Seeds’ Early Traits as Predictors of Performance in Direct Seeding Restoration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Species Selection and Seed Preparation
2.3. Species Selection and Seed Preparation
2.4. Data Analysis
3. Results
3.1. Species Assemblage, Germinability, and Emergence
3.2. The Trajectory of Direct Seeding
3.3. Early Seed Traits and the Ability for Direct Seeding
4. Discussion
Implications for Direct Seeding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lebrija-Trejos, E.; Perez-Garcia, E.A.; Meave, J.A.; Bongers, F.; Poorter, L. Functional traits and environmental filtering drive community assembly in a species-rich tropical system. Ecology 2010, 91, 386–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraft, N.J.B.; Valencia, R.; Ackerly, D.D. Functional traits and niche-based tree community assembly in an amazonian forest. Science 2008, 322, 580–582. Available online: https://www.science.org/doi/10.1126/science.1160662 (accessed on 20 June 2022). [CrossRef] [PubMed] [Green Version]
- Mouillot, D.; Mason, N.W.H.; Wilson, J.B. Is the abundance of species determined by their functional traits? A new method with a test using plant communities. Oecologia 2007, 152, 729–737. [Google Scholar] [CrossRef]
- Fernández-Pascual, E.; Jiménez-Alfaro, B.; Bueno, A. Comparative seed germination traits in alpine and subalpine grasslands: Higher elevations are associated with warmer germination temperatures. Plant Biol. 2017, 19, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brancalion, P.H.S.; Gandolfi, S.; Rodrigues, R.R. Procedimentos operacionais para aplicação de métodos de restauração florestal. In Restauração Florestal; Brancalion, P.H.S., Gandolfi, S., Rodrigues, R.R., Eds.; Oficina de Textos: São Paulo, Brazil, 2015; pp. 251–285. Available online: http://www.lcb.esalq.usp.br/sites/default/files/publicacao_arq/978-85-7975-019-9.pdf (accessed on 20 June 2022).
- Tymus, J.R.C.; Lenti, F.E.B.; Silva, A.P.M.; Isernhagen, I. Restauração da Vegetação Nativa no Brasil. Caracterização de Técnicas e Estimativas de Custo. Relatório de Pesquisa. 2018. Available online: https://www.tnc.org.br/content/dam/tnc/nature/en/documents/brasil/restauracao-da-vegetacao-nativa-no-brasil.pdf (accessed on 20 June 2022).
- Trentin, B.E.; Estevan, D.A.; Rossetto, E.F.S.; Gorenstein, M.R.; Brizola, G.P.; Bechara, F.C. Restauração florestal na Mata Atlântica: Passiva, nucleação e plantio de alta diversidade. Ciência Florest. 2018, 28, 160–174. [Google Scholar] [CrossRef] [Green Version]
- Meli, P.; Isernhagen, I.; Brancalion, P.H.S.; Isernhagen, E.C.C.; Behling, M.; Rodrigues, R.R. Optimizing seeding density of fast-growing native trees for restoring the Brazilian Atlantic Forest. Restor. Ecol. 2017, 26, 212–219. [Google Scholar] [CrossRef]
- Ferreira, R.A.; Santos, P.L.; Aragão, A.G.; Santos, T.I.S.; Santos-Neto, E.M.; Rezende, A.M.D.S. Semeadura direta com espécies florestais na implantação de mata ciliar no Baixo São Francisco em Sergipe. Braz. Sci. For. 2009, 37, 37–46. Available online: https://www.ipef.br/publicacoes/scientia/nr81/cap04.pdf (accessed on 20 June 2022).
- Aguirre, A.G.; Lima, J.T.; Teixeira, J.; Gandolfi, S. Potencial da semeadura direta na restauração florestal de pastagem abandonada no município de Piracaia, SP, Brasil. Hoehnea 2015, 42, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Freitas, M.G.; Rodrigues, S.B.; Campos-Filho, E.M.; Carmo, G.H.P.; Veiga, J.M.; Junqueira, R.G.P.; Vieira, D.L.M. Evaluating the success of direct seeding for tropical forest restoration for over ten years. For. Ecol. Manag. 2019, 438, 224–232. [Google Scholar] [CrossRef]
- Florentine, S.K.; Graz, F.P.; Ambrose, G.; O’brien, L. The current status of different age, direct-seeded revegetation sites in an agricultural landscape in the Burrumbeet Region, Victoria, Australia. Land Degrad. Dev. 2013, 24, 81–89. [Google Scholar] [CrossRef]
- Pilon, N.A.L.; Durigan, G. Critérios para indicação de espécies prioritárias para a restauração da vegetação de cerrado. Sci. For. 2013, 41, 389–399. Available online: https://www.ipef.br/publicacoes/scientia/nr99/cap10.pdf (accessed on 20 June 2022).
