Effect of Pb Stress on Ionome Variations and Biomass in Rhus chinensis Mill
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Experimental Method
2.3. Biomass Measurements
2.4. Analysis of Elemental Concentrations of R. chinensis
2.5. Statistical Analysis of Data
3. Results
3.1. Biomass and Tolerance Index
3.2. Accumulation and Translocation of Pb
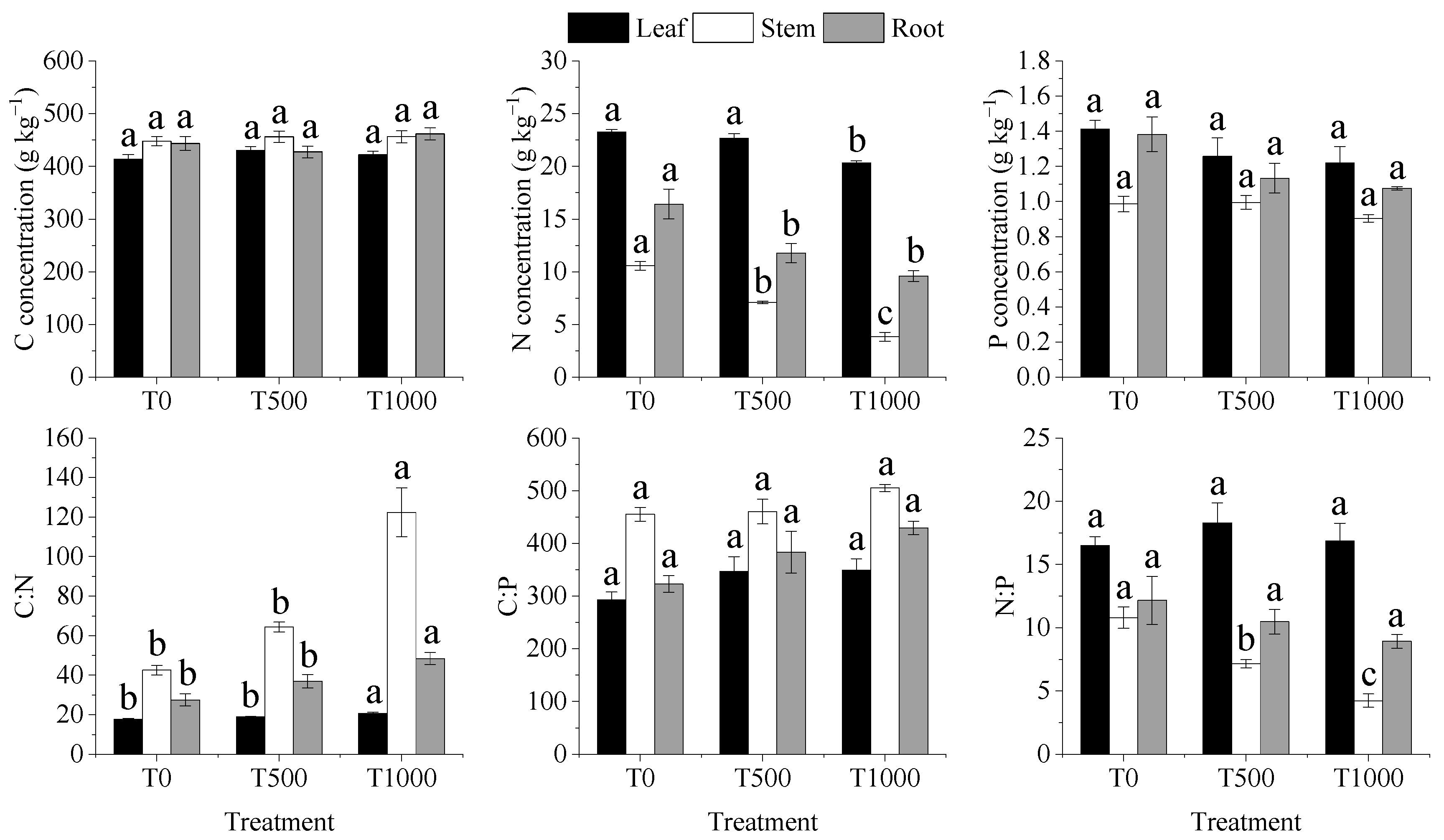
3.3. Stoichiometric Patterns of C, N, and P
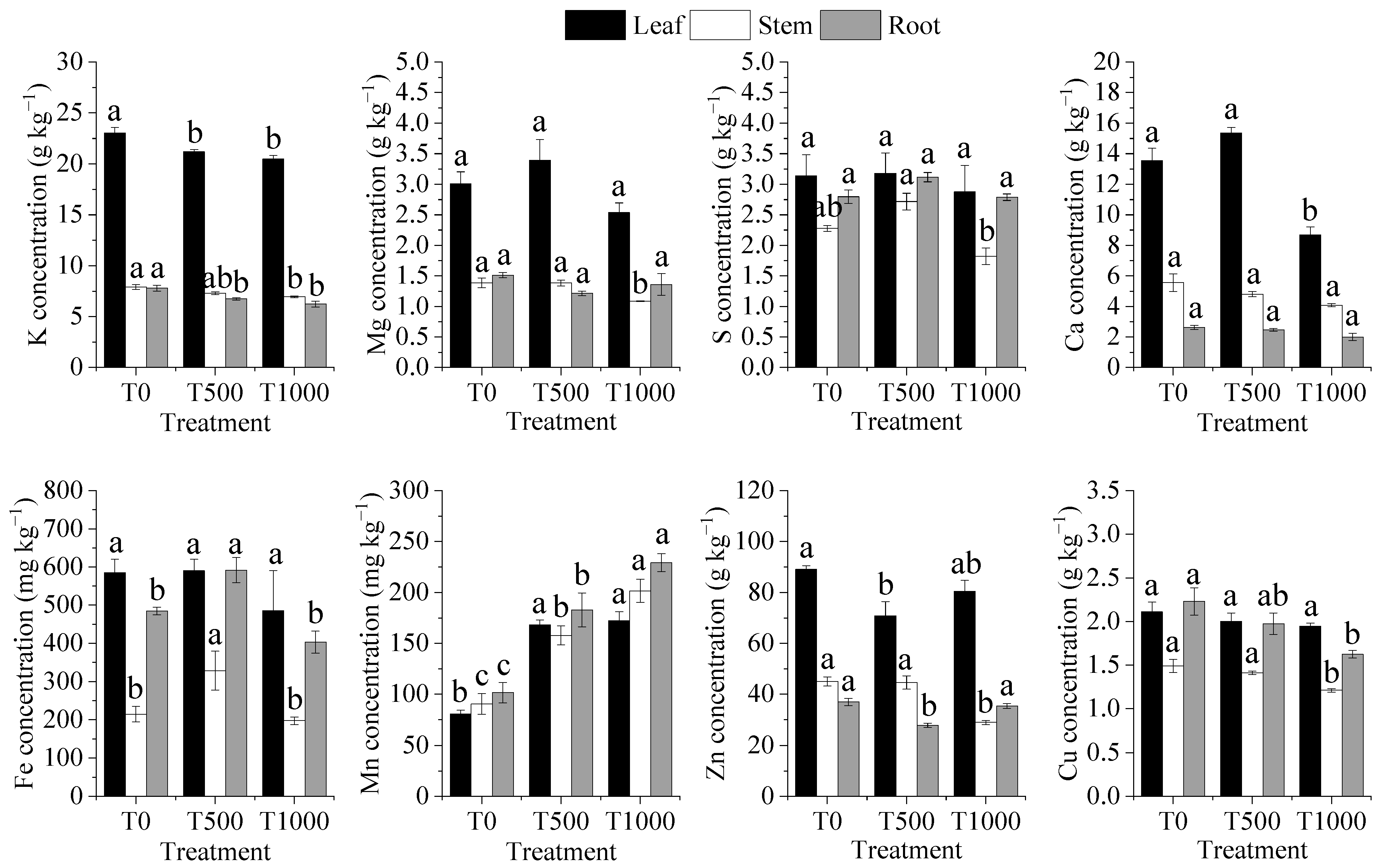
3.4. Concentration of the Elements in R. chinensis Plants
3.5. Changes of Ionomic Profiles in Different Types of Organs of R. chinensis Plants under Pb Stress
4. Discussion
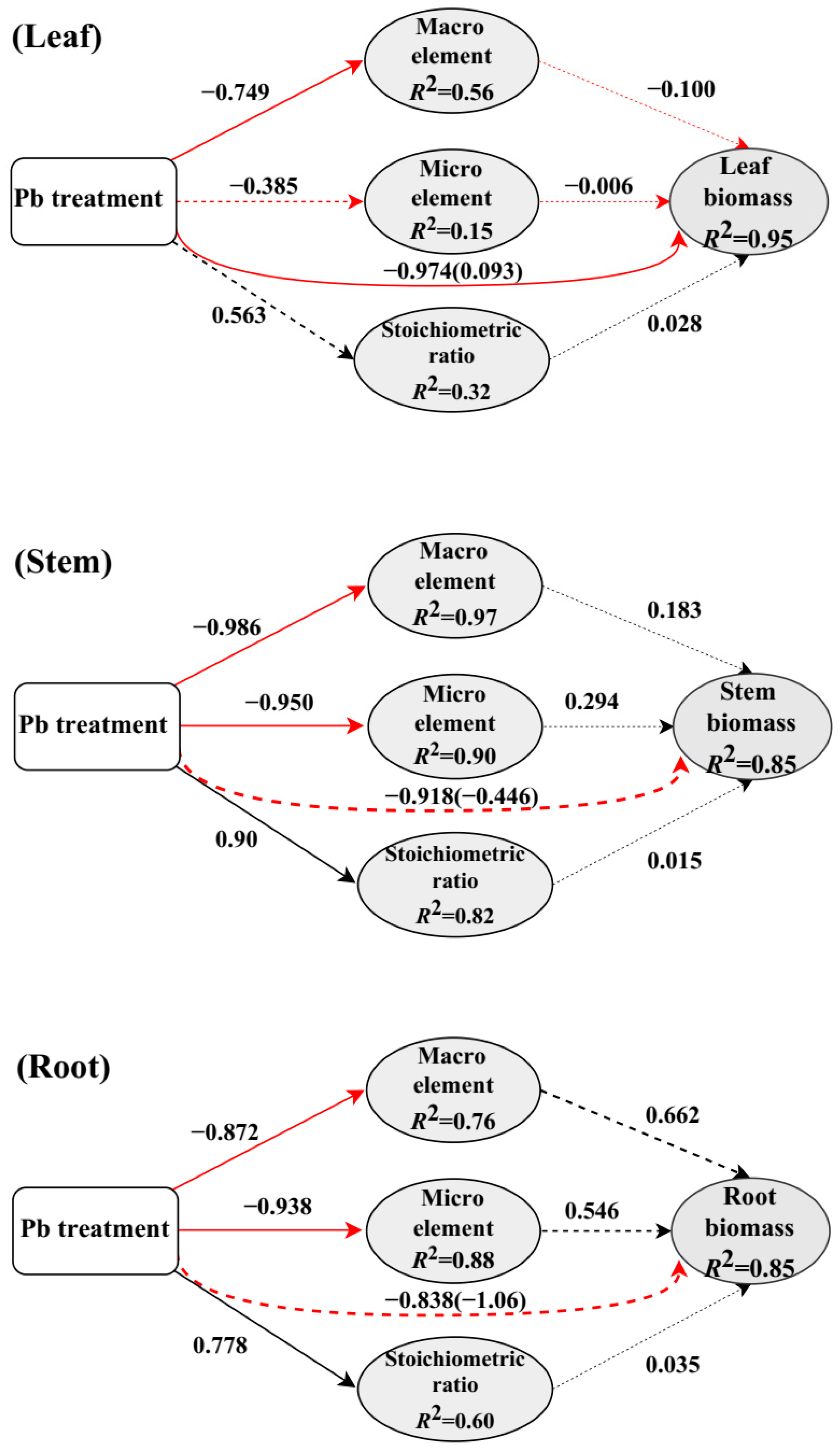
4.1. Change of the Ionome of R. chinensis Plants under Pb Stress
4.2. Major Factors Regulating Biomass
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zulfiqar, U.; Farooq, M.; Hussain, S.; Maqsood, M.; Hussain, M.; Ishfaq, M.; Anjum, M.Z. Lead toxicity in plants: Impacts and remediation. J. Environ. Manag. 2019, 250, 109557. [Google Scholar] [CrossRef]
- Ferrer, M.A.; Cimini, S.; López-Orenes, A.; Calderón, A.A.; De Gara, L. Differential Pb tolerance in metallicolous and non-metallicolous Zygophyllum fabago populations involves the strengthening of the antioxidative pathways. Environ. Exp. Bot. 2018, 150, 141–151. [Google Scholar] [CrossRef]
- Gupta, D.K.; Huang, H.G.; Corpas, F.J. Lead tolerance in plants: Strategies for phytoremediation. Environ. Sci. Pollut. Res. 2013, 20, 2150–2161. [Google Scholar] [CrossRef]
- Hanumanth Kumar, G.; Pramoda Kumari, J. Heavy metal lead influative toxicity and its assessment in phytoremediating plants—A review. Water Air Soil Pollut. 2015, 226, 324. [Google Scholar] [CrossRef]
- Zeng, P.; Guo, Z.; Xiao, X.; Peng, C.; Liu, L.; Yan, D.; He, Y. Physiological stress responses, mineral element uptake and phytoremediation potential of Morus alba L. in cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2020, 189, 109973. [Google Scholar] [CrossRef]
