Climate Seasonality Mediates Global Patterns of Foliar Carbon and Nitrogen Isotopes
Abstract
1. Introduction
2. Materials and Methods
2.1. Foliar δ13C and δ15N Values of Woody Plants
2.2. Climate and Soil Properties Matched with Each Site
2.3. Data Analyses
2.4. Determining Whether Climate Variables Are Related to Foliar Δ13C and δ15N
2.5. Determining Whether Climate Seasonality Explains Foliar Δ13C and δ15N
3. Results
3.1. Effect of Annual Climate on Foliar Δ13C and δ15N
3.2. Effect of Growth-Season Climate Variables on Foliar Δ13C and δ15N
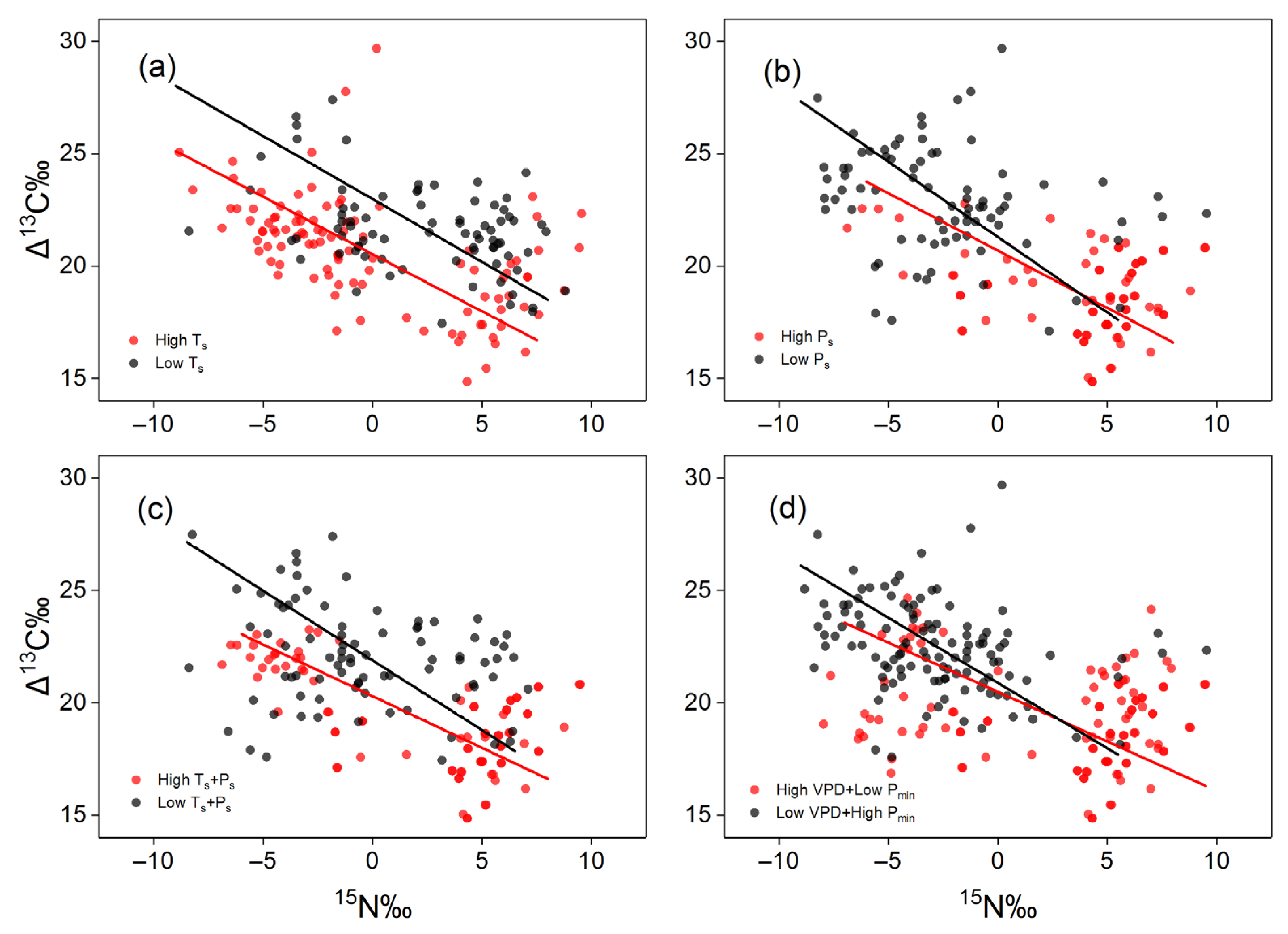
3.3. Effect of Seasonality on Determining Foliar Δ13C and δ15N
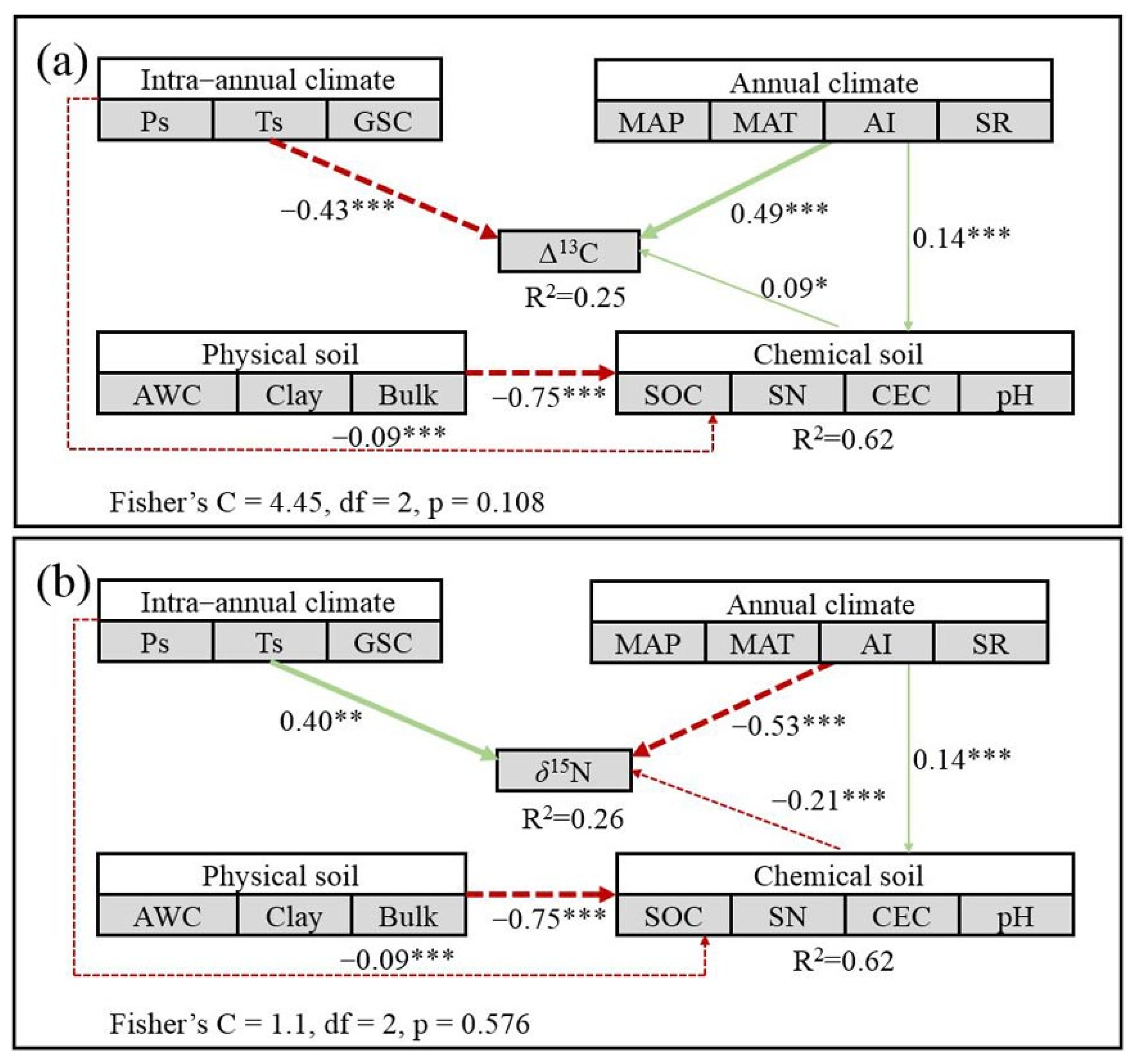
3.4. Effect of Intra-Annual Climate Compared with Annual Climate and Soil Properties
4. Discussion
4.1. Effect of Climate Seasonality on Foliar Δ13C and δ15N
4.2. Effect of Growth-Season Climate on Foliar Δ13C and δ15N
4.3. Knowledge Gap and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berry, J.A.; Beerling, D.J.; Franks, P.J. Stomata: Key players in the earth system, past and present. Curr. Opin. Plant. Biol. 2010, 13, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Chapin, F.S., III; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors: Physiological ecology provides tools for studying how interacting environmental resources control plant growth. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Piao, S.; Sun, Y.; Ciais, P.; Cheng, L.; Mao, J.; Poulter, B.; Shi, X.; Zeng, Z.; Wang, Y. Change in terrestrial ecosystem water-use efficiency over the last three decades. Glob. Chang. Biol. 2015, 21, 2366–2378. [Google Scholar] [CrossRef] [PubMed]
- Knauer, J.; Zaehle, S.; Reichstein, M.; Medlyn, B.E.; Forkel, M.; Hagemann, S.; Werner, C. The response of ecosystem water-use efficiency to rising atmospheric CO2 concentrations: Sensitivity and large-scale biogeochemical implications. New Phytol. 2017, 213, 1654–1666. [Google Scholar] [CrossRef]
- Birami, B.; Naegele, T.; Gattmann, M.; Preisler, Y.; Gast, A.; Arneth, A.; Ruehr, N.K. Hot drought reduces the effects of elevated CO2 on tree water-use efficiency and carbon metabolism. New Phytol. 2020, 226, 1607–1621. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M.J.S. The carbon and nitrogen isotope composition of Australian grasses in relation to climate. Funct. Ecol. 2009, 23, 1040–1049. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, T.; Lu, X.; Ellsworth, D.S.; BassiriRad, H.; You, C.; Wang, D.; He, P.; Deng, Q.; Liu, H.; et al. Global response patterns of plant photosynthesis to nitrogen addition: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3585–3600. [Google Scholar] [CrossRef]