- Palma, A.C.; Laurance, S.G.W. A review of the use of direct seeding and seedling plantings in restoration: What do we know and where should we go? Appl. Veg. Sci. 2015, 18, 561–568. [Google Scholar] [CrossRef]
- Havens, K.; Vitt, P.; Still, S.; Kramer, A.T.; Fant, J.B.; Schatz, K. Seed sourcing for restoration in an era of climate change. Nat. Areas J. 2015, 35, 122–133. [Google Scholar] [CrossRef]
- Freire, J.M.; Urzedo, D.I.; Piña-Rodrigues, F.C.M. A realidade das sementes nativas no Brasil: Desafios e oportunidades para a produção em larga escala. Seed News 2017, 21, 24–28. [Google Scholar] [CrossRef]
- Urzedo, D.I.; Fisher, R.; Piña-Rodrigues, F.C.M.; Freire, J.M.; Junqueira, R.G.P. How policies constrain native seed supply for restoration in Brazil. Restor. Ecol. 2019, 27, 768–774. [Google Scholar] [CrossRef]
- Urzedo, D.I.; Piña-Rodrigues, F.C.M.; Feltran-Barbieri, R.; Junqueira, R.G.P.; Fisher, R. Seed networks for upscaling forest landscape restoration: Is it possible to expand native plant sources in Brazil? Forests 2020, 11, 259. [Google Scholar] [CrossRef] [Green Version]
- Campos-Filho, E.M.; Costa, J.N.M.N.; Sousa, O.L.; Junqueira, R.G.P. Mechanized direct-seeding of native forests in Xingu, central Brazil. J. Sustain. For. 2013, 32, 702–727. Available online: https://www.tandfonline.com/doi/abs/10.1080/10549811.2013.817341 (accessed on 20 June 2022). [CrossRef]
- Ceccon, E.; González, E.J.; Martorell, C. Is direct seeding a biologically viable strategy for restoring forest ecosystems? Evidences from a meta-analysis. Land Degrad. Dev. 2016, 27, 511–520. [Google Scholar] [CrossRef]
- Villela, F.N.J.; Manfredini, S.; Corrêa, A.J.M.; Carmo, J.B. Morfopedologia e zoneamento voltado à ocupação. Rev. Dep. Geogr.-USP 2015, 30, 179–192. [Google Scholar] [CrossRef]
- Corrêa, L.S.; Cardoso-Leite, E.; Castello, A.C.D.; Coelho, S.; Kortz, A.R.; Villela, F.N.J.; Koch, I. Estrutura, composição florística e caracterização sucessional em remanescente de floresta estacional semidecidual no sudeste do Brasil. Rev. Árvore 2014, 38, 799–809. [Google Scholar] [CrossRef] [Green Version]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Ko¨ppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Inmet-Instituto Nacional de Meteorologia, 2020. Banco de Dados Meteorológicos. Available online: https://bdmep.inmet.gov.br/ (accessed on 18 May 2021).
- Galetti, G.; Silva, J.M.S.; Piña-Rodrigues, F.C.M.; Piotrowiski, I. Análise multicriterial da estabilidade ecológica em três modelos de restauração florestal. Rev. Bras. Ciências Ambient. 2018, 48, 142–157. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.M.; Shirasuna, R.T.; Lima, F.C.; Ortiz, P.R.T. Lista de Espécies Indicadas Para Restauração Ecológica Para Diversas Regiões do Estado de São Paulo; Instituto de Botânica: São Paulo, Brazil, 2017; pp. 303–436. Available online: https://www.infraestruturameioambiente.sp.gov.br/institutodebotanica/wp-content/uploads/sites/235/2019/10/lista-especies-rad-2019.pdf (accessed on 20 June 2022).
- Brasil., 2013. Ministério da Agricultura, Pecuária e Abastecimento. Instruções Para Análise de Sementes de Espécies Florestais. Brasília. Available online: https://www.gov.br/agricultura/pt-br/assuntos/laboratorios/arquivos-publicacoes-laboratorio/florestal_documento_pdf-ilovepdf-compressed.pdf (accessed on 18 November 2019).
- Marcos-Filho, J. Seed vigor testing: An overview of the past, present and future perspective. Sci. Agric. 2015, 72, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Mori, E.S.; Piña-Rodrigues, F.C.M.; Freitas, N.P. Guia Para Germinação de 100 Espécies Nativas, 1st ed.; Instituto Refloresta: São Paulo, Brazil, 2012; Available online: https://esalqlastrop.com.br/img/publicacoes/C2.pdf (accessed on 18 February 2018).
- Piotrowski, I.; Silva, J.M.S.; Piña-Rodrigues, F. Linha do Tempo de Implantação e Manejo de Áreas Restauradas por Semeadura Direta. 2020. Available online: https://www.researchgate.net/publication/348235852_Sistematica_para_implantacao_e_manejo_de_areas_restauradas_por_semeadura_direta_em_Floresta_Decidual (accessed on 23 January 2023).
- Magurran, A.E. Measuring Biological Diversity; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Clarke, K.R.; Warwick, R.M. A further biodiversity index applicable to species lists: Variation in taxonomic distinctness. Mar. Ecol. Prog. Ser. 2001, 216, 265–278. [Google Scholar] [CrossRef]
- Price, A.R.G.; Keeling, M.J.; O’callaghan, C.J. Ocean-scale patterns of ‘biodiversity’ of Atlantic asteroids determined from taxonomic distinctness and other measures. Biol. J. Linn. Soc. Lond. 1999, 66, 187–203. [Google Scholar] [CrossRef] [Green Version]
- Liao, X.; Li, Q.; Yang, X.; Zhang, W.; Li, W. Multi-objective optimization for crash safety design of vehicles using stepwise regression model. Chin. J. Mech. Eng. 2007, 35, 561–569. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 2nd ed.; Sage publications: Thousand Oaks, CA, USA, 2010; p. 449. ISBN 978-1-4129-7514-8. [Google Scholar]
- Pearson, K. On lines and planes of closest fit to systems of points in space. Philos. Mag. 1901, 2, 559–572. Available online: http://pbil.univ-lyon1.fr/R/pearson1901.pdf (accessed on 18 March 2020). [CrossRef] [Green Version]
- Johnson, R.A.; Wichern, D.W. Applied Multivariate Statistical Analysis; Pearson: Prentice Hall, NJ, USA, 2002. [Google Scholar]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.6. 2019. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 18 February 2020).
