Effects of Pruning on Vegetation Growth and Soil Properties in Poplar Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. StudyArea
2.2. Experimental Design
2.3. Field Investigation
2.3.1. Growth Characteristic Investigation
2.3.2. Soil Sampling and Laboratory Analysis
2.4. Data Calculations and Statistical Analysis
2.4.1. Data Calculations
2.4.2. Statistical Analysis
3. Results
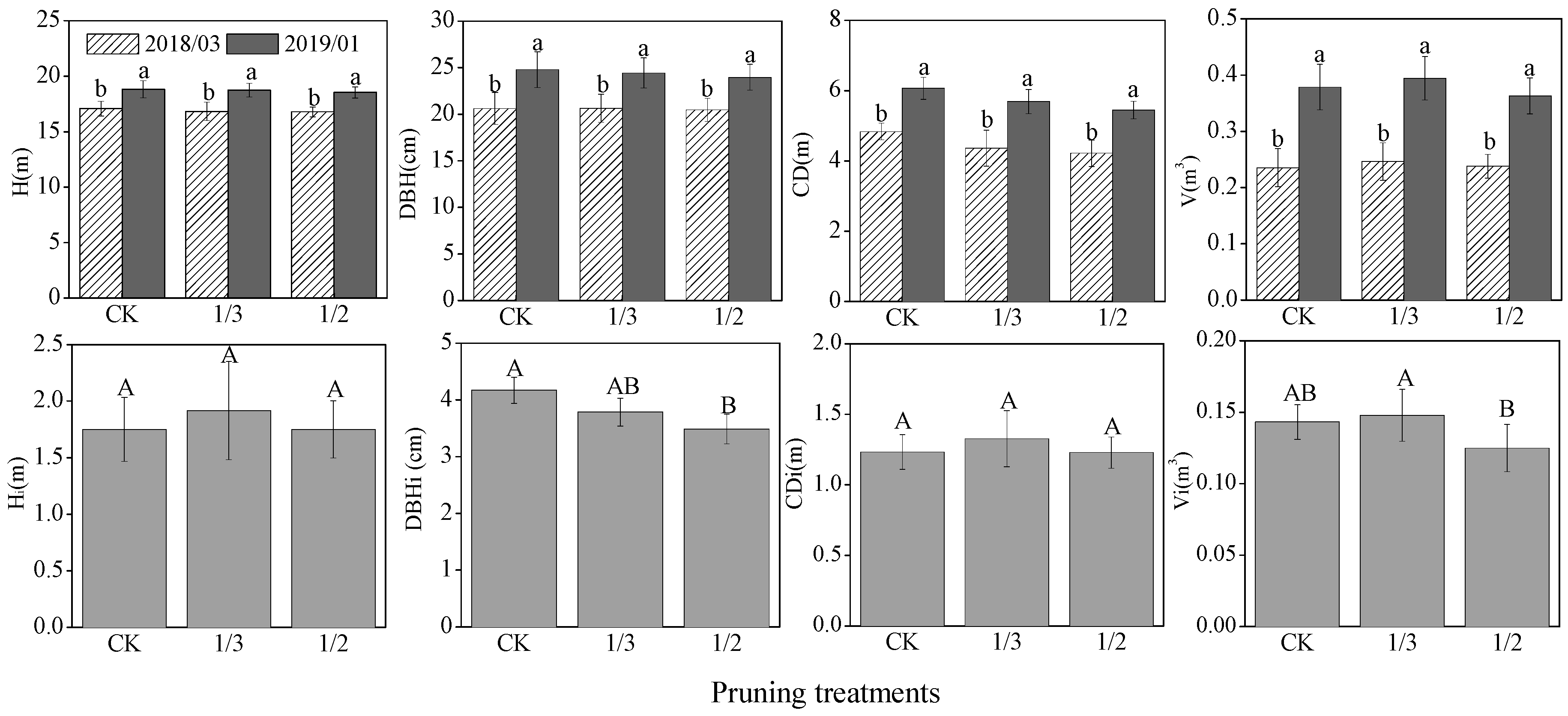
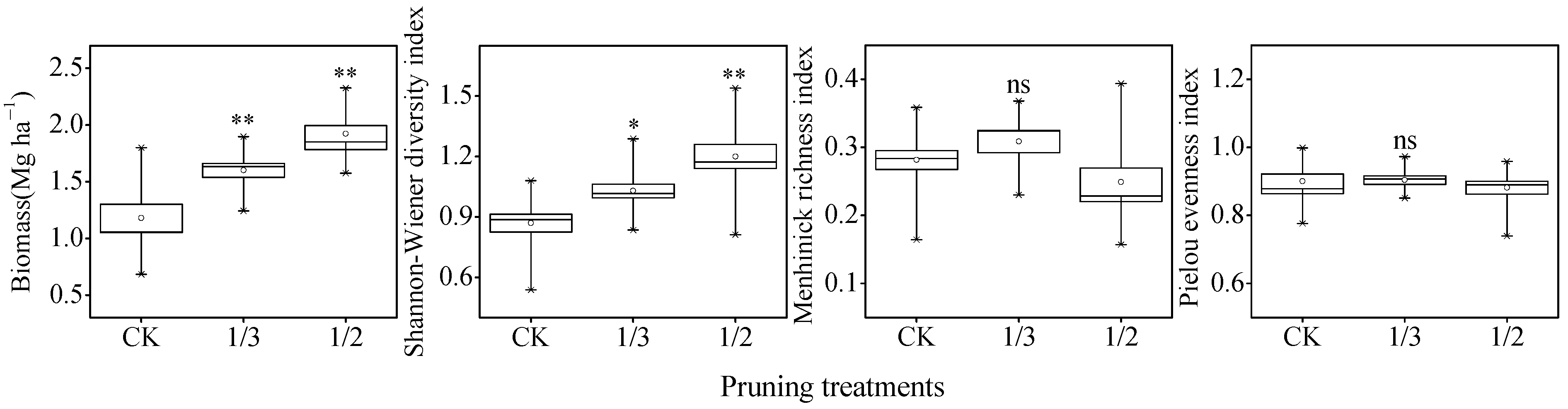
3.1. Growth of Poplar and Understory Vegetation Diversity
3.2. Soil Properties
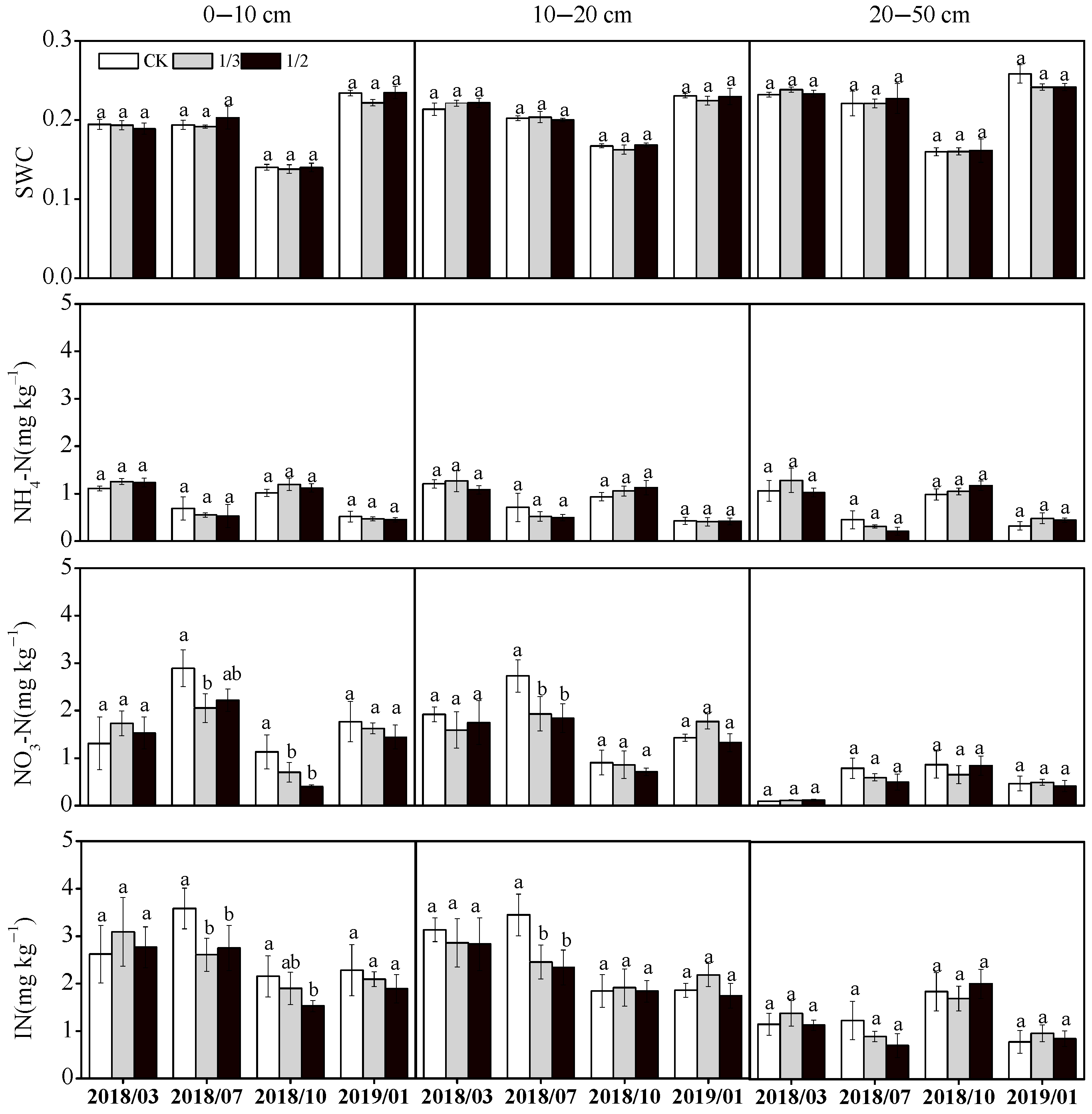
3.2.1. Soil Water and Inorganic Nitrogen Contents
3.2.2. Soil Microbial Biomass
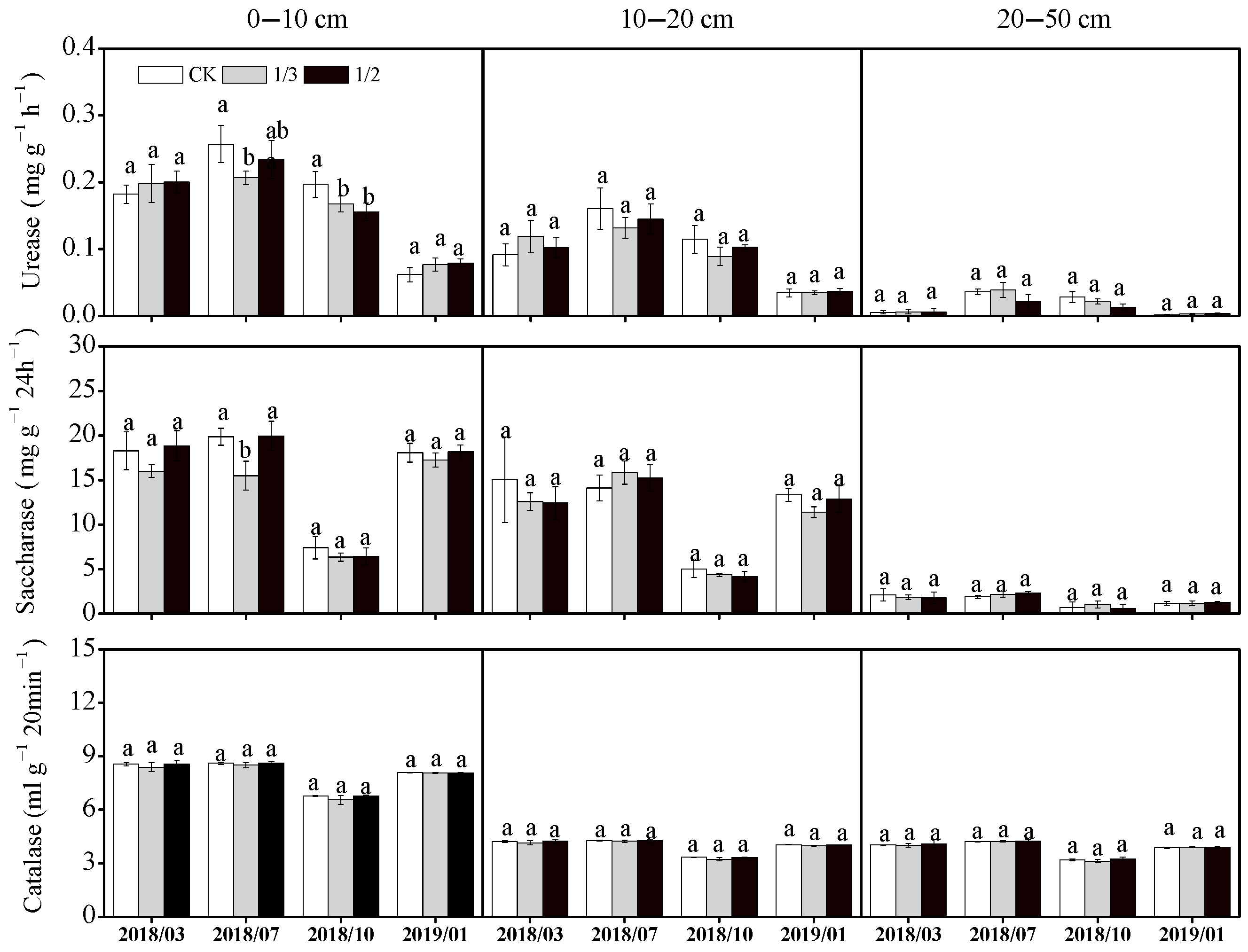
3.2.3. Soil Enzyme Activities
3.2.4. Nonmetric Multidimensional Scaling Analysis of Soil Properties and Enzyme Activities
3.3. Relationship between Soil Properties and Soil Enzyme Activities
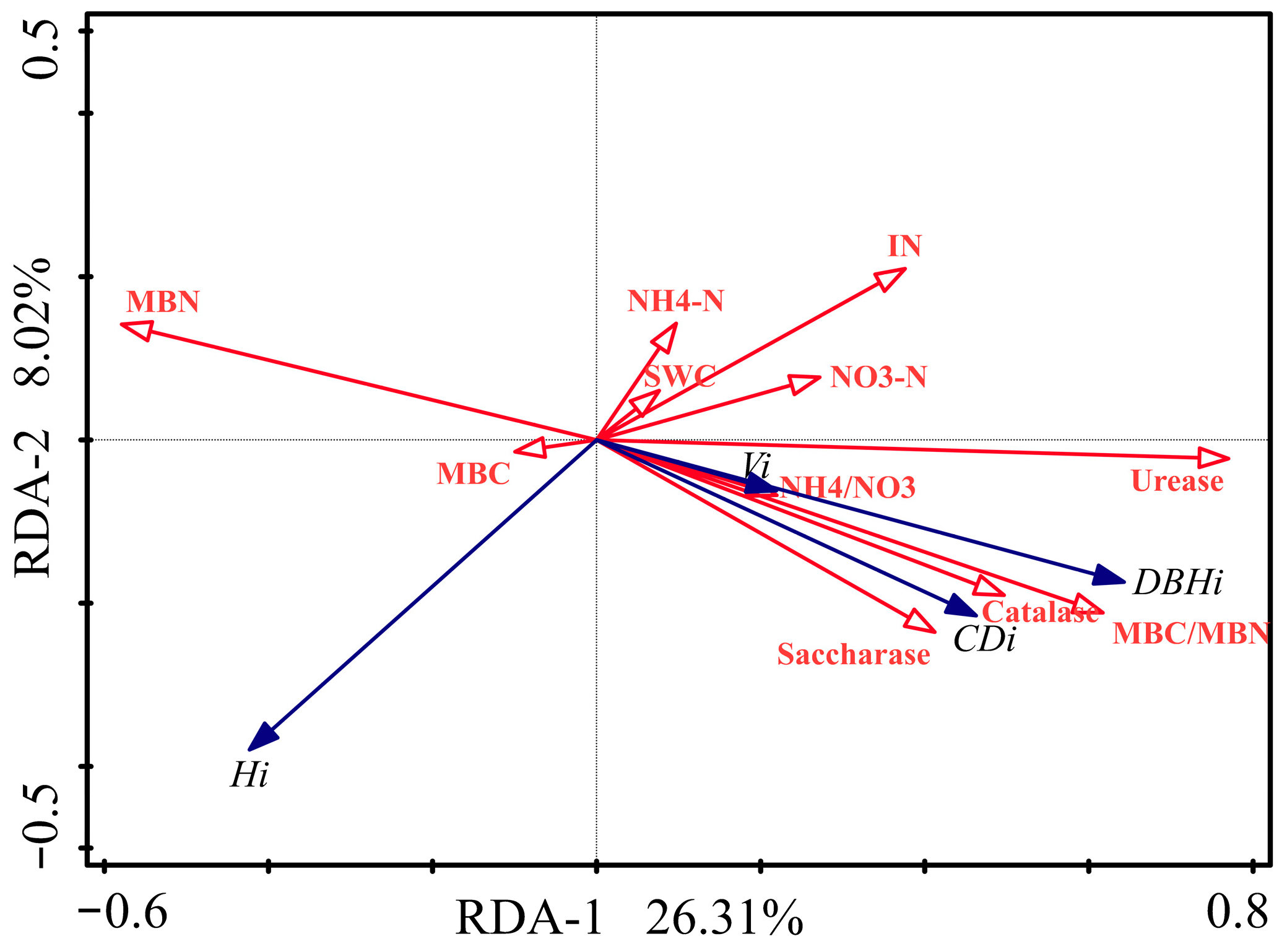
3.4. Relationships among Poplar Growth, Understory Vegetation Characteristics and Soil Properties
4. Discussion
4.1. Effects of Pruning Intensity on Poplar Growthand Understory Vegetation
4.2. Effects of Pruning Intensity on Soil Properties in Poplar Plantations
4.3. Relationships between Soil Properties and the Growth of Poplar Trees and Understory Vegetation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Liu, L.Q. Effects of pruning intensity on nonstructural carbohydrates of Populus alba × P. talassica in the arid desert region of Northwest China. J. For. Res. 2021, 32, 823–830. [Google Scholar] [CrossRef]
- Arnold, R.J.; Xie, Y.J.; Luo, J.Z.; Wang, H.R.; Midgley, S.J. A tale of two genera: Exotic Eucalyptus and Acacia species in China. 2. Plantation resource development. Int. Forest. Rev. 2020, 22, 153–168. [Google Scholar] [CrossRef]
- Maurin, V.; DesRochers, A. Physiological and growth responses to pruning season and intensity of hybrid poplar. For. Ecol. Manag. 2013, 304, 399–406. [Google Scholar] [CrossRef]
- Desrochers, A.; Maurin, V.; Tarroux, E. Production and role of epicormic shoots in pruned hybrid poplar: Effects of clone, pruning season and intensity. Ann. For. Sci. 2015, 72, 425–434. [Google Scholar] [CrossRef]
- Ishii, H.; Clement, J.P.; Shaw, D.C. Branch growth and crown form in old coastal Douglas-fir. For. Ecol. Manag. 2000, 131, 81–91. [Google Scholar] [CrossRef]