- Cornwell, W.K.; Wright, I.J.; Turner, J.; Maire, V.; Barbour, M.M.; Cernusak, L.A.; Dawson, T.; Ellsworth, D.; Farquhar, G.D.; Griffiths, H.; et al. Climate and soils together regulate photosynthetic carbon isotope discrimination within C-3 plants worldwide. Glob. Ecol. Biogeogr. 2018, 27, 1056–1067. [Google Scholar] [CrossRef]
- Farquhar, G.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Farquhar, G.; O’Leary, M.; Berry, J. On the relationship between carbon isotope discrimination and the intercellular carbon dioxide concentration in leaves. Funct. Plant Biol. 1982, 9, 121–137. [Google Scholar] [CrossRef]
- Klaus, V.H.; Hoelzel, N.; Prati, D.; Schmitt, B.; Schoening, I.; Schrumpf, M.; Solly, E.F.; Haensel, F.; Fischer, M.; Kleinebecker, T. Plant diversity moderates drought stress in grasslands: Implications from a large real-world study on 13C natural abundances. Sci. Total Environ. 2016, 566, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Elmore, A.J.; Aidar, M.P.M.; Bustamante, M.; Dawson, T.E.; Hobbie, E.A.; Kahmen, A.; Mack, M.C.; McLauchlan, K.K.; Michelsen, A.; et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009, 183, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Aranibar, J.; Bauters, M.; Boeckx, P.; Crowley, B.E.; Dawes, M.A.; Delzon, S.; Fajardo, A.; et al. Isotopic evidence for oligotrophication of terrestrial ecosystems. Nat. Ecol. Evol. 2018, 2, 1735–1744. [Google Scholar] [CrossRef]
- Craine, J.M.; Elmore, A.J.; Wang, L.; Augusto, L.; Baisden, W.T.; Brookshire, E.N.; Cramer, M.D.; Hasselquist, N.J.; Hobbie, E.A.; Kahmen, A.; et al. Convergence of soil nitrogen isotopes across global climate gradients. Sci. Rep. 2015, 5, 8280. [Google Scholar] [CrossRef]
- Elmore, A.J.; Nelson, D.M.; Craine, J.M. Earlier springs are causing reduced nitrogen availability in North American eastern deciduous forests. Nat. Plants 2016, 2, 16133. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Hogberg, P. Nitrogen isotopes link mycorrhizal fungi and plants to nitrogen dynamics. New Phytol. 2012, 196, 367–382. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef]
- Zhang, Q.; Ficklin, D.L.; Manzoni, S.; Wang, L.; Way, D.; Phillips, R.P.; Novick, K.A. Response of ecosystem intrinsic water use efficiency and gross primary productivity to rising vapor pressure deficit. Environ. Res. Lett. 2019, 14, 074023. [Google Scholar] [CrossRef]