- Merritt, D.J.; Dixon, K.W. Restoration seed banks—A matter of scale. Science 2011, 332, 424–425. Available online: https://www.science.org/doi/10.1126/science.1203083 (accessed on 18 February 2018). [CrossRef]
- Schmidt, I.B.; Ferreira, M.C.; Sampaio, A.B.; Walter, B.M.T.; Vieira, D.L.M.; Holl, K.D. Tailoring restoration interventions to the grassland-savanna-forest complex in central Brazil. Restor. Ecol. 2019, 27, 942–948. [Google Scholar] [CrossRef]
- Isernhagen, I. Uso de Semeadura Direta de Espécies Arbóreas Nativas Para Restauração Florestal de Áreas Agrícolas, Sudeste do Brasil. 2010. Tese (Doutorado em Ciências-Recursos Florestais)-Departamento de Ciências Florestais, Universidade de São Paulo-Escola Superior de Agricultura “Luiz de Queiroz”, Piracicaba, 2010. Available online: https://www.teses.usp.br/teses/disponiveis/11/11150/tde-20102010-155109/pt-br.php (accessed on 16 August 2020).
- Corsini, C.R.; Scolforo, J.R.S.; Oliveira, A.D.; Mello, J.M.; Machado, E.L.M. Diversidade e similaridade de fragmentos florestais nativos situados na região nordeste de Minas Gerais. Cerne 2014, 20, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Shoo, L.P.; Freebody, K.; Kanowski, J.; Catterall, C.P. Slow recovery of tropical old-field rainforest regrowth and the value and limitations of active restoration. Conserv. Biol. 2016, 30, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Elliot, S.; Navakitbumrung, P.; Kuarak, C.; Zangkum, S.; Anusarnsunthorn, V.; Blakesley, D. Selecting framework tree species for restoring seasonally dry tropical forests in northern Thailand based on field performance. For. Ecol. Manag. 2003, 184, 177–191. [Google Scholar] [CrossRef]
- Rodrigues, S.B.; Freitas, M.G.; Campos-filho, E.M.; do Carmo, G.H.P.; da Veiga, J.M.; Junqueira, R.G.P.; Vieira, D.L.M. Direct seeded and colonizing species guarantee successful early restoration of South Amazon forests. For. Ecol. Manag. 2019, 451, 117559. [Google Scholar] [CrossRef]
- Pérez, D.R.; González, F.; Ceballos, C.; Oneto, M.E.; Aronson, J. Direct seeding and outplantings in drylands of Argentinean Patagonia: Estimated costs, and prospects for large-scale restoration and rehabilitation. Restor. Ecol. 2019, 27, 1105–1116. [Google Scholar] [CrossRef]
- Pietro-Souza, W.; Silva, N.M. Plantio manual de muvuca de sementes no contexto da restauração ecológica de áreas de preservação permanente degradadas. Rev. Bras. Agroecol. 2014, 9, 63–74. Available online: http://revistas.aba-agroecologia.org.br/index.php/rbagroecologia/article/view/15350 (accessed on 18 February 2018).
- Oliveira, M.C.; Leite, J.B.; Galdino, P.S.; Ogata, R.S.; Silva, D.A.; Ribeiro, J.F. Sobrevivência e crescimento de espécies nativas do Cerrado após semeadura direta na recuperação de pastagem abandonada. Neotrop. Biol. Conserv. 2019, 14, 313–327. [Google Scholar] [CrossRef]
- Pellizzaro, K.F.; Cordeiro, A.O.O.; Alves, M.; Motta, C.P.; Rezende, G.M.; Silva, R.R.P.; Ribeiro, J.F.; Sampaio, A.B.; Vieira, D.L.M.; Schmidt, I.B. “Cerrado” restoration by direct seeding: Field establishment and initial growth of 75 trees, shrubs and grass species. Braz. J. Bot. 2017, 40, 681–693. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Garza, C.; Bongers, F.; Poorter, L. Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? For. Ecol. Manag. 2013, 303, 35–45. [Google Scholar] [CrossRef]
- Soler-Guilhen, J.H.; Bernardes, C.O.; de Souza Marçal, T.; Oliveira, W.B.d.S.; Ferreira, M.F.d.S.; Ferreira, A. Euterpe edulis seed germination parameters and genotype selection. Acta Scientiarum. Agronomy 2019, 42, e42461. [Google Scholar] [CrossRef] [Green Version]
- Leitão, F.H.M.; Marques, M.C.M.; Ceccon, E. Young restored forests increase seedling recruitment in abandoned pastures in the Southern Atlantic rainforest. Rev. Biol. Trop. 2010, 58, 1271–1282. Available online: https://www.scielo.sa.cr/scielo.php?pid=S0034-77442010000400019&script=sci_arttext&tlng=en (accessed on 18 February 2018). [PubMed] [Green Version]
- Doust, S.J.; Erskine, P.D.; Lamb, D. Restoring rainforest species by direct seeding: Tree seedling establishment and growth performance on degraded land in the wet tropics of Australia. For. Ecol. Manag. 2008, 256, 1178–1188. [Google Scholar] [CrossRef]
- Souza, D.C.; ENGEL, V.L. Direct seeding reduces costs, but it is not promising for restoring tropical seasonal forests. Ecol. Eng. 2018, 116, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ceccon, E. Restauración en Bosques Tropicales: Fundamentos Ecológicos, Prácticos y Sociales; Ediciones Diaz Santos: Mexico City, Mexico, 2014; 288p, Available online: https://www.crim.unam.mx/web/sites/default/files/Muestra_Restauracion%20de%20bosques.pdf (accessed on 18 February 2018).