- Yang, W.; Liu, D.; Wang, Y.; Hussain, B.; Zhao, F.; Ding, Z.; Yang, X.; Dawood, M. Variations in phytoremediation potential and phytoavailability of heavy metals in different Salix genotypes subjected to seasonal flooding. J. Environ. Manag. 2021, 299, 113632. [Google Scholar] [CrossRef]
- Cai, X.; Fu, J.; Li, X.; Peng, L.; Yang, L.; Liang, Y.; Jiang, M.; Ma, J.; Sun, L.; Guo, B.; et al. Low-molecular-weight organic acid-mediated tolerance and Pb accumulation in centipede grass under Pb stress. Ecotoxicol. Environ. Saf. 2022, 241, 113755. [Google Scholar] [CrossRef]
- Wang, S.; Volk, T.A.; Xu, J. Variability in growth and cadmium accumulation capacity among willow hybrids and their parents: Implications for yield-based selection of Cd-efficient cultivars. J. Environ. Manag. 2021, 299, 113643. [Google Scholar] [CrossRef]
- Feng, X.; Han, L.; Chao, D.; Liu, Y.; Zhang, Y.; Wang, R.; Guo, J.; Feng, R.; Xu, Y.; Ding, Y.; et al. Ionomic and transcriptomic analysis provides new insight into the distribution and transport of cadmium and arsenic in rice. J. Hazard. Mater. 2017, 331, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Leitenmaier, B.; Küpper, H. Compartmentation and complexation of metals in hyperaccumulator plants. Front. Plant. Sci. 2013, 4, 374. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Xia, Y.; Hu, C.; Yu, M.; Shabala, S.; Wu, S.; Tam, Q.; Xu, S.; Sun, X. Ionomics analysis provides new insights into the co-enrichment of cadmium and zinc in wheat grains. Ecotoxicol. Environ. Saf. 2021, 223, 112623. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Ding, Z.; Wang, H.; Song, L.; Jia, S.; Ma, D. Zinc stress affects ionome and metabolome in tea plants. Plant Physiol. Biochem. 2017, 111, 318–328. [Google Scholar] [CrossRef]
- Huang, X.Y.; Salt, D.E. Plant Ionomics: From elemental profiling to environmental adaptation. Mol. Plant 2016, 9, 787–797. [Google Scholar] [CrossRef] [Green Version]
- Baxter, I.; Dilkes, B.P. Elemental profiles reflect plant adaptations to the environment. Science 2012, 336, 1661–1663. [Google Scholar] [CrossRef] [Green Version]