- Dănescu, A.; Ehring, A.; Bauhus, J.; Albrecht, A.; Hein, S. Modelling discoloration and duration of branch occlusion following green pruning in Acer pseudoplatanus and Fraxinus excelsior. For. Ecol. Manag. 2015, 335, 87–98. [Google Scholar] [CrossRef]
- Wang, C.S.; Hein, S.; Zhao, Z.G.; Guo, J.J.; Zeng, J. Branch occlusion and discoloration of Betula alnoides under artificial and natural pruning. For. Ecol. Manag. 2016, 375, 200–210. [Google Scholar] [CrossRef]
- York, R.A. Long-term taper and growth reductions following pruning intensity treatments in giant sequoia (Sequoiadendron giganteum). Can. J. For. Res. 2019, 49, 1189–1197. [Google Scholar] [CrossRef] [Green Version]
- Li, R.S.; Han, J.M.; Guan, X.; Chi, Y.G.; Zhang, W.D.; Chen, L.C.; Wang, Q.K.; Xu, M.; Yang, Q.P.; Wang, S.L. Crown pruning and understory removal did not change the tree growth rate in a Chinese fir (Cunninghamia lanceolata) plantation. For. Ecol. Manag. 2020, 464, 118056. [Google Scholar] [CrossRef]
- Binkley, D.; Laclau, J.P.; Sterba, H. Why one tree grows faster than another: Patterns of light use and light use efficiency at the scale of individual trees and stands. For. Ecol. Manag. 2013, 288, 1–4. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, Y.K.; Wang, X.; Nie, Z.Y.; Gao, Z.Y.; Zhang, L.L. Effects of branch removal on water use of rain-fed jujube (Ziziphus jujuba Mill.) plantations in Chinese semiarid Loess Plateau region. Agric. Water Manag. 2016, 178, 258–270. [Google Scholar] [CrossRef]
- Ortega-Vargas, E.; Burgueño-Ferreira, J.A.; Ávila-Reséndiz, C.; Campbell, W.B.; Jarillo-Rodríguez, J.; López-Ortiz, S. Morphological and physiological responses of Guazuma ulmifolia Lam. to different pruning dates. Agroforest. Syst. 2019, 93, 461–470. [Google Scholar] [CrossRef]
- Alcorn, P.J.; Bauhus, J.; Thomas, D.S.; James, R.N.; Smith, R.G.B.; Nicotra, A.B. Photosynthetic response to green crown pruning in young plantation-grown Eucalyptus pilularis and E. cloeziana. For. Ecol. Manag. 2008, 255, 3827–3838. [Google Scholar] [CrossRef]
- Ge, X.M.; Tian, Y.; Tang, L.Z. Nutrient distribution indicated whole-tree harvesting as a possible factor restricting the sustainable productivity of a poplar plantation system in China. PLoS ONE 2015, 10, e0125303. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, M.; Deng, J.Y.; Wang, B.T. Afforestation affects soil seed banks by altering soil properties and understory plants on the eastern Loess Plateau, China. Ecol. Indic. 2021, 126, 107670. [Google Scholar] [CrossRef]