- Guerin, N.; Isernhagen, I.; Vieira, D.L.M.; Campos-Filho, E.M.; Campos, R.J.B. Avanços e próximos desafios da semeadura direta para restauração ecológica. Restauração Ecológica Ecossistemas Degrad. 2015, 2, 331–376. Available online: https://www.researchgate.net/publication/273381379_Avancos_e_Proximos_Desafios_da_Semeadura_Direta_para_Restauracao_Ecologica#fullTextFileContent (accessed on 16 August 2020).
- Santos, P.L.; Ferreira, R.A.; Aragão, A.G.; Amaral, L.A.; Oliveira, A.S. Estabelecimento de espécies florestais nativas por meio de semeadura direta para recuperação de áreas degradadas. Rev. Árvore 2012, 36, 237–245. [Google Scholar] [CrossRef] [Green Version]
- de Souza, T.V.; Torres, I.C.; Steiner, N.; Paulilo, M.T.S. Dormência de sementes em espécies arbóreas da Mata Atlântica Tropical Brasileira e suas relações com características de sementes e condições ambientais. Braz. J. Bot. 2015, 38, 243–264. [Google Scholar] [CrossRef]
- Cole, R.J.; Holl, K.D.; Keene, C.L.; Zahawi, R.A. Direct seeding of late-successional trees to restore tropical montane forest. For. Ecol. Manag. 2011, 261, 1590–1597. [Google Scholar] [CrossRef]
- Jurado, E.; Flores, J. Is seed dormancy under environmental control or bound to plant traits? J. Veg. Sci. 2005, 16, 559–564. [Google Scholar] [CrossRef]
- Lavorel, S.; Garnier, E. Predicting changes in community composition and ecosystem functioning from plant traits: Revisiting the Holy Grail. Funct. Ecol. 2002, 16, 545–556. [Google Scholar] [CrossRef]
- Silva, R.R.P.; Vieira, D.L.M. Direct seeding of 16 Brazilian savanna trees: Responses to seed burial, mulching and an invasive grass. Appl. Veg. Sci. 2017, 20, 410–421. [Google Scholar] [CrossRef] [Green Version]
