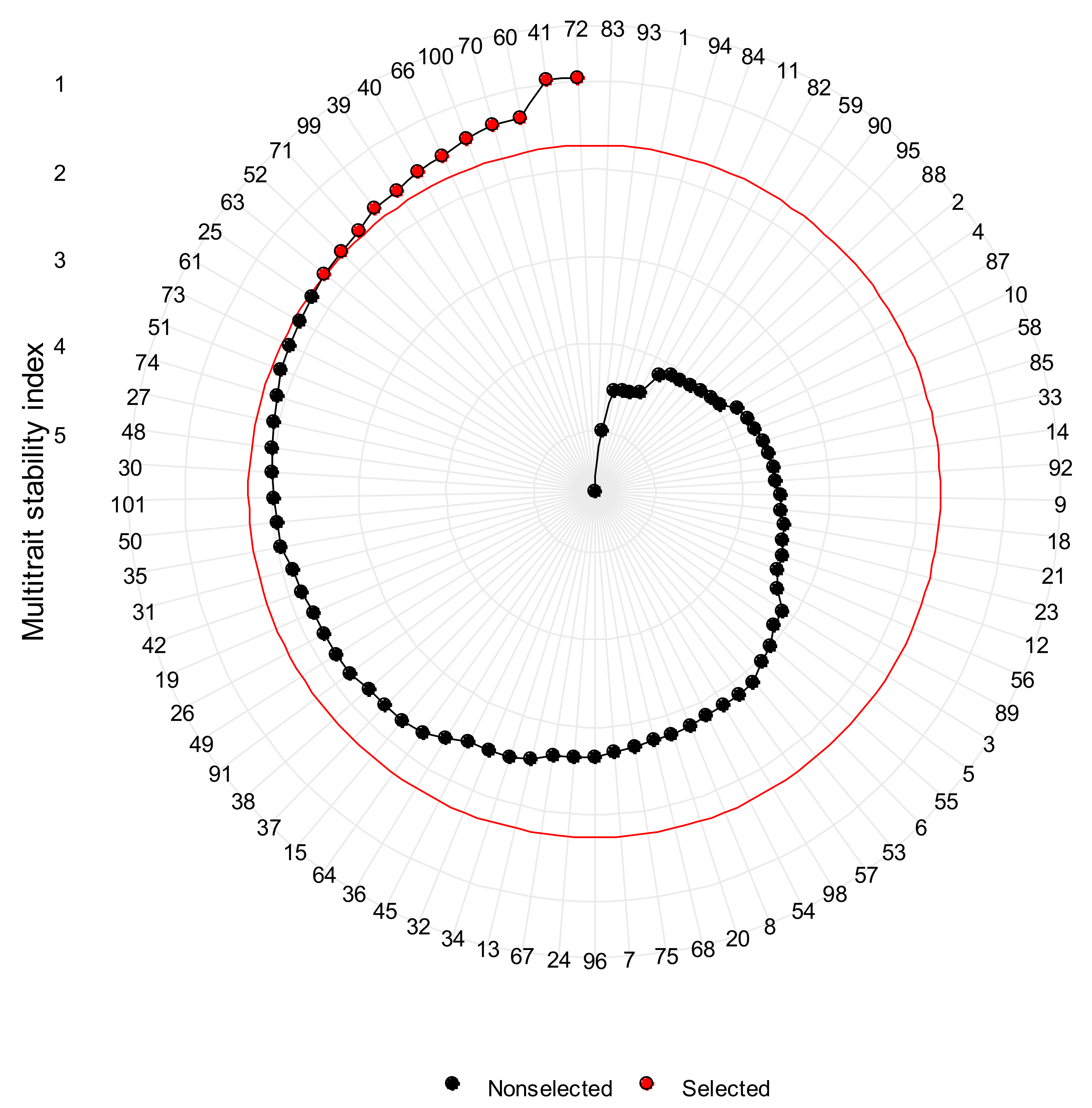
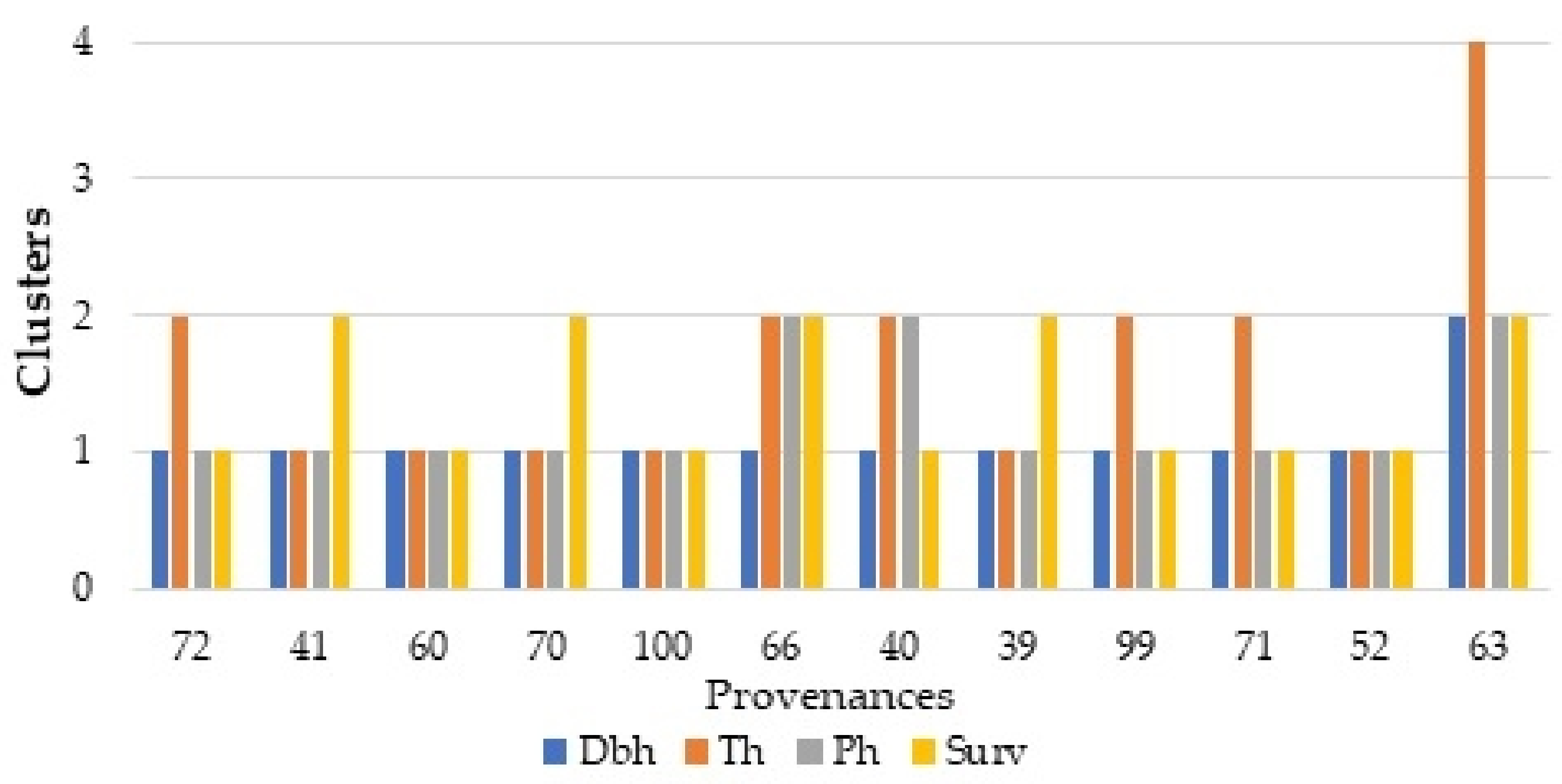
3.3. The Multi-Trait Selection in Each Provenance Trial
3.4. The Multi-Trait Selection across Provenance Trials
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jansen, S.; Konrad, H.; Geburek, T. The extent of historic translocation of Norway spruce forest reproductive material in Europe. Ann. For. Sci. 2017, 74, 56. [Google Scholar] [CrossRef] [Green Version]
- Rezultate IFN—Ciclul II|Inventarul Forestier National 2018. Available online: http://roifn.ro/site/rezultate-ifn-2/ (accessed on 3 February 2020).