- Hung Dinh, V.; Kwak, J.-H.; Lee, K.-S.; Lim, S.-S.; Matsushima, M.; Chang, S.X.; Lee, K.-H.; Choi, W.-J. Foliar chemistry and tree ring delta C-13 of Pinus densiflora in relation to tree growth along a soil pH gradient. Plant Soil 2013, 363, 101–112. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, L.; Guan, W.; Yu, F.-H.; van Kleunen, M. Latitudinal and longitudinal clines of phenotypic plasticity in the invasive herb Solidago canadensis in China. Oecologia 2016, 182, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.C. The latitudinal gradient in geographical range: How so many species coexist in the tropics. Am. Nat. 1989, 133, 240–256. [Google Scholar] [CrossRef]
- Trabucco, A.; Zomer, R.J.; Global Aridity Index and Potential Evapo-Transpiration (ET0) Climate Database v2. CGIAR Consortium for Spatial Information (CGIAR-CSI). 2019. Available online: https://cgiarcsi.community (accessed on 24 January 2019).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Campbell, J.N. An Introduction to Environmental Biophysics; Springer: New York, NY, USA, 1998. [Google Scholar]
- Batjes, N.H. World Soil Property Estimates for Broad-Scale Modelling (WISE30sec, ver. 1.0). Report 2015/01, ISRIC—World Soil Information, Wageningen. 2015. Available online: http://www.isric.org/data/datadownload (accessed on 1 July 2015).
- Ehleringer, J.R.; Hall, A.E.; Farquhar, G.D. Introduction: Water use in relation to productivity. In Stable Isotopes and Plant Carbon–Water Relations; Academic Press: New York, NY, USA, 1993; pp. 3–8. [Google Scholar]
- Feng, X. Long-term ci/ca response of trees in Western North America to atmospheric CO2 concentration derived from carbon isotope chronologies. Oecologia 1998, 117, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Hogberg, P.; Hogbom, L.; Schinkel, H.; Hogberg, M.; Johannisson, C.; Wallmark, H. 15N abundance of surface soils, roots and mycorrhizas in profiles of European forest soils. Oecologia 1996, 108, 207–214. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environmentfor Statistical Computing (Version 3.6.1); R Foundation for Statistical Computing: Vienna, Austria, 2019; Available online: https://www.R-project.org/ (accessed on 1 July 2015).
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. smatr 3-an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modeling in R for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Choat, B.; Brodribb, T.J.; Brodersen, C.R.; Duursma, R.A.; López, R.; Medlyn, B.E. Triggers of tree mortality under drought. Nature 2018, 558, 531–539. [Google Scholar] [CrossRef]