- Masullo, L.S.; Piña-Rodrigues, F.C.M.; Figliolia, M.B.; Américo, C.C. Otimização de testes de tetrazólio para avaliar a qualidade de Platymiscium floribundum, Lonchocarpus muehlbergianus e Acacia polyphylla DC. Sementes. J. Seed Sci. 2017, 39, 189–197. [Google Scholar] [CrossRef]
- Ranal, M.A.; Santana, D.G. How and Why to Measure the Germination Process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
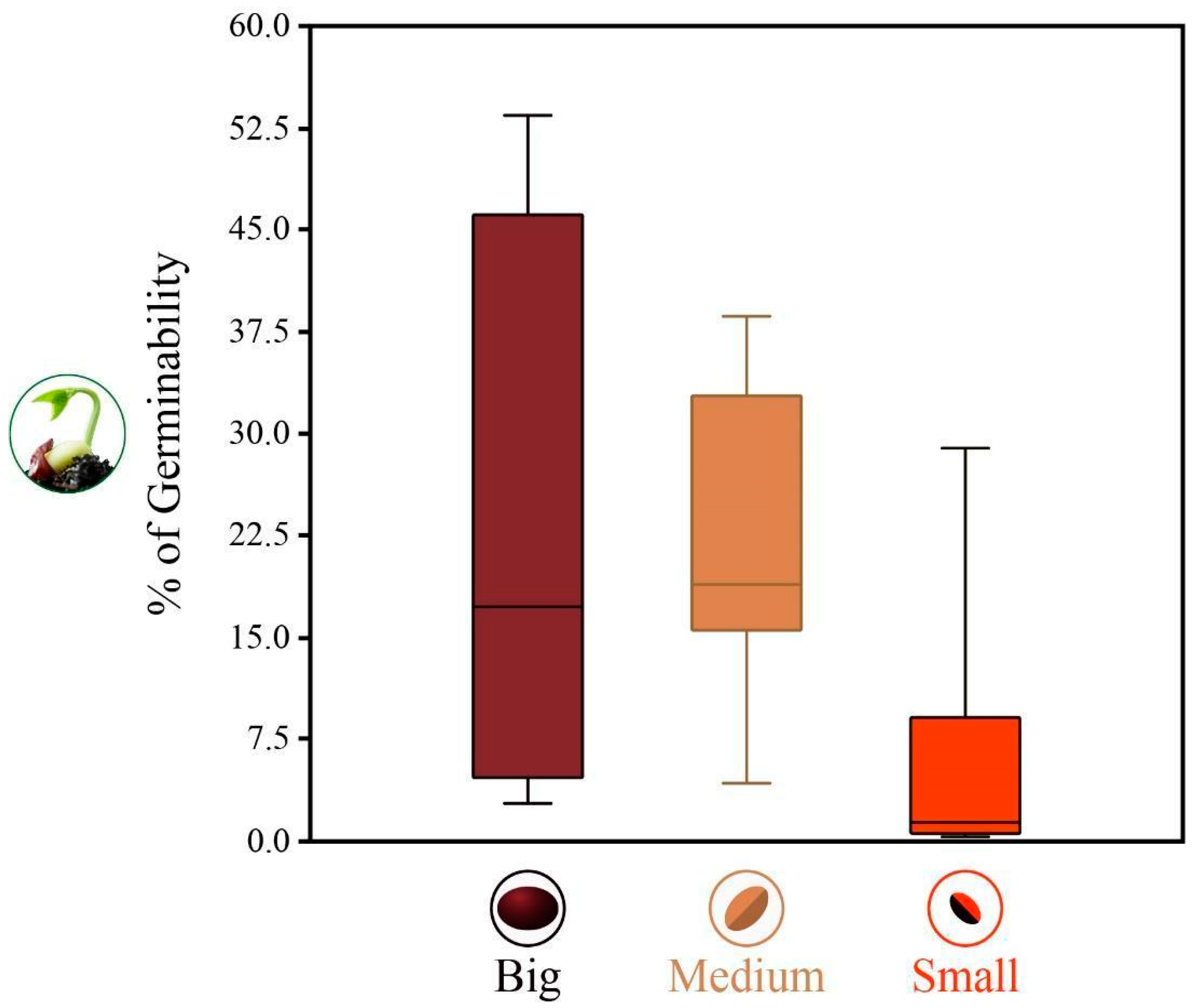


| Scientific Name | Acronyms | Family | Sz | EG | Disp | NS Kg−1 | NS Plots−1 | G (%) | Gd (%) | E | Den (ind.hec) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Senegalia polyphylla (DC.) Britton & Rose | SENPOL | Fabaceae | S | P | Aut | 13,829 | 835 | 77 | 28.9 | 241 | 2510 |
| Platypodium elegans Vogel | PLAELE | Fabaceae | L | NP | Ane | 885 | 326 | 59 | 53.4 | 174 | 1813 |
| Handroanthus heptaphyllus (Vell.) Mattos | HANHEP | Bignoniaceae | M | NP | Ane | 40,000 | 835 | 76 | 16.3 | 136 | 1417 |
| Mabea fistulifera Mart. | MABFIS | Euphorbiaceae | M | P | Aut | 9600 | 326 | 57 | 38.7 | 126 | 1313 |
| Copaifera langsdorffii Desf. | COPLAN | Fabaceae | M | NP | Zoo | 1720 | 326 | 66 | 33.1 | 108 | 1125 |
| Jacaranda cuspidifolia Mart. | JACCUS | Bignoniaceae | S | NP | Ane | 33,000 | 835 | 78 | 12.8 | 107 | 1115 |
| Poecilanthe parviflora Benth. | POEPAR | Fabaceae | M | NP | Aut | 2350 | 326 | 43 | 31.9 | 104 | 1083 |
| Hymenaea courbaril L. | HYMCOU | Fabaceae | L | NP | Zoo | 325 | 326 | 29 | 24.2 | 79 | 823 |
| Bixa orellana L. | BIXORE | Bixaceae | S | P | Zoo | 31,000 | 835 | 56 | 9.2 | 77 | 802 |