- Șofletea, N.; Curtu, A.L. Dendrologie; Editura Universităţii Transilvania: Brasov, Romania, 2007; ISBN 973-635-885-2. [Google Scholar]
- Jansson, G.; Danusevičius, D.; Grotehusman, H.; Kowalczyk, J.; Krajmerova, D.; Skrøppa, T.; Wolf, H. Norway Spruce (Picea abies (L.) H.Karst.). In Forest Tree Breeding in Europe: Current State-of-the-Art and Perspectives; Pâques, L.E., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 123–176. [Google Scholar] [CrossRef]
- Krutzsch, P.H. IUFRO’s role in coniferous tree improvement: Norway spruce (Picea abies (L.) Karst.). Silvae Genetica 1992, 41, 143–150. [Google Scholar]
- Nitu, C. Cercetari privind Comportarea Provenientelor de Molid Testate in Diferite Conditii Stationale (Researches Concerning the Behavior of Norway Spruce Provenances Tested in Different Site Conditions); Redactia de Propaganda Tehnica Agricola: Bucuresti, Romania, 1984; p. 36. [Google Scholar]
- Mihai, G. Surse de Seminţe Testate Pentru Principalele Specii de Arbori Forestieri din România [Tested Seed Sources for the Main Forest tree Species from Romania]; Editura Silvică: Bucureşti, Romania, 2009. [Google Scholar]
- Campbell, B.T.; Jones, M.A. Assessment of genotype × environment interactions for yield and fiber quality in cotton performance trials. Euphytica 2005, 144, 69–78. [Google Scholar] [CrossRef]
- Aitken, S.N.; Yeaman, S.; Holliday, J.A.; Wang, T.; Curtis-McLane, S. Adaptation, migration or extirpation: Climate change outcomes for tree populations. Evol. Appl. 2008, 1, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Adaptive versus Non-Adaptive Phenotypic Plasticity and the Potential for Contemporary Adaptation in New Environments—GHALAMBOR-2007—Functional Ecology—Wiley Online Library. Available online: https://besjournals.onlinelibrary.wiley.com/doi/10.1111/j.1365-2435.2007.01283.x (accessed on 12 December 2022).
- Codesido, V.; Fernández-López, J. Implication of genotype × site interaction on Pinus radiata breeding in Galicia. New For. 2009, 37, 17–34. [Google Scholar] [CrossRef]
- Haapanen, M. Impact of Family-by-trial Interaction on the Utility of Progeny Testing Methods for Scots Pine. Silvae Genet. 1996, 45, 130–135. [Google Scholar]
- Costa e Silva, J.; Potts, B.M.; Dutkowski, G.W. Genotype by environment interaction for growth of Eucalyptus globulus in Australia. Tree Genet. Genomes 2006, 2, 61–75. [Google Scholar] [CrossRef]
- Campbell, R.K. Genotype * Environment Interaction: A Case Study for Douglas-fir in Western Oregon|Pacific Northwest Research Station|PNW—US Forest Service. Available online: https://www.fs.usda.gov/pnw/publications/genotype-environment-interaction-case-study-douglas-fir-western-oregon (accessed on 12 December 2022).
- Longauer, R.; Pacalaj, M.; Gömöry, D.; Strmeň, S.; Krajmerová, D. Growth and survival of Norway spruce in the provenance experiment IUFRO 1972 at the age of 38 year. Acta Fac. For. Zvolen Slovak. 2012, 54, 93–110. [Google Scholar]
- Lundströmer, J.; Karlsson, B.; Berlin, M. Strategies for deployment of reproductive material under supply limitations—A case study of Norway spruce seed sources in Sweden. Scand. J. For. Res. 2020, 35, 495–505. [Google Scholar] [CrossRef]
- Sixto, H.; Salvia, J.; Barrio, M.; Pilar Ciria, M.; Canellas, I. Genetic variation and genotype-environment interactions in short rotation Populus plantations in southern Europe. New For. 2011, 42, 163–177. [Google Scholar] [CrossRef]
- Heinrich, G.M.; Francis, C.A.; Eastin, J.D. Stability of Grain Sorghum Yield Components Across Diverse Environments1. Crop Sci. 1983, 23, 209–212. [Google Scholar] [CrossRef]
- Laing, D.R. Adaptabilidad y Estabilidad en el Comportamiento de Plantas de Frijol Comun. 1978. Available online: https://cgspace.cgiar.org/handle/10568/69890 (accessed on 12 December 2022).