- Tetemke, B.A.; Birhane, E.; Rannestad, M.M.; Eid, T. Species diversity and stand structural diversity of woody plants predominantly determine aboveground carbon stock of a dry Afromontane forest in Northern Ethiopia. For. Ecol. Manag. 2021, 500, 119634. [Google Scholar] [CrossRef]
- Danilović, M.; Sarić, R.; Cirović, V.; Pudja, V. The impact of pruning on tree development in poplar Populus × canadensis “I-214” plantations. iForest 2022, 15, 33–37. [Google Scholar] [CrossRef]
- Brookes, P.C.; Landman, A.; Pruden, G.; Jenkinson, D.S. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, S. Nitrate reductase, arginine deaminase, urease and dehydrogenase activities in natural soil (ridges with forest) and in cotton soil after acetamiprid treatments. Chemosphere 2008, 71, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, G.B.; Xue, S.; Song, Z.L. Rhizosphere soil microbial activity under different vegetation types on the Loess Plateau, China. Geoderma 2011, 161, 115–125. [Google Scholar] [CrossRef]
- Tang, L.Z.; Wu, L.; Ge, X.M.; Tian, Y.; Dun, X.J.; Liu, D.; Fang, S.Z. Comparative studies on the growth of different poplar clones on beach land of Yangtse River. For. Res. 2013, 26, 123–128. [Google Scholar] [CrossRef]
- Shaheen, H.; Ullah, Z.; Khan, S.M.; Harper, D.M. Species composition and community structure of western Himalayan moist temperate forests in Kashmir. For. Ecol. Manag. 2012, 278, 138–145. [Google Scholar] [CrossRef]
- Ares, A.; Brauer, D. Aboveground biomass partitioning in loblolly pine silvopastoral stands: Spatial configuration and pruning effects. For. Ecol. Manag. 2005, 219, 176–184. [Google Scholar] [CrossRef]
- Neilsen, W.A.; Pinkard, E.A. Effects of green pruning on growth of Pinus radiata. Can. J. For. Res. 2003, 33, 2067–2073. [Google Scholar] [CrossRef]
- Pinkard, E.A. Effects of pattern and severity of pruning on growth and branch development of pre-canopy closure Eucalyptus nitens. For. Ecol. Manag. 2002, 157, 217–230. [Google Scholar] [CrossRef]
- Pinkard, E.A. Physiological and growth responses related to pattern and severity of green pruning in young Eucalyptus globulus. For. Ecol. Manag. 2003, 182, 231–245. [Google Scholar] [CrossRef]
- Víquez, E.; Pérez, D. Effect of pruning on tree growth, yield, and wood properties of Tectona grandis plantations in Costa Rica. Silva Fenn. 2005, 39, 381–390. [Google Scholar] [CrossRef]