- Hess, L.J.T.; Austin, A.T. Pinus ponderosa alters nitrogen dynamics anddiminishes the climate footprint in natural ecosystems of Patagonia. J. Ecol. 2014, 102, 610–621. [Google Scholar] [CrossRef]
- Hallik, L.; Niinemets, Ü.; Wright, I.J. Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol. 2009, 184, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.R.; Lambers, H. Soil-plant-atmosphere interactions: Structure, function, and predictive scaling for climate change mitigation. Plant Soil 2021, 461, 5–27. [Google Scholar] [CrossRef]
- Shan, Y.; Huang, M.; Suo, L.; Zhao, X.; Wu, L. Composition and variation of soil δ15N stable isotope in natural ecosystems. Catena 2019, 183, 104236. [Google Scholar] [CrossRef]
- Yan, G.; Han, S.; Zhou, M.; Sun, W.; Huang, B.; Wang, H.; Xing, Y.; Wang, Q. Variations in the natural 13C and 15N abundance of plants and soils under long-term N addition and precipitation reduction: Interpretation of C and N dynamics. For. Ecosyst. 2020, 7, 49. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]
- Hobbie, E.A.; Hobbie, J.E. Natural abundance of 15N in nitrogen-limited forests and tundra can estimate nitrogen cycling through mycorrhizal fungi: A review. Ecosystems 2008, 11, 815–830. [Google Scholar] [CrossRef]
- Kohzu, A.; Matsui, K.; Yamada, T.; Sugimoto, A.; Fujita, N. Significance of rooting depth in mire plants: Evidence from natural 15N abundance. Ecol. Res. 2003, 18, 257–266. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Koba, K.; Sasaki, Y.; Fang, Y.T.; Yoh, M. The natural abundance of 15N in plant and soil-available nitrogen indicates a shift of main plant nitrogen resources to NO3 from NH4 along the nitrogen leaching gradient. Rapid Commun. Mass Spectrom. 2010, 24, 1001–1008. [Google Scholar] [CrossRef]
- Bol, R.; Ostle, N.J.; Chenu, C.C.; Petzke, K.-J.; Werner, R.A.; Balesdent, J. Long term changes in the distribution and delta N-15 values of individual soil amino acids in the absence of plant and fertiliser inputs. Isot. Environ. Healt. 2004, 40, 243–256. [Google Scholar] [CrossRef]
- Yano, Y.; Shaver, G.R.; Giblin, A.E.; Rastetter, E.B. Depleted 15N in hydrolysable-N of arctic soils and its implication for mycorrhizal fungi–plant interaction. Biogeochemistry 2010, 97, 183–194. [Google Scholar] [CrossRef]