- Baxter, I. Should we treat the ionome as a combination of individual elements, or should we be deriving novel combined traits? J. Exp. Bot. 2015, 66, 2127–2131. [Google Scholar] [CrossRef] [Green Version]
- Filipiak, M.; Filipiak, Z.M. Application of ionomics and ecological stoichiometry in conservation biology: Nutrient demand and supply in a changing environment. Biol. Conserv. 2022, 272, 109622. [Google Scholar] [CrossRef]
- Fernández-Martínez, M. From atoms to ecosystems: Elementome diversity meets ecosystem functioning. New Phytol. 2022, 234, 35–42. [Google Scholar] [CrossRef]
- Mleczek, M.; Gąsecka, M.; Waliszewska, B.; Magdziak, Z.; Szostek, M.; Rutkowski, P.; Kaniuczak, J.; Zborowska, M.; Budzyńska, S.; Mleczek, P.; et al. Salix viminalis L.—A highly effective plant in phytoextraction of elements. Chemosphere 2018, 212, 67–78. [Google Scholar] [CrossRef]
- Fan, W.; Xia, Z.; Liu, C.; Ma, S.; Liu, S.; Wu, Y.; Zhu, B.; Xu, C.; Zhao, A. Ionomics, transcriptomics and untargeted metabolomics analyses provide new insights into the Cd response and accumulation mechanisms of mulberry. Environ. Exp. Bot. 2022, 196, 104821. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Y.; Qin, X.; Zhao, L.; Liang, X.; Sun, Y.; Xu, Y. Soil application of manganese sulfate effectively reduces Cd bioavailability in Cd-contaminated soil and Cd translocation and accumulation in wheat. Sci. Total Environ. 2022, 814, 152765. [Google Scholar] [CrossRef]
- Mišúthová, A.; Slováková, Ľ.; Kollárová, K.; Vaculík, M. Effect of silicon on root growth, ionomics and antioxidant performance of maize roots exposed to As toxicity. Plant Physiol. Biochem. 2021, 168, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Xu, X.; Zhu, H.; Ren, Y.; Niu, H.; Hou, R.; Wan, X.; Cai, H. Metabolics and ionomics responses of tea leaves (Camellia sinensis (L.) O. Kuntze) to fluoride stress. Plant Physiol. Biochem. 2021, 158, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.U.; Nawaz, M.F.; Gul, S.; Yasin, G.; Hussain, B.; Li, Y.; Chen, H. State-of-the-art OMICS strategies against toxic effects of heavy metals in plants: A review. Ecotoxicol. Environ. Saf. 2022, 242, 113952. [Google Scholar] [CrossRef]