| Pterocarpus violaceus Vogel | PTEVIO | Fabaceae | M | NP | Ane | 2571 | 326 | 22 | 21.5 | 70 | 729 |
| Astronium urundeuva (M. Allemão) Engl. | ASTURU | Anacardiaceae | S | NP | Aut | 55,500 | 835 | 85 | 8.0 | 67 | 698 |
| Enterolobium contortisiliquum (Vell.) Morong | ENTCON | Fabaceae | M | P | Aut | 3000 | 326 | 35 | 16.3 | 53 | 552 |
| Ceiba speciosa (A.St.-Hil.) Ravenna | CEISPE | Malvaceae | M | NP | Ane | 5700 | 326 | 74 | 15.3 | 50 | 521 |
| Centrolobium robustum (Vell.) Mart. Ex Benth. | CENROB | Fabaceae | L | NP | Ane | 98 | 326 | ---- | 10.4 | 34 | 354 |
| Peltophorum dubium (Spreng.) Taub. | PELDUB | Fabaceae | S | P | Aut | 20,850 | 835 | 79 | 3.7 | 31 | 323 |
| Pterogyne nitens Tul. | PTENIT | Fabaceae | M | NP | Ane | 5250 | 326 | 17 | 4.3 | 14 | 146 |
| Mimosa bimucronata (DC.) Kuntze | MIMBIM | Fabaceae | S | P | Aut | 88,500 | 835 | 41 | 1.4 | 12 | 125 |
| Genipa americana L. | GENAME | Rubiaceae | S | NP | Zoo | 14,250 | 835 | 46 | 1.3 | 11 | 115 |
| Syagrus romanzoffiana (Cham.) Glassman | SYAROM | Arecaceae | L | NP | Zoo | 800 | 326 | 10 | 2.8 | 9 | 94 |
| Piptadenia gonoacantha (Mart.) J.F.Macbr. | PIPGON | Fabaceae | S | P | Aut | 21,500 | 835 | 3 | 0.6 | 5 | 52 |
| Tabebuia roseoalba (Ridl.) Sandwith | TABROS | Bignoniaceae | S | NP | Ane | 69,000 | 835 | 90 | 0.6 | 5 | 52 |
| Cedrela fissilis Vell. | CEDFIS | Meliaceae | S | NP | Ane | 36,000 | 326 | 65 | 1.2 | 4 | 42 |
| Citharexylum myrianthum Cham. | CITMYR | Verbenaceae | S | P | Zoo | 19,000 | 835 | 18 | 0.4 | 3 | 31 |
| Albizia niopoides (Spruce ex Benth.) Burkart | ALBNIO | Fabaceae | S | P | Aut | 36,000 | 835 | 15 | 0 | 0 | 0 |
| Apeiba tibourbou Aubl. | APETIB | Malvaceae | Ss | P | Zoo | 265,000 | 835 | 28 | 0 | 0 | 0 |