- Zeng, B. Aboveground biomass partitioning and leaf development of Chinese subtropical trees following pruning. For. Ecol. Manag. 2003, 173, 135–144. [Google Scholar] [CrossRef]
- Hevia, A.; Álvarez-González, J.G.; Majada, J. Comparison of pruning effects on tree growth, productivity and dominance of two major timber conifer species. For. Ecol. Manag. 2016, 374, 82–92. [Google Scholar] [CrossRef]
- Springmann, S.; Rogers, R.; Spiecker, H. Impact of artificial pruning on growth and secondary shoot development of wild cherry (Prunus avium L.). For. Ecol. Manag. 2011, 261, 764–769. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Warren, C.R.; Baker, T.G. Effect of thinning, pruning and nitrogen fertiliser application on transpiration, photosynthesis and water-use efficiency in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2012, 266, 286–300. [Google Scholar] [CrossRef]
- Forrester, D.I.; Collopy, J.J.; Beadle, C.L.; Baker, T.G. Effect of thinning, pruning and nitrogen fertiliser application on light interception and light-use efficiency in a young Eucalyptus nitens plantation. For. Ecol. Manag. 2013, 288, 21–30. [Google Scholar] [CrossRef]
- Lisboa, M.; Acuña, E.; Cancino, J.; Chao, F.; Muñoz, F.; Rodríguez, R.; Volker, P. Physiological response to pruning severity in Eucalyptus regnans plantations. New Forests 2014, 45, 753–764. [Google Scholar] [CrossRef]
- Beadle, C.; Barry, K.; Hardiyanto, E.; Irianto, R.; Junarto; Mohammed, C.; Rimbawanto, A. Effect of pruning Acacia mangium on growth, form and heart rot. For. Ecol. Manag. 2007, 238, 261–267. [Google Scholar] [CrossRef]
- Berrill, J.P.; Dagley, C.M.; Coppeto, S.A.; Gross, S.E. Curtailing succession: Removing conifers enhances understory light and growth of young aspen in mixed stands around Lake Tahoe, California and Nevada, USA. For. Ecol. Manag. 2017, 400, 511–522. [Google Scholar] [CrossRef]
- Schmidt, T.L.; Wardle, T.D. Impact of pruning eastern redcedar (Juniperus virginiana). West. J Appl. For. 2002, 17, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Torras, O.; Saura, S. Effects of silvicultural treatments on forest biodiversity indicators in the Mediterranean. For. Ecol. Manag. 2008, 255, 3322–3330. [Google Scholar] [CrossRef]
- Zhao, N.X.; Xu, Y.J.; Chen, L.P.; Jiang, M.; Dong, K.; Wang, J.L.; Gao, Y.B. Species identities impact the responses of intensity and importance of competition to the soil fertility changes. Glob. Ecol. Conserv. 2021, 27, e01519. [Google Scholar] [CrossRef]