- Cseh, E.; Fodor, F.; Varga, A.; Záray, G. Effect of lead treatment on the distribution of essential elements in cucumber. J. Plant Nutr. 2000, 23, 1095–1105. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhang, S.; Wang, P.; Hou, J.; Qian, J. Effects of Pb stress on nutrient uptake and secondary metabolism in submerged macrophyte Vallisneria natans. Ecotoxicol. Environ. Saf. 2011, 74, 1297–1303. [Google Scholar] [CrossRef] [PubMed]
- Kısa, D.; Öztürk, L.; Doker, S.; Gökçe, İ. Expression analysis of metallothioneins and mineral contents in tomato (Lycopersicon esculentum) under heavy metal stress. J. Sci. Food Agric. 2017, 97, 1916–1923. [Google Scholar] [CrossRef]
- Mondaca, P.; Valenzuela, P.; Quiroz, W.; Valdenegro, M.; Abades, S.; Celis-Diez, J.L. Environmental conditions and plant physiology modulate Cu phytotoxicity in field-contaminated soils. Ecotoxicol. Environ. Saf. 2022, 246, 114179. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.T.; Wang, S.F.; Li, J.C. Pb, Zn accumulation and nutrient uptake of 15 plant species grown in abandoned mine tailings. Environ. Sci. 2012, 33, 2021–2027. [Google Scholar]
- Shi, X.; Wang, S.; Wang, D.; Sun, H.; Chen, Y.; Liu, J.; Jiang, Z. Woody species Rhus chinensis Mill. seedlings tolerance to Pb: Physiological and biochemical response. J. Environ. Sci. 2019, 78, 63–73. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, M.; Ren, H.; Yu, J.; Wu, J.; Ma, X. Bioaccumulation and detoxification mechanisms for lead uptake identified in Rhus chinensis Mill. seedlings. Ecotoxicol. Environ. Saf. 2017, 142, 59–68. [Google Scholar] [CrossRef]
- Cao, Y.; Ma, C.; Tong, R.; Chen, J.; Li, X.; Chen, G. Flooding alleviates copper stress on Salix: Evidence from stoichiometric patterns among plant tissues. Plant Soil 2022, 478, 545–558. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Khan, M.A.; Luo, W.; Xiang, Z.; Xu, W.; Zhong, B.; Ma, J.; Ye, Z.; Zhu, Y.; et al. Effect of plant extracts and citric acid on phytoremediation of metal-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 211, 111902. [Google Scholar] [CrossRef] [PubMed]