| Cecropia pachystachya Trécul | CECPAC | Urticaceae | Ss | P | Zoo | 1,172,000 | 835 | 43 | 0 | 0 | 0 |
| Cupania vernalis Cambess. | CUPVER | Sapindaceae | M | NP | Zoo | 3500 | 326 | 4 | 0 | 0 | 0 |
| Gallesia integrifolia (Spreng.) Harms. | GALINT | Phytolaccaceae | S | NP | Ane | 19,500 | 835 | 81 | 0 | 0 | 0 |
| Guazuma ulmifolia Lam. | GUAULM | Malvaceae | Ss | P | Zoo | 132,000 | 835 | 59 | 0 | 0 | 0 |
| Maclura tinctoria (L.) D.Don ex Steud. | MACTIN | Moraceae | Ss | NP | Zoo | 364,300 | 835 | 75 | 0 | 0 | 0 |
| Myroxylon peruiferum L.f. | MYRPER | Fabaceae | M | NP | Ane | 1800 | 326 | 71 | 0 | 0 | 0 |
| Psidium myrtoides O.Berg | PSIMYR | Myrtaceae | S | NP | Zoo | 23,645 | 326 | 36 | 0 | 0 | 0 |
| Psidium rufum Mart. Ex DC. | PSIRUF | Myrtaceae | S | NP | Zoo | 12,600 | 835 | 81 | 0 | 0 | 0 |
| Schinus terebinthifolia Raddi | SCHTER | Anacardiaceae | S | P | Zoo | 40,500 | 835 | 2 | 0 | 0 | 0 |
| Senna multijuga (Rich.) H.S.Irwin & Barneby | SENMUL | Fabaceae | S | P | Zoo | 89,000 | 835 | 32 | 0 | 0 | 0 |
| Myrsine coriacea (Sw.) R.Br. ex Roem. & Schult. | MYRCOR | Primulaceae | S | P | Zoo | 20,500 | 835 | 0 | 0 | 0 | 0 |
| Croton floribundus (Spreng.) Harms | CROFLOR | Euphorbiaceae | Ss | P | Aut | 31,150 | 835 | 0 | 0 | 0 | 0 |
| Croton urucurana Baill. | CROURU | Euphorbiaceae | Ss | P | Aut | 120,000 | 835 | 0 | 0 | 0 | 0 |
| Model | AIC | |
|---|---|---|
| PBS~E + Gd + S | AIC = −217.54 | |
| PBS~Gd + S | AIC = −219.13 | |
| Variables | Coefficient | Significance |
| Intercept | β0 = −0.021696 | 0.0335 * |
| Germinability (Gd%) | β1 = 0.008474 | <2 × 10−16 *** |
| Survival (S%) | β2 = 0.029903 | 0.0194 * |