- Becker, H.C.; Léon, J. Stability Analysis in Plant Breeding. Plant Breed. 1988, 101, 1–23. [Google Scholar] [CrossRef]
- Gianoli, E.; Valladares, F. Studying phenotypic plasticity: The advantages of a broad approach. Biol. J. Linn. Soc. 2012, 105, 1–7. [Google Scholar] [CrossRef] [Green Version]
- White, T.L.; Adams, W.T.; Neale, D.B. Forest Genetics. 2007. Available online: https://books.google.ro/books?id=UHZCeg4BqtkC (accessed on 12 December 2022).
- Falconer, D.S. Introduction to Quantitative Genetics; Prentice Hall: Harlow, UK, 1996; ISBN 978-0-582-24302-6. [Google Scholar]
- Raymond, C.A.; Namkoong, G. Optimizing breeding zones: Genetic flexibility or maximum value. Silvae Genet. 1990, 39, 110–113. [Google Scholar]
- Stojnic, S.; Orlović, S.; Ballian, D.; Ivankovic, M.; Sijacic/Nikolic, M.; Pilipovic, A.; Bogdan, S.; Kvesic, S.; Mataruga, M.; Daničić, V.; et al. Provenance by site interaction and stability analysis of European beech (Fagus sylvatica L.) provenances grown in common garden experiments. Silvae Genet. 2015, 64, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Chmura, D.J.; Matras, J.; Barzdajn, W.; Buraczyk, W.; Kowalkowski, W.; Kowalczyk, J.; Rożkowski, R.; Szeligowski, H. Variation in growth of Norway spruce in the IUFRO 1972 provenance experimental series. Silvae Genet. 2016, 67, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.F. A Discriminant Function for Plant Selection. Ann. Eugen. 1936, 7, 240–250. [Google Scholar] [CrossRef]
- Hazel, L.N. The Genetic Basis for Constructing Selection Indexes. Genetics 1943, 28, 476–490. [Google Scholar] [CrossRef]
- Olivoto, T.; Nardino, M. MGIDI: Toward an effective multivariate selection in biological experiments. Bioinformatics 2021, 37, 1383–1389. [Google Scholar] [CrossRef]
- Allen, M.P. (Ed.) The problem of multicollinearity. In Understanding Regression Analysis; Springer: Boston, MA, USA, 1997; pp. 176–180. ISBN 978-0-585-25657-3. [Google Scholar]
- Prunier, J.G.; Colyn, M.; Legendre, X.; Nimon, K.F.; Flamand, M.C. Multicollinearity in spatial genetics: Separating the wheat from the chaff using commonality analyses. Mol. Ecol. 2015, 24, 263–283. [Google Scholar] [CrossRef]
- Bizari, E.H.; Val, B.H.P.; Pereira, E.d.M.; Mauro, A.O.D.; Unêda-Trevisoli, S.H. Selection indices for agronomic traits in segregating populations of soybean1. Rev. Ciênc. Agronômica 2017, 48, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Aitken, S.N.; Rozenberg, P.; Carlson, M.R. Selection for height growth and Pilodyn pin penetration in lodgepole pine: Effects on growth traits, wood properties, and their relationships. Can. J. For. Res. 1999, 29, 434–445. [Google Scholar] [CrossRef]
- Christophe, C.; Birot, Y. Genetic structures and expected genetic gains from multitrait selection in wild populations of Douglas fir and Sitka spruce. II. Practical application of index selection on several populations. Silvae Genet. 1983, 32, 173–181. [Google Scholar]
- White, T.L.; Hodge, G.R. Best linear prediction of breeding values in a forest tree improvement program. Theor. Appl. Genet. 1988, 76, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Chollet, F.; Roman-Amat, B. Determination of economic coefficients for multi- trait selection on maritime pine (Pinus pinaster AIT.). In Proceedings of the IUFRO Conference, a Joint Meeting of Working Parties on Breeding Theory, Progeny Testing and Seed Orchards, Williamsburg, VA, USA, 13–17 October 1986; North Carolina State University-Industry Cooperative Tree Improvement Program, Publ.: Williamsburg, VA, USA, 1986; pp. 567–581. [Google Scholar]
- Carreras, R.; Bessega, C.; López, C.R.; Saidman, B.O.; Vilardi, J.C. Developing a breeding strategy for multiple trait selection in Prosopis alba Griseb., a native forest species of the Chaco Region in Argentina. For. Int. J. For. Res. 2017, 90, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Missanjo, E.; Matsumura, J. Multiple Trait Selection Index for Simultaneous Improvement of Wood Properties and Growth Traits in Pinus kesiya Royle ex Gordon in Malawi. Forests 2017, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Zhao, X.; Liu, H.; Wang, S.; Song, Z.; Ma, X.; Li, K. Preliminary study on genetic variation of growth traits and wood properties and superior clones selection of Populus ussuriensis Kom. IForest-Biogeosci. For. 2019, 12, 459. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z. Quantitative Genetics of Norway Spruce in Sweden. Available online: https://pub.epsilon.slu.se/13331/ (accessed on 16 February 2022).