- Bachofen, C.; D’Odorico, P.; Buchmann, N. Light and VPD gradients drive foliar nitrogen partitioning and photosynthesis in the canopy of European beech and silver fir. Oecologia 2020, 192, 323–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ye, Q.; Gleason, S.M.; He, P.; Yin, D. Weak tradeoff between xylem hydraulic efficiency and safety: Climatic seasonality matters. New Phytol. 2021, 229, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Oren, R.; Sperry, J.S.; Katul, G.G.; Pataki, D.E.; Ewers, B.E.; Phillips, N.; Schäfer, K.V.R. Survey and synthesis of intra- and interspecific variation in stomatal sensitivity to vapour pressure deficit. Plant Cell Environ. 1999, 22, 1515–1526. [Google Scholar] [CrossRef]
- de Carcer, P.S.; Vitasse, Y.; Penuelas, J.; Jassey, V.E.J.; Buttler, A.; Signarbieux, C. Vapor-pressure deficit and extreme climatic variables limit tree growth. Glob. Chang. Biol. 2018, 24, 1108–1122. [Google Scholar] [CrossRef]
- Ocheltree, T.W.; Nippert, J.B.; Prasad, P.V.V. Stomatal responses to changes in vapor pressure deficit reflect tissue-specific differences in hydraulic conductance. Plant Cell Environ. 2014, 37, 132–139. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, L.; Xu, Q.; Lundgren, M.R.; Yang, K.; Zhao, P.; Ye, Q. Ecophysiological responses of two closely related Magnoliaceae genera to seasonal changes in subtropical China. J. Plant Ecol. 2018, 11, 434–444. [Google Scholar] [CrossRef]
- Klein, T. The variability of stomatal sensitivity to leaf water potential across tree species indicates a continuum between isohydric and anisohydric behaviours. Funct. Ecol. 2014, 28, 1313–1320. [Google Scholar] [CrossRef]
- Gleason, S.M.; Westoby, M.; Jansen, S.; Choat, B.; Hacke, U.G.; Pratt, R.B.; Bhaskar, R.; Brodribb, T.J.; Bucci, S.J.; Cao, K.-F.; et al. Weak tradeoff between xylem safety and xylem-specific hydraulic efficiency across the world’s woody plant species. New Phytol. 2016, 209, 123–136. [Google Scholar] [CrossRef]
- Gleason, S.M.; Butler, D.W.; Waryszak, P. Shifts in leaf and stem hydraulic traits across aridity gradients in eastern Australia. Int. J. Plant Sci. 2013, 174, 1292–1301. [Google Scholar] [CrossRef]
- Ens, E.; Hutley, L.B.; Rossiter-Rachor, N.A.; Douglas, M.M.; Setterfield, S.A. Resource-use efficiency explains grassy weed invasion in a low-resource savanna in north Australia. Front. Plant Sci. 2015, 6, 560. [Google Scholar] [CrossRef]
- Idol, T.; Baker, P.J.; Meason, D. Indicators of forest ecosystem productivity and nutrient status across precipitation and temperature gradients in Hawaii. J. Trop. Ecol. 2007, 23, 693–704. [Google Scholar] [CrossRef]