| Fitting equation coefficients | ||
| R2 (%) | 99.62 | |
| R2aj (%) | 99.59 | |
| Sxy% | 0.008 | |
| p-value | 2.2 × 10−16 *** | |
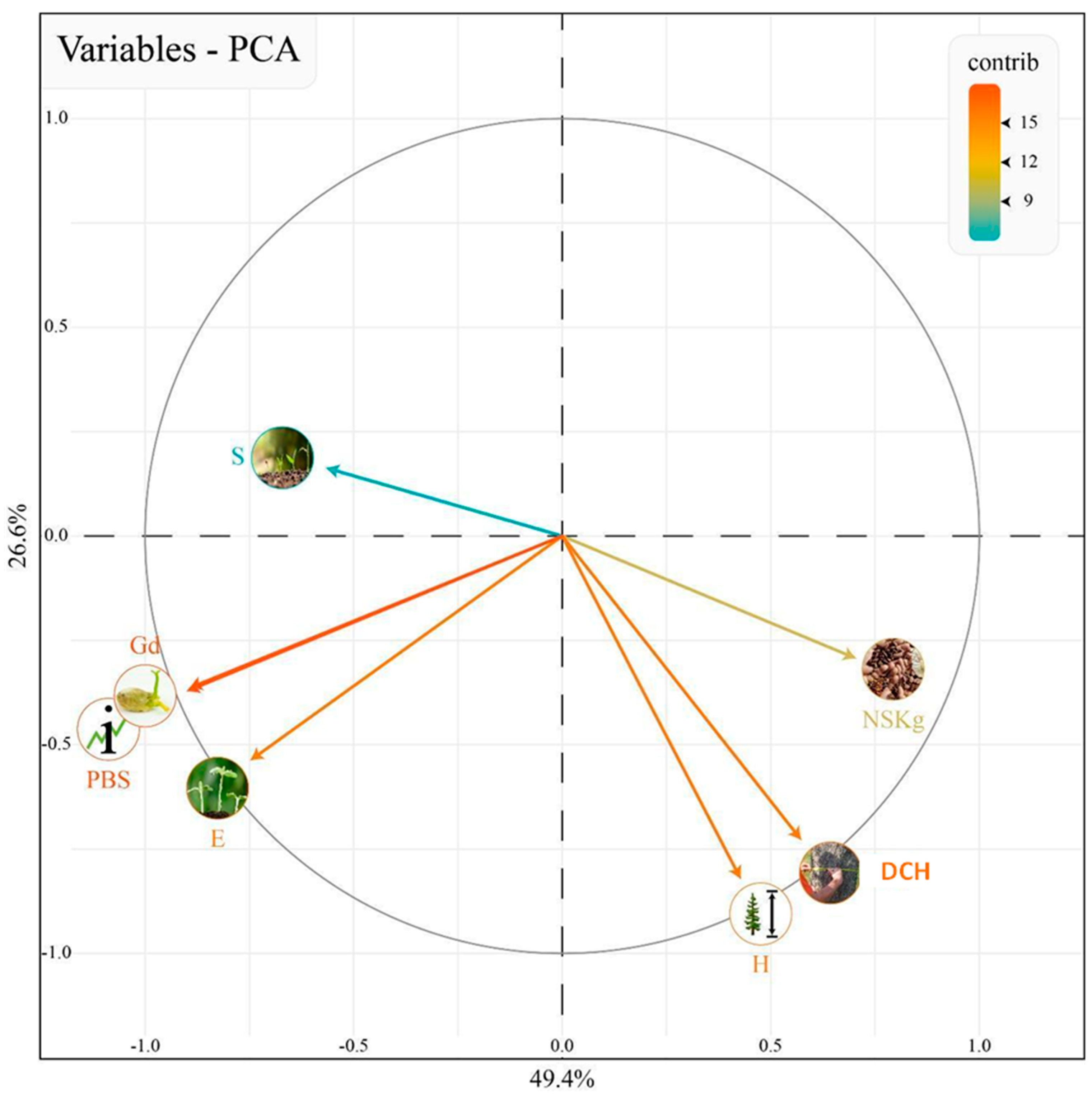
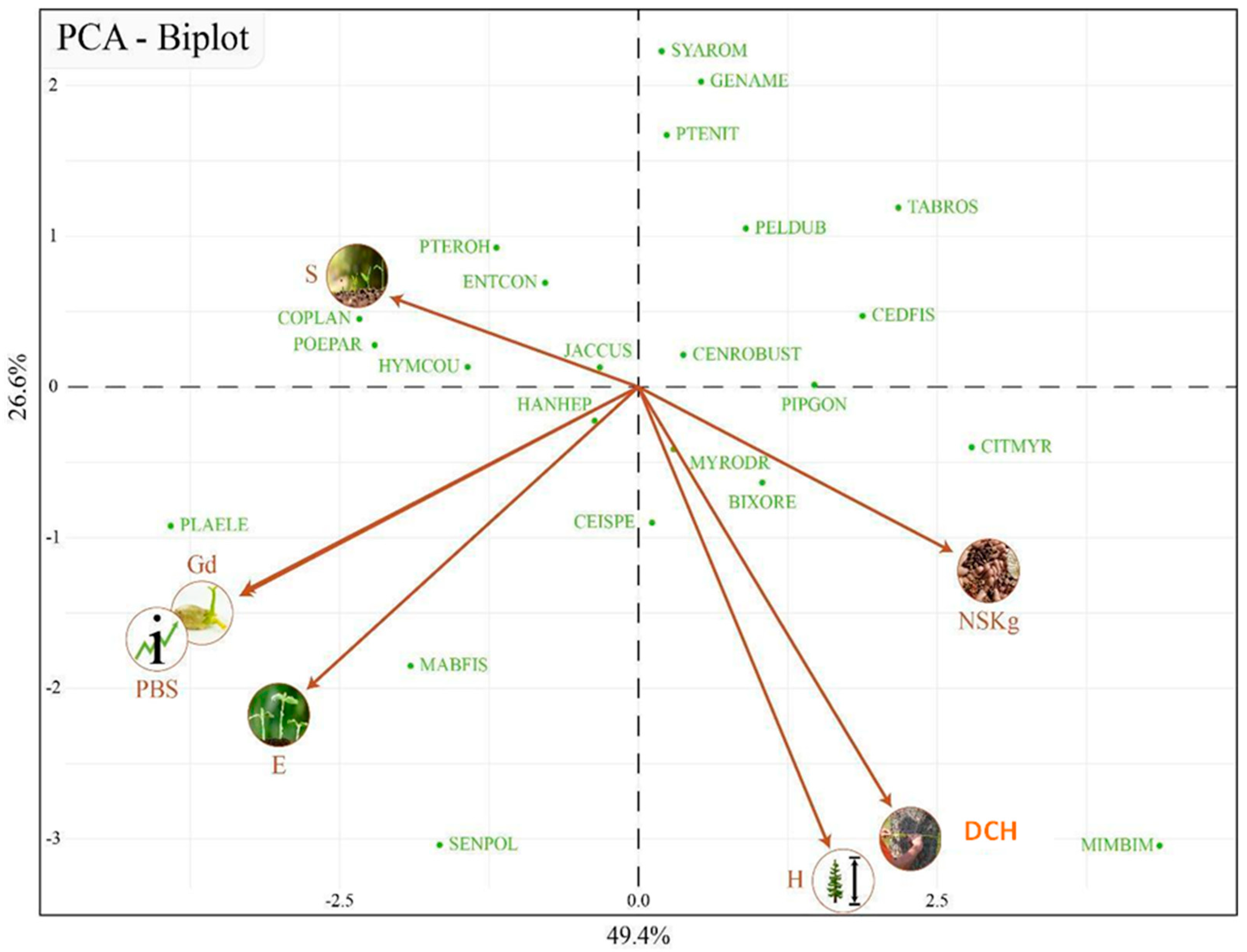
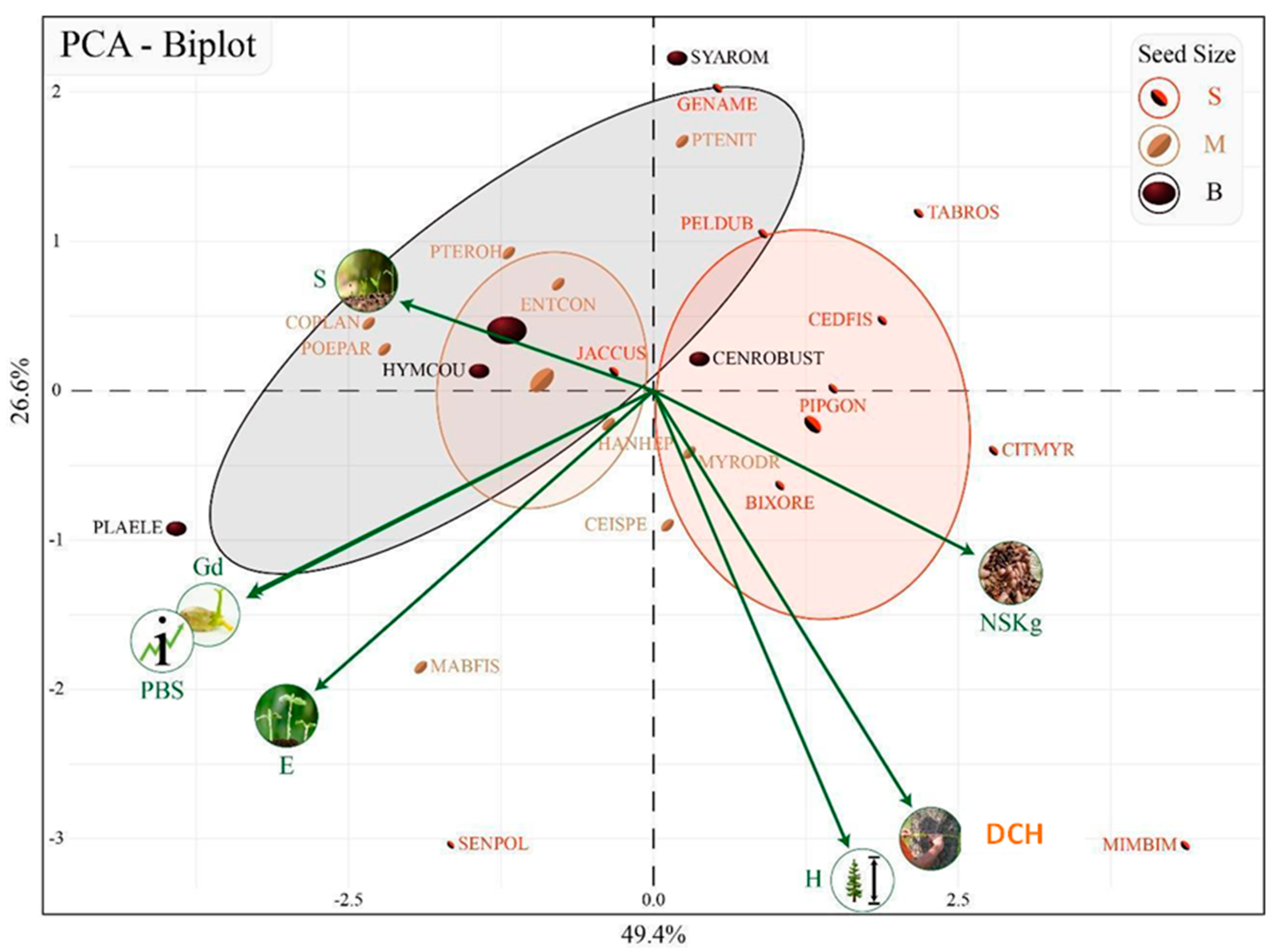
| PC1 | PC2 | PC3 | ||
|---|---|---|---|---|
| Cumulative proportion | 0.4943 | 0.7603 | 0.8919 | |
| Weighting coefficients | Correlation coefficients | |||
| Variables | PC1 | PC2 | PC1 | PC2 |
| E | −0.40 | −0.39 | −0.74 | −0.54 |
| H | 0.23 | −0.60 | 0.43 | −0.82 |
| DCH | 0.31 | −0.53 | 0.57 | −0.72 |
| NSKg | 0.38 | −0.21 | 0.70 | −0.29 |
| Gd | −0.48 | −0.27 | −0.89 | −0.36 |
| S | −0.30 | 0.12 | −0.56 | 0.16 |
| PBS | −0.48 | −0.27 | −0.90 | −0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowski, I.; Paladines, H.M.; de Almeida, L.S.; López, A.M.T.; Dutra, F.B.; Francisco, B.S.; da Silva, J.M.S.; Piña-Rodrigues, F.C.M. Seeds’ Early Traits as Predictors of Performance in Direct Seeding Restoration. Forests 2023, 14, 547. https://doi.org/10.3390/f14030547
Piotrowski I, Paladines HM, de Almeida LS, López AMT, Dutra FB, Francisco BS, da Silva JMS, Piña-Rodrigues FCM. Seeds’ Early Traits as Predictors of Performance in Direct Seeding Restoration. Forests. 2023; 14(3):547. https://doi.org/10.3390/f14030547
Chicago/Turabian StylePiotrowski, Ivonir, Harvey Marin Paladines, Lausanne Soraya de Almeida, Alex Mauri Tello López, Felipe Bueno Dutra, Bruno Santos Francisco, José Mauro Santana da Silva, and Fatima C. Márquez Piña-Rodrigues. 2023. "Seeds’ Early Traits as Predictors of Performance in Direct Seeding Restoration" Forests 14, no. 3: 547. https://doi.org/10.3390/f14030547





