Growth of Populus × euramericana Plantlet under Different Light Durations
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Growth of Plantlets
3.2. Construction of Growth Curves
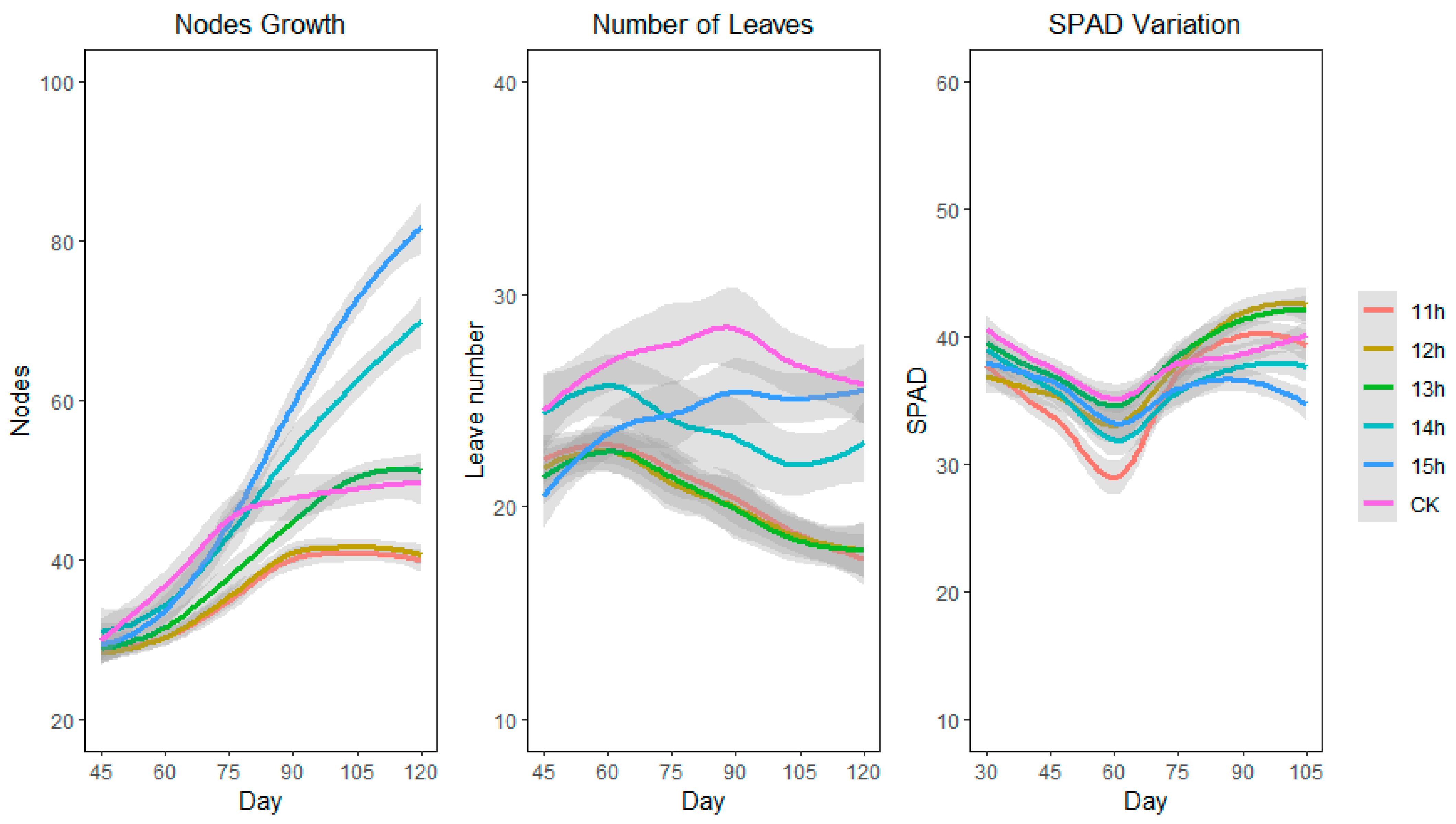
3.3. Number of Nodes and Leaves and SPAD Value
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tripathi, S.; Hoang, Q.T.N.; Han, Y.J.; Kim, J.I. Regulation of photomorphogenic development by plant phytochromes. Int. J. Mol. Sci. 2019, 20, 6165. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Terzaghi, W.; Gong, Y.; Li, C.; Ling, J.J.; Fan, Y.; Qin, N.; Gong, X.; Zhu, D.; Deng, X.W. Modulation of BIN2 kinase activity by HY5 controls hypocotyl elongation in the light. Nat. Commun. 2020, 11, 1592. [Google Scholar] [CrossRef] [PubMed]
- Young, E.; Hanover, J.W. Effects of quality, intensity, and duration of light breaks during a long night on dormancy in blue spruce (Picea pungens Engelm.) seedlings. Plant Physiol. 1977, 60, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Xu, D.; Li, J.; Deng, X.W. COP9 Signalosome: Discovery