- Amissah, L.; Mohren, G.M.J.; Kyereh, B.; Agyeman, V.K.; Poorter, L. Rainfall seasonality and drought performance shape the distribution of tropical tree species in Ghana. Ecol. Evol. 2018, 8, 8582–8597. [Google Scholar] [CrossRef]
- Kreft, H.; Jetz, W. Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. USA 2007, 104, 5925–5930. [Google Scholar] [CrossRef]
- Nolan, C.; Overpeck, J.T.; Allen, J.R.M.; Anderson, P.M.; Betancourt, J.L.; Binney, H.A.; Brewer, S.; Bush, M.B.; Chase, B.M.; Cheddadi, R.; et al. Past and future global transformation of terrestrial ecosystems under climate change. Science 2018, 361, 920–923. [Google Scholar] [CrossRef]
- Tharammal, T.; Bala, G.; Devaraju, N.; Nemani, R. A review of the major drivers of the terrestrial carbon uptake: Model-based assessments, consensus, and uncertainties. Environ. Res. Lett. 2019, 14, 093005. [Google Scholar] [CrossRef]
- Amundson, R.; Berhe, A.A.; Hopmans, J.W.; Olson, C.; Sztein, A.E.; Sparks, D.L. Soil and human security in the 21st century. Science 2015, 348, 1261071. [Google Scholar] [CrossRef]
- Liles, G.C.; Maxwell, T.M.; Silva, L.C.R.; Zhang, J.W.; Horwath, W.R. Two decades of experimental manipulation reveal potential for enhanced biomass accumulation and water use efficiency in Ponderosa Pine plantations across climate gradients. J. Geophys. Res. Biogeo. 2019, 124, 2321–2334. [Google Scholar] [CrossRef]
- Franklin, J.F.; Johnson, K.N. Lessons in policy implementation from experiences with the Northwest Forest Plan, USA. Biodivers. Conserv. 2014, 23, 3607–3613. [Google Scholar] [CrossRef]
- Perry, T.D.; Jones, J.A. Summer streamflow deficits from regenerating Douglas-fir forest in the Pacific Northwest, USA. Ecohydrology 2017, 10, e1790. [Google Scholar] [CrossRef]
- Stevens, J.T.; Safford, H.D.; Harrison, S.; Latimer, A.M. Forest disturbance accelerates thermophilization of understory plant communities. J. Ecol. 2015, 103, 1253–1263. [Google Scholar] [CrossRef]
- Brice, M.-H.; Cazelles, K.; Legendre, P.; Fortin, M.-J. Disturbances amplify tree community responses to climate change in the temperate-boreal ecotone. Glob. Ecol. Biogeogr. 2019, 28, 1668–1681. [Google Scholar] [CrossRef]
- Hallema, D.W.; Sun, G.; Caldwell, P.V.; Norman, S.P.; Cohen, E.C.; Liu, Y.; Bladon, K.D.; McNulty, S.G. Burned forests impact water supplies. Nat. Commun. 2018, 9, 1307. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, T.M.; Silva, L.C.R.; Horwath, W.R. Integrating effects of species composition and soil properties to predict shifts in montane forest carbon-water relations. Proc. Natl. Acad. Sci. USA 2018, 115, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Giguere-Croteau, C.; Boucher, E.; Bergeron, Y.; Girardin, M.P.; Drobyshev, I.; Silva, L.C.R.; Helie, J.