- Ares, A.; Louis, D.S.; Brauer, D. Trends in tree growth and understory yield in silvopastoral practices with southern pines. Agroforest. Syst. 2003, 59, 27–33. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Ecroyd, C.E.; Leckie, A.C.; Kimberley, M.O. Diversity and succession of adventive and indigenous vascular understorey plants in Pinus radiata plantation forests in New Zealand. For. Ecol. Manag. 2003, 185, 307–326. [Google Scholar] [CrossRef]
- Halpern, C.B.; Halaj, J.; Evans, S.A.; Dovčiak, M. Level and pattern of overstory retention interact to shape long-term responses of understories to timber harvest. Ecol. Appl. 2012, 22, 2049–2064. [Google Scholar] [CrossRef]
- Roberts, M.W.; D’Amato, A.W.; Kern, C.C.; Palik, B.J. Long-term impacts of variable retention harvesting on ground-layer plant communities in Pinus resinosa forests. J. Appl. Ecol. 2016, 53, 1106–1116. [Google Scholar] [CrossRef] [Green Version]
- Lull, C.; Bautista, I.; Lidón, A.; del Campo, A.D.; González-Sanchis, M.; García-Prats, A. Temporal effects of thinning on soil organic carbon pools, basal respiration and enzyme activities in a Mediterranean Holm oak forest. For. Ecol. Manag. 2020, 464, 118088. [Google Scholar] [CrossRef]
- Ren, C.J.; Kang, D.; Wu, J.P.; Zhao, F.Z.; Yang, G.H.; Han, X.H.; Feng, Y.Z.; Ren, G.X. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Jin, S.S.; Wang, Y.K.; Wang, X.; Bai, Y.H.; Shi, L.G. Effect of pruning intensity on soil moisture and water use efficiency in jujube (Ziziphus jujube Mill.) plantations in the hilly Loess Plateau Region, China. J. Arid Land. 2019, 11, 446–460. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, A. Effect of tree canopy on the spatial distribution of soil nutrients in a Mediterranean Dehesa. Pedobiologia 2003, 47, 117–125. [Google Scholar] [CrossRef]
- Andivia, E.; Vázquez-Piqué, J.; Fernández, M.; Alejano, R. Litter production in holm oak trees subjected to different pruning intensities in Mediterranean dehesas. Agroforest. Syst. 2013, 87, 657–666. [Google Scholar] [CrossRef]
- Ou, Z.Y.; Pang, S.L.; He, Q.F.; Peng, Y.H.; Huang, X.R.; Shen, W.H. Effects of vegetation restoration and environmental factors on understory vascular plants in a typical karst ecosystem in southern China. Sci. Rep. 2020, 10, 12011. [Google Scholar] [CrossRef]