- Rashidi-Jouybari, I.; Lenz, P.; Beaulieu, J.; Nadeau, S.; Bousquet, J.; Achim, A. Multi-trait selection for improved solid wood physical and flexural properties in white spruce. For. Int. J. For. Res. 2022, 95, 492–503. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, J.; Yun, H.; Yang, G.; Ma, J.; Ma, W.; Qu, G. Genetic Evaluation and Combined Selection for the Simultaneous Improvement of Growth and Wood Properties in Catalpa bungei Clones. Forests 2021, 12, 868. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Sari, B.G.; Diel, M.I. Mean Performance and Stability in Multi-Environment Trials II: Selection Based on Multiple Traits. Agron. J. 2019, 111, 2961–2969. [Google Scholar] [CrossRef]
- Nițu, C.; Benea, V.; Duran, V.; Florescu, I.; Gruescu, A.; Marcu, A.; Răescu, V. Aspecte privind variabilitatea genetică a unor proveniențe de molid. An. Inst. Cercet. Amenaj. Silv. 1974, 31, 49–58. [Google Scholar]
- Meteo Romania | Clima Romaniei. Available online: https://www.meteoromania.ro/clima/clima-romaniei/ (accessed on 14 February 2023).
- Badea, L. Geografia României; Editura Academiei Republicii Socialiste România: Bucuresti, Romania, 1983. [Google Scholar]
- Kapeller, S.; Lexer, M.J.; Geburek, T.; Hiebl, J.; Schueler, S. Intraspecific variation in climate response of Norway spruce in the eastern Alpine range: Selecting appropriate provenances for future climate. For. Ecol. Manag. 2012, 271, 46–57. [Google Scholar] [CrossRef]
- Liepe, K.J.; van der Maaten, E.; van der Maaten-Theunissen, M.; Liesebach, M. High Phenotypic Plasticity, but Low Signals of Local Adaptation to Climate in a Large-Scale Transplant Experiment of Picea abies (L.) Karst. in Europe. Front. For. Glob. Chang. 2022, 5, 804857. [Google Scholar] [CrossRef]
- Aitken, S.N.; Bemmels, J.B. Time to get moving: Assisted gene flow of forest trees. Evol. Appl. 2016, 9, 271–290. [Google Scholar] [CrossRef]
- Nanson, A. Génétique et Amélioration des Arbres Forestiers; Les Presses Agronomiques de Gembloux: Gembloux, Belgium, 2004; ISBN 978-2-87016-070-1. [Google Scholar]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B.; Jensen, S.P. lmerTest: Tests in Linear Mixed Effects Models 2020. Available online: https://CRAN.R-project.org/package=lmerTest (accessed on 8 December 2022).
- Olivoto, T.; Lúcio, A.D.C.; da Silva, J.A.G.; Marchioro, V.S.; de Souza, V.Q.; Jost, E. Mean Performance and Stability in Multi-Environment Trials I: Combining Features of AMMI and BLUP Techniques. Agron. J. 2019, 111, 2949–2960. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D. metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef] [Green Version]
- R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 12 June 2022).