-F.; Garneau, M. North America’s oldest boreal trees are more efficient water users due to increased CO2, but do not grow faster. Proc. Natl. Acad. Sci. USA 2019, 116, 2749–2754. [Google Scholar] [CrossRef] [PubMed]
- HÖGberg, P. 15N natural abundance in soil-plant systems. New Phytol. 1997, 137, 179–203. [Google Scholar] [CrossRef]
- Pardo, L.H.; Templer, P.H.; Goodale, C.L.; Duke, S.; Groffman, P.M.; Adams, M.B.; Boeckx, P.; Boggs, J.; Campbell, J.; Colman, B.; et al. Regional assessment of N saturation using foliar and root delta N-15. Biogeochemistry 2006, 80, 143–171. [Google Scholar] [CrossRef]
- Dorado Linan, I.; Gutierrez, E.; Heinrich, I.; Andreu-Hayles, L.; Muntan, E.; Campelo, F.; Helle, G. Age effects and climate response in trees: A multi-proxy tree-ring test in old-growth life stages. Eur. J. For. Res. 2012, 131, 933–944. [Google Scholar] [CrossRef]
- Fang, Y.; Yoh, M.; Koba, K.; Zhu, W.; Takebayashi, Y.; Xiao, Y.; Lei, C.; Mo, J.; Zhang, W.; Lu, X. Nitrogen deposition and forest nitrogen cycling along an urban-rural transect in southern China. Glob. Chang. Biol. 2011, 17, 872–885. [Google Scholar] [CrossRef]
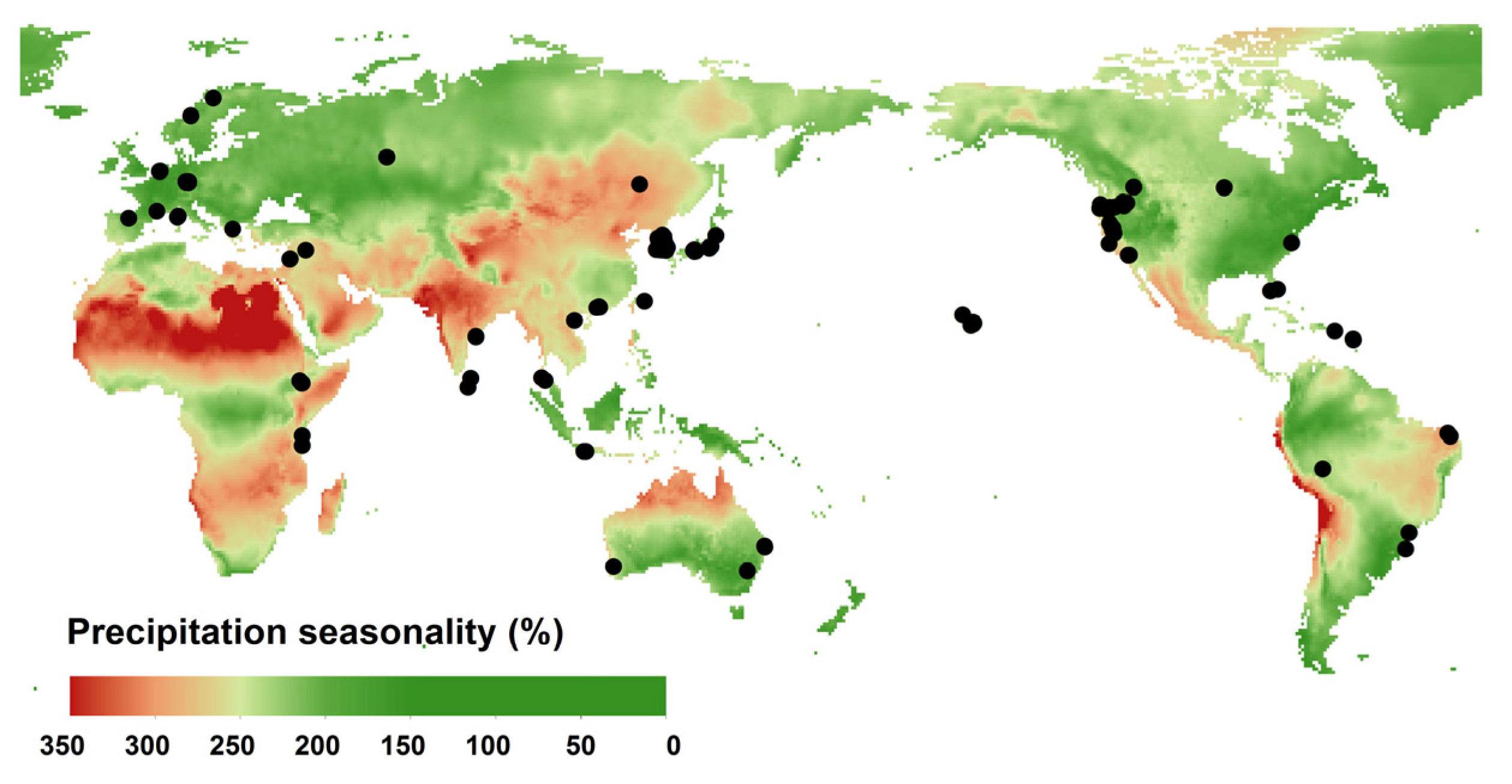

| Full Name | Unit | Abbreviation | Note |
|---|---|---|---|
| Annual climatic variables | |||
| Elevation | m a.s.l. | El | |
| Aridity index | unitless | AI | MAP/PET |
| Mean annual precipitation | mm | MAP | |
| Precipitation seasonality | unitless | Ps | SD/mean of 12 months |
| Precipitation of wettest quarter | mm | Pwet | |
| Precipitation of driest quarter | mm | Pdry | |
| Precipitation of wettest month | mm | Pmax | |
| Precipitation of driest month | mm | Pmin | |
| Precipitation of warmest quarter | mm | Pwarm | |
| Precipitation of coldest quarter | mm | Pcold | |
| Mean annual temperature | °C | MAT | |
| Temperature seasonality | unitless | Ts | SD of monthly temperature |
| Temperature of warmest month | °C | Tmax | |
| Temperature of coldest month | °C | Tmin | |
| Temperature of warmest quarter | °C | Twarm | |
| Temperature of coldest quarter | °C | Tcold | |
| Temperature of wettest quarter | °C | Twet | |
| Temperature of driest quarter | °C | Tdry | |
| Vapor pressure deficit | kPa | VPD | |
| Potential evapotranspiration | mm·y−1 | PET | |
| Mean annual solar radiation | kJ·m−2·d−1 | SR | |
| Monthly climatic variables | |||