- Monaci, F.; Ancora, S.; Paoli, L.; Loppi, S.; Franzaring, J. Differential elemental stoichiometry of two Mediterranean evergreen woody plants over a geochemically heterogeneous area. Perspect. Plant Ecol. 2022, 55, 125672. [Google Scholar] [CrossRef]
- Yamashita, H.; Fukuda, Y.; Yonezawa, S.; Morita, A.; Ikka, T. Tissue ionome response to rhizosphere pH and aluminum in tea plants (Camellia sinensis L.), a species adapted to acidic soils. Plant-Environ. Interact. 2020, 1, 152–164. [Google Scholar] [CrossRef]
- Lai, J.; Liu, Z.; Luo, X. A metabolomic, transcriptomic profiling, and mineral nutrient metabolism study of the phytotoxicity mechanism of uranium. J. Hazard. Mater. 2020, 386, 121437. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, R.; Raut, V.V.; Pandey, M.; Jeyakumar, S.; Verulkar, S.; Suprasanna, P.; Srivastava, K.A. Arsenic and cadmium induced macronutrient deficiencies trigger contrasting gene expression changes in rice. Environ. Pollut. 2022, 300, 118923. [Google Scholar] [CrossRef]
- Qin, S.; Liu, H.; Nie, Z.; Rengel, Z.; Gao, W.; Li, C.; Zhao, P. Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review. Pedosphere 2020, 30, 168–180. [Google Scholar] [CrossRef]
- Borišev, M.; Pajević, S.; Nikolić, N.; Orlović, S.; Župunski, M.; Pilipović, A.; Kebert, M. Magnesium and iron deficiencies alter Cd accumulation in Salix viminalis L. Int. J. Phytorem. 2016, 18, 164–170. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Liang, X.; Wang, L.; Sun, Y.; Huang, Q.; Qin, X.; Zhao, L. Soil application of manganese sulfate could reduce wheat Cd accumulation in Cd contaminated soil by the modulation of the key tissues and ionomic of wheat. Sci. Total Environ. 2021, 770, 145328. [Google Scholar] [CrossRef]
- Upadhyay, R.; Panda, S.K. Zinc reduces copper toxicity induced oxidative stress by promoting antioxidant defense in freshly grown aquatic duckweed Spirodela polyrhiza L. J. Hazard. Mater. 2010, 175, 1081–1084. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Guo, D.; Zhang, Y. Leaf nitrogen and phosphorus stoichiometry across 753 terrestrial plant species in China. New Phytol. 