- Budeanu, M.; Șofletea, N.; Petritan, I. Among-population Variation in Quality Traits in Two Romanian Provenance Trials with Picea abies L. Balt. For. 2014, 20, 37–47. [Google Scholar]
- Chen, Z.-Q.; Karlsson, B.; Wu, H.X. Patterns of additive genotype-by-environment interaction in tree height of Norway spruce in southern and central Sweden. Tree Genet. Genomes 2017, 13, 25. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Suontama, M.; Burdon, R.D.; Dungey, H.S. Genotype by environment interactions in forest tree breeding: Review of methodology and perspectives on research and application. Tree Genet. Genomes 2017, 13, 60. [Google Scholar] [CrossRef] [Green Version]
- Skrøppa, T.; Steffenrem, A. Performance and Phenotypic Stability of Norway Spruce Provenances, Families, and Clones Growing under Diverse Climatic Conditions in Four Nordic Countries. Forests 2021, 12, 230. [Google Scholar] [CrossRef]
- Mihai, G.; Teodosiu, M.; Birsan, M.-V.; Alexandru, A.-M.; Mirancea, I.; Apostol, E.-N.; Garbacea, P.; Ionita, L. Impact of Climate Change and Adaptive Genetic Potential of Norway Spruce at the South–eastern Range of Species Distribution. Agric. For. Meteorol. 2020, 291, 108040. [Google Scholar] [CrossRef]
- Pigliucci, M.; Murren, C.J.; Schlichting, C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006, 209, 2362–2367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T. Breeding strategies for forest trees: Concepts and challenges. South. Afr. For. J. 2001, 190, 31–42. [Google Scholar] [CrossRef]
- Matras, J. Growth and development of Polish provenances of Picea abies in the IUFRO 1972 experiment. Dendrobiology 2009, 61, 145–158. [Google Scholar]
- Budeanu, M.; Șofletea, N.; Pârnuta, G. Testing Romanian seed sources of Norway spruce (Picea abies): Results on growth traits and survival at age 30. Ann. For. Res. 2012, 55, 43–52. [Google Scholar] [CrossRef]
- Schueler, S.; Kapeller, S.; Konrad, H.; Geburek, T.; Mengl, M.; Bozzano, M.; Koskela, J.; Lefèvre, F.; Hubert, J.; Kraigher, H.; et al. Adaptive genetic diversity of trees for forest conservation in a future climate: A case study on Norway spruce in Austria. Biodivers. Conserv. 2013, 22, 1151–1166. [Google Scholar] [CrossRef]
- Zeltiņš, P.; Katrevičs, J.; Gailis, A.; Maaten, T.; Desaine, I.; Jansons, Ā. Adaptation Capacity of Norway Spruce Provenances in Western Latvia. Forests 2019, 10, 840. [Google Scholar] [CrossRef] [Green Version]
- Giertych, M. Genetics. In Biology and Ecology of Norway Spruce; Tjoelker, M.G., Boratyński, A., Bugała, W., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 115–155. ISBN 978-1-4020-4841-8. [Google Scholar]
- Fowler, D.P.; Coles, J.F. Norway Spruce Provenance Experiments in the Maritimes Region of Canada. For. Chron. 1980, 56, 155–160. [Google Scholar] [CrossRef] [Green Version]
- Skrøppa, T. Forsøk med Rumenske Granprovenienser Trials with Norway Spruce Provenances from Romania; NIBIO: Postboks, Norway, 2021; ISBN 978-82-17-02926-7. [Google Scholar]
| Trait | LRTp | Vp | Vr | MS g | MS b | Mean ± SD | |
|---|---|---|---|---|---|---|---|
| Dorna Candrenilor | Dbh | 5.07 * | 0.56 | 28.87 | 264.06 *** | 15.19 ns | 23.75 ± 5.58 |