- Jennings, T.N.; Smith, J.E.; Cromack, K.; Sulzman, E.W.; McKay, D.; Caldwell, B.A.; Beldin, S.I. Impact of postfire logging on soil bacterial and fungal communities and soil biogeochemistry in a mixed-conifer forest in central Oregon. Plant Soil. 2012, 350, 393–411. [Google Scholar] [CrossRef]
- Pramanik, P.; Phukan, M.; Ghosh, S.; Goswami, A.J. Pruned tea bushes secrete more root exudates to influence microbiological properties in soil. Arch. Agron. Soil Sci. 2018, 64, 1172–1180. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.Y.; Zhang, C.; Wang, H.M.; Fu, X.L.; Chen, F.S.; Wan, S.Z.; Sun, X.M.; Wen, X.F.; Wang, J.F. Understory vegetation plays the key role in sustaining soil microbial biomass and extracellular enzyme activities. Biogeosciences 2018, 15, 4481–4494. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.L.; Wang, D.; Chen, X.; Wang, J.; Diao, J.J.; Zhang, J.Y.; Guan, Q.W. Soil microbial functional diversity and biomass as affected by different thinning intensities in a Chinese fir plantation. Appl. Soil Ecol. 2015, 92, 35–44. [Google Scholar] [CrossRef]
- Deng, J.; Chong, Y.J.; Zhang, D.; Ren, C.J.; Zhao, F.Z.; Zhang, X.X.; Han, X.H.; Yang, G.H. Temporal variations in soil enzyme activities and responses to land use change in the Loess Plateau, China. Appl. Sci. 2019, 9, 3129. [Google Scholar] [CrossRef] [Green Version]
- Li, X.F.; Zhang, Y.; Ding, C.X.; Liu, Y.; Wu, K.X.; Jiang, F.Z.; Su, D.R. Water addition promotes vegetation recovery of degraded alpine meadows by regulating soil enzyme activity and nutrients in the Qinghai–Tibetan Plateau. Ecol. Eng. 2020, 158, 106047. [Google Scholar] [CrossRef]
- Li, J.; Zhao, B.Q.; Li, X.Y.; Jiang, R.B.; So, H.B. Effects of long-term combined application of organic and mineral fertilizers on microbial biomass, soil enzyme activities and soil fertility. Agric. Sci. China 2008, 7, 336–343. [Google Scholar] [CrossRef]
- Rivaie, A.A. The effects of understory vegetation on P availability in Pinus radiata forest stands: A review. J. For. Res. 2014, 25, 489–500. [Google Scholar] [CrossRef]
- Su, X.P.; Wang, M.H.; Huang, Z.Q.; Fu, S.L.; Chen, H.Y.H. Forest understorey vegetation: Colonization and the availability and heterogeneity of resources. Forests 2019, 10, 944. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.T.; Zhang, X.N.; Xu, C.Y.; Hao, M.H.; Choe, C.; He, H.J. Thinning can increase shrub diversity and decrease herb diversity by regulating light and soil environments. Front. Plant Sci. 2022, 13, 948648. [Google Scholar] [CrossRef] [PubMed]