2005, 168, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Fagan, W.F.; Denno, R.F.; Dobberfuhl, D.R.; Folarin, A.; Huberty, A.; Interlandi, S.; Kilham, S.S.; McCauley, E.; Schulz, K.L.; et al. Nutritional constraints in terrestrial and freshwater food webs. Nature 2000, 408, 578–580. [Google Scholar] [CrossRef] [PubMed]
- Koerselman, W.; Meuleman, A.F. The vegetation N: P ratio: A new tool to detect the nature of nutrient limitation. J. Appl. Ecol. 1996, 33, 1441–1450. [Google Scholar] [CrossRef]
- Collin, S.; Baskar, A.; Geevarghese, D.M.; Ali, M.N.V.S.; Bahubali, P.; Choudhary, R.; Lvov, V.; Tovar, G.I.; Senatov, F.; Koppala, S.; et al. Bioaccumulation of lead (Pb) and its effects in plants: A review. J. Hazard. Mater. Lett. 2022, 3, 100064. [Google Scholar] [CrossRef]
- Muratore, C.; Espen, L.; Prinsi, B. Nitrogen uptake in plants: The plasma membrane root transport systems from a physiological and proteomic perspective. Plants 2021, 10, 681. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Zhao, Q.; Cao, W.; Chen, X.; Zou, C. Health risk assessment associated with heavy metal accumulation in wheat after long-term phosphorus fertilizer application. Environ. Pollut. 2020, 262, 114348. [Google Scholar] [CrossRef]
- de Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular-physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Wang, S.; Shi, X.; Sun, H.; Chen, Y.; Pan, H.; Yang, X.; Rafiq, T. Variations in metal tolerance and accumulation in three hydroponically cultivated varieties of Salix integra treated with lead. PLoS ONE 2014, 9, e108568. [Google Scholar] [CrossRef]
- Eissa, M.A.; Roshdy, N.M.K. Nitrogen fertilization: Effect on Cd-phytoextraction by the halophytic plant quail bush [Atriplex lentiformis (Torr.) S. Wats]. S. Afr. J. Bot. 2018, 115, 126–131. [Google Scholar] [CrossRef]
- Kerdsomboon, K.; Techo, T.; Limcharoensuk, T.; Tatip, S.; Auesukaree, C. Low phosphate mitigates cadmium-induced oxidative stress in Saccharomyces cerevisiae by enhancing endogenous antioxidant defence system. Environ. Microbiol. 2022, 24, 707–720. [Google Scholar] [CrossRef]