| Monthly temperature | °C | T1~T12 | 1~12 corresponding to 12 months |
| Monthly precipitation | mm | P1~P12 | months |
| Monthly solar radiation | kJ·m−2·d−1 | SR1~SR12 | |
| Monthly vapor pressure deficit | kPa | VPD1~VPD12 | |
| Monthly potential evapotranspiration | mm·yr−1 | PET1~PET12 | |
| Physical soil | |||
| Soil available water capacity | cm·m−1 | AWC | |
| Soil clay | % | Clay | |
| Soil bulk density | kg·dm−3 | Bulk | |
| Chemical soil | |||
| Soil pH | unitless | pH | |
| Soil organic carbon | g·kg−1 | SOC | |
| Soil total nitrogen | g·kg−1 | SN | |
| Soil cation exchange capacity | cmolc·kg−1 | CEC |
| High Seasonality | Low Seasonality | Model Comparison | Changing Patterns | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Slope | Inter. | R2 | p | Slope | Inter. | R2 | p | A-Slope | B-Inter. | C-Shift | ||
| Ts | −0.51 | 20.53 | 0.28 | <0.001 | −0.56 | 22.98 | 0.21 | <0.001 | LR= 0.4528 | F = 45.33 | F = 0.01 | High Ts group has a smaller y intercept |
| p = 0.501 | p < 0.001 | p = 0.91 | ||||||||||
| Ps | −0.51 | 20.69 | 0.08 | 0.008 | −0.67 | 21.29 | 0.04 | 0.01 | LR = 3.294 | F = 1.172 | F = 153.6 | High Ps group has a more positive shift along x axis |
| p = 0.07 | p = 0.28 | p < 0.001 | ||||||||||
| Ts and Ps | −0.46 | 20.29 | 0.36 | <0.001 | −0.62 | 21.88 | 0.14 | 0.005 | LR = 5 | F = 13.06 | F = 49.89 | High Ts and Ps group has a smaller intercept, a less negative slope, and a more positive shift along x axis |
| p = 0.03 | p < 0.001 | p < 0.001 | ||||||||||
| VPD and Pmin | −0.44 | 20.48 | 0.05 | 0.02 | −0.58 | 20.88 | 0.10 | <0.001 | LR = 4.59 | F = 1.273 | F = 152.5 | High VPD and Pmin group has a less negative slope and a more positive shift along x axis |
| p = 0.03 | p = 0.26 | p < 0.001 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, L.; Li, Y.; Zheng, X. Climate Seasonality Mediates Global Patterns of Foliar Carbon and Nitrogen Isotopes. Forests 2023, 14, 461. https://doi.org/10.3390/f14030461
Du L, Li Y, Zheng X. Climate Seasonality Mediates Global Patterns of Foliar Carbon and Nitrogen Isotopes. Forests. 2023; 14(3):461. https://doi.org/10.3390/f14030461
Chicago/Turabian StyleDu, Lan, Yan Li, and Xinjun Zheng. 2023. "Climate Seasonality Mediates Global Patterns of Foliar Carbon and Nitrogen Isotopes" Forests 14, no. 3: 461. https://doi.org/10.3390/f14030461
APA StyleDu, L., Li, Y., & Zheng, X. (2023). Climate Seasonality Mediates Global Patterns of Foliar Carbon and Nitrogen Isotopes. Forests, 14(3), 461. https://doi.org/10.3390/f14030461