- Pohl, M.; Alig, D.; Körner, C.; Rixen, C. Higher plant diversity enhances soil stability in disturbed alpine ecosystems. Plant Soil. 2009, 324, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Siefert, A.; Ravenscroft, C.; Althoff, D.; Alvarez-Yépiz, J.C.; Carter, B.E.; Glennon, K.L.; Heberling, J.M.; Jo, I.S.; Pontes, A.; Sauer, A.; et al. Scale dependence of vegetation–environment relationships: A meta-analysis of multivariate data. J. Veg. Sci. 2012, 23, 942–951. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, K.D.; Zhao, X.J.; She, J.W.; Zheng, X.; Tang, L.Z. Effects of pruning on microclimate and understory vegetation in a poplar plantation. Ecol. Environ. Sci. 2019, 28, 1548–1556. [Google Scholar] [CrossRef]
- Choe, C.H.; Kim, J.H.; Xu, C.Y.; Choe, J.S.; Man, H.S.; Jo, S.M. Effect of soil nutrient spatial heterogeneity by cutting disturbance on understory plant diversity in broadleaved-Korean pine forest in Changbai Mountain, China. Eur. J. For. Res. 2021, 140, 603–613. [Google Scholar] [CrossRef]
- Chytrý, M.; Tichý, L.; Roleček, J. Local and regional patterns of species richness in Central European vegetation types along the pH/calcium gradient. Folia Geobot. 2003, 38, 429–442. [Google Scholar] [CrossRef]
- Weiher, E.; Forbes, S.; Schauwecker, T.; Grace, J.B. Multivariate control of plant species richness and community biomass in blackland prairie. Oikos 2004, 106, 151–157. [Google Scholar] [CrossRef]




| T | DBH (cm) | H (m) | HCB (m) | CD (m) | |
|---|---|---|---|---|---|
| EW | NS | ||||
| CK | 20.89 ± 0.75a | 17.08 ± 0.39a | 2.35 ± 0.16a | 4.94 ± 0.18a | 4.73 ± 0.18a |
| 1/3 | 21.52 ± 0.68a | 16.83 ± 0.48a | 2.58 ± 0.17a | 5.06 ± 0.36a | 4.87 ± 0.17a |
| 1/2 | 21.20 ± 0.48a | 16.79 ± 0.26a | 2.50 ± 0.17a | 4.74 ± 0.19a | 4.73 ± 0.21a |
| Mean | 21.20 ± 0.25 | 16.90 ± 0.13 | 2.49 ± 0.10 | 4.91 ± 0.13 | 4.78 ± 0.06 |
| T | D | BD (g·cm−3) | P (%) | TN (g·kg−1) | TP (g·kg−1) | TK (g·kg−1) | OM (g·kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 0–10 cm | 1.23 ± 0.08Aa | 54.50 ± 1.73Aa | 0.90 ± 0.14Aa | 0.88 ± 0.05Aa | 16.24 ± 0.79Aa | 28.61 ± 2.09Aa |
| 10–20 cm | 1.31 ± 0.01Aa | 49.88 ± 2.64Aa | 0.55 ± 0.06Ba | 0.74 ± 0.03Ba | 15.69 ± 0.35Aa | 19.89 ± 0.99Ba | |
| 20–50 cm | - | - | 0.22 ± 0.02Ca | 0.59 ± 0.02Ca | 13.22 ± 0.39Ba | 7.00 ± 0.64Ca | |
| 1/3 | 0–10 cm | 1.13 ± 0.08Aa | 57.15 ± 1.75Aa | 0.81 ± 0.19Aa | 0.87 ± 0.11Aa | 16.63 ± 0.29Aa | 29.04 ± 1.52Aa |