| Pb/(mg kg−1) | Available Pb/(mg kg−1) | Total Pb /(mg kg−1) | Hydrolysable Nitrogen/(mg kg−1) | Available Phosphorus/(mg kg−1) | Available Potassium/(mg kg−1) | pH |
|---|---|---|---|---|---|---|
| 0 | 24.0 ± 4.7 | 40.4 ± 1.1 | 56.3 ± 2.5 | 3.53 ± 0.06 | 124.3 ± 0.6 | 5.59 ± 0.02 |
| 500 | 254.7 ± 9.9 | 409 ± 32.5 | 92.7 ± 2.1 | 5.62 ± 0.03 | 130.0 ± 1.0 | 5.66 ± 0.02 |
| 1000 | 630.3 ± 32.5 | 867.7 ± 19.1 | 76.8 ± 1.0 | 5.51 ± 0.02 | 135.0 ± 3.0 | 5.39 ± 0.02 |
| Leaf | Reduction% | Stem | Reduction% | Root | Reduction% | TI | |
|---|---|---|---|---|---|---|---|
| T0 | 2.75 ± 0.07 a | 1.81 ± 0.04 a | 1.82 ± 0.04 a | ||||
| T500 | 2.02 ± 0.22 b | 26.53 | 1.44 ± 0.15 b | 20.42 | 1.32 ± 0.13 b | 27.46 | 0.75 |
| T1000 | 1.35 ± 0.16 c | 51.11 | 1.22 ± 0.14 b | 32.68 | 1.24 ± 0.21 b | 31.69 | 0.60 |
| T0 | T500 | T1000 | |
|---|---|---|---|
| Pb-Leaf | 14.71 ± 1.62 c | 50.84 ± 5.15 b | 64.70 ± 1.36 a |
| Pb-Stem | 7.23 ± 1.00 b | 78.22 ± 14.97 a | 86.67 ± 3.92 a |
| Pb-Root | 28.53 ± 1.72 c | 270.76 ± 11.34 b | 699.50 ± 51.10 a |
| BCF-shoot | 0.291 ± 0.028 a | 0.154 ± 0.021 b | 0.087 ± 0.003 c |
| BCF-root | 0.706 ± 0.043 b | 0.669 ± 0.028 b | 0.806 ± 0.059 a |
| TF | 0.411 ± 0.018 a | 0.230 ± 0.031 b | 0.108 ± 0.005 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Wang, S.; Wang, Y.; Lu, M.; Shi, X. Effect of Pb Stress on Ionome Variations and Biomass in Rhus chinensis Mill. Forests 2023, 14, 528. https://doi.org/10.3390/f14030528
He W, Wang S, Wang Y, Lu M, Shi X. Effect of Pb Stress on Ionome Variations and Biomass in Rhus chinensis Mill. Forests. 2023; 14(3):528. https://doi.org/10.3390/f14030528
Chicago/Turabian StyleHe, Wenxiang, Shufeng Wang, Yangdong Wang, Mengzhu Lu, and Xiang Shi. 2023. "Effect of Pb Stress on Ionome Variations and Biomass in Rhus chinensis Mill" Forests 14, no. 3: 528. https://doi.org/10.3390/f14030528
APA StyleHe, W., Wang, S., Wang, Y., Lu, M., & Shi, X. (2023). Effect of Pb Stress on Ionome Variations and Biomass in Rhus chinensis Mill. Forests, 14(3), 528. https://doi.org/10.3390/f14030528