| Th | 55.39 *** | 0.70 | 8.14 | 108.68 *** | 62.44 *** | 25.90 ± 3.24 | |
| Ph | 152.53 *** | 0.62 | 3.44 | 33.74 *** | 104.27 *** | 16.74 ± 2.21 | |
| Surv | 12.31 *** | 34.31 | 104.5 | 680.76 *** | 533.53 ** | 49.97 ± 13.73 | |
| Zărnești | Dbh | 0.44 ns | 0.14 | 22.24 | 49.73 * | 82.91 * | 21.14 ± 4.76 |
| Th | 21.55 *** | 0.6 | 11.12 | 50.90 *** | 206.67 *** | 21.43 ± 3.53 | |
| Ph | 98.84 *** | 1.16 | 6.31 | 44.59 *** | 322.91 *** | 10.96 ± 2.95 | |
| Surv | 0 ns | 0 | 178.2 | 365.38 * | 504.92 ns | 40.66 ± 13.71 | |
| Turda | Dbh | 0 ns | 0 | 30.12 | 151.61 *** | 25.25 ns | 21.57 ± 5.57 |
| Th | 14.35 *** | 0.49 | 9.45 | 64.60 *** | 58.99 ** | 21.48 ± 3.28 | |
| Ph | 55.53 *** | 0.94 | 7.04 | 18.22 * | 24.78 * | 11.21 ± 2.86 | |
| Surv | 0.16 ns | 4.5 | 160.27 | 880.02 *** | 888.61 ** | 36.78 ± 14.22 |
| Trait | LRTg | LRTge | Vg | Vge | Vr | MS Env | MS B (Env) | Mean ± SD |
|---|---|---|---|---|---|---|---|---|
| Dbh | 39.55 *** | 0.12 ns | 1.25 | 0.06 | 27.17 | 917.8 *** | 42.72 ns | 22.30 ± 5.46 |
| Th | 63.70 *** | 40.93 *** | 1.18 | 0.47 | 9.45 | 2109 *** | 108 *** | 23.23 ± 3.99 |
| Ph | 43.04 *** | 173.02 *** | 0.9 | 0.79 | 5.36 | 2134 *** | 149.3 *** | 13.34 ± 3.83 |
| Surv | 33.6 *** | 0.45 ns | 35.39 | 4.48 | 150.1 | 3859 *** | 480.9 *** | 42.47 ± 14.95 |
| Traits | Site Mean | Selected Provenances Mean | Selection Differential% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dorna Candrenilor | Zarnesti | Turda | Dorna Candrenilor | Zarnesti | Turda | Dorna Candrenilor | Zarnesti | Turda | |
| Diameter at breast height (cm) | 23.8 | 21.1 | 21.6 | 25.7 | 21.5 | 22.2 | 8.05 | 1.62 | 2.85 |
| Total height (m) | 25.9 | 21.4 | 21.5 | 28.1 | 22.7 | 22.8 | 8.39 | 5.86 | 6.1 |
| Pruned height (m) | 16.7 | 11 | 11.2 | 18.2 | 12.8 | 12.4 | 8.97 | 16.5 | 10.3 |
| Survival (%) | 50 | 40.7 | 36.8 | 56 | 40.7 | 40.2 | 12 | 0.175 | 9.29 |
| Traits | Site Mean | Selected Provenances Mean | Selection Differential% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dorna Candrenilor | Zarnesti | Turda | Dorna Candrenilor | Zarnesti | Turda | Dorna Candrenilor | Zarnesti | Turda | |
| Diameter at breast height (cm) | 23.8 | 21.1 | 21.6 | 25.47 | 22.45 | 23.17 | 7.02 | 6.42 | 7.25 |
| Total height (m) | 25.9 | 21.4 | 21.5 | 27.68 | 23.24 | 22.97 | 6.87 | 8.61 | 6.86 |
| Pruned height (m) | 16.7 | 11.0 | 11.2 | 17.99 | 12.74 | 12.27 | 7.71 | 15.83 | 9.58 |
| Survival (%) | 50 | 40.7 | 36.8 | 57.21 | 44.79 | 43.35 | 14.42 | 10.04 | 17.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexandru, A.-M.; Mihai, G.; Stoica, E.; Curtu, A.L. Multi-Trait Selection and Stability in Norway Spruce (Picea abies) Provenance Trials in Romania. Forests 2023, 14, 456. https://doi.org/10.3390/f14030456
Alexandru A-M, Mihai G, Stoica E, Curtu AL. Multi-Trait Selection and Stability in Norway Spruce (Picea abies) Provenance Trials in Romania. Forests. 2023; 14(3):456. https://doi.org/10.3390/f14030456
Chicago/Turabian StyleAlexandru, Alin-Madalin, Georgeta Mihai, Emanuel Stoica, and Alexandru Lucian Curtu. 2023. "Multi-Trait Selection and Stability in Norway Spruce (Picea abies) Provenance Trials in Romania" Forests 14, no. 3: 456. https://doi.org/10.3390/f14030456