| 10–20 cm | 1.29 ± 0.06Aa | 51.59 ± 5.77Aa | 0.50 ± 0.10Aa | 0.72 ± 0.04Aa | 16.56 ± 0.53Aa | 18.71 ± 1.41Ba | |
| 20–50 cm | - | - | 0.24 ± 0.06Ba | 0.55 ± 0.03Ba | 13.49 ± 0.69Ba | 10.02 ± 1.60Ca | |
| 1/2 | 0–10 cm | 1.18 ± 0.12Aa | 55.39 ± 2.31Aa | 0.92 ± 0.15Aa | 0.91 ± 0.03Aa | 15.86 ± 0.45Aa | 31.27 ± 1.41Aa |
| 10–20 cm | 1.30 ± 0.07Aa | 50.94 ± 1.94Aa | 0.64 ± 0.04Ba | 0.77 ± 0.05Ba | 15.71 ± 0.54ABa | 20.72 ± 2.15Ba | |
| 20–50 cm | – | – | 0.28 ± 0.06Ca | 0.57 ± 0.02Ca | 14.14 ± 1.03Ba | 6.68 ± 1.24Ca |
| Source | F(p) Value SWC | NH4-N | NO3-N | IN | MBC | MBN | Catalase | Saccharase | Urease |
|---|---|---|---|---|---|---|---|---|---|
| T | 0.629 (0.536) | 4.665 (0.012) | 9.247 (<0.001) | 8.053 (0.001) | 5.805 (0.005) | 6.730 (0.003) | 17.002 (<0.001) | 10.822 (<0.001) | 9.382 (<0.001) |
| S | 248.398 (<0.001) | 307.926 (<0.001) | 41.898 (<0.001) | 19.866 (<0.001) | 4.702 (0.005) | 65.714 (<0.001) | 2414.374 (<0.001) | 429.663 (<0.001) | 242.015 (<0.001) |
| D | 51.448 (<0.001) | 14.178 (<0.001) | 130.396 (<0.001) | 127.363 (<0.001) | 78.109 (<0.001) | 236.031 (<0.001) | 90.699 (<0.001) | 2193.513 (<0.001) | 1152.01 (<0.001) |
| T × S | 0.939 (0.473) | 8.245 (<0.001) | 2.307 (0.043) | 4.382 (0.001) | 0.744 (0.616) | 1.499 (0.191) | 2.504 (0.029) | 1.895 (0.094) | 3.14 (0.009) |
| T × D | 0.224 (0.924) | 1.256 (0.295) | 1.444 (0.228) | 1.32 (0.271) | 3.21 (0.018) | 0.478 (0.752) | 1.606 (0.182) | 6.98 (<0.001) | 2.069 (0.094) |
| S × D | 5.762 (<0.001) | 3.313 (0.006) | 16.377 (<0.001) | 16.278 (<0.001) | 1.293 (0.271) | 25.984 (<0.001) | 4.975 (<0.001) | 83.914 (<0.001) | 40.196 (<0.001) |
| T × S × D | 0.389 (0.963) | 1.235 (0.277) | 1.321 (0.226) | 1.656 (0.095) | 0.623 (0.815) | 0.722 (0.725) | 0.244 (0.955) | 3.288 (<0.001) | 2.364 (0.013) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, K.; Xu, C.; Qian, Z.; Zhang, K.; Tang, L. Effects of Pruning on Vegetation Growth and Soil Properties in Poplar Plantations. Forests 2023, 14, 501. https://doi.org/10.3390/f14030501
Huang K, Xu C, Qian Z, Zhang K, Tang L. Effects of Pruning on Vegetation Growth and Soil Properties in Poplar Plantations. Forests. 2023; 14(3):501. https://doi.org/10.3390/f14030501
Chicago/Turabian StyleHuang, Kaidong, Cheng Xu, Zhuangzhuang Qian, Kang Zhang, and Luozhong Tang. 2023. "Effects of Pruning on Vegetation Growth and Soil Properties in Poplar Plantations" Forests 14, no. 3: 501. https://doi.org/10.3390/f14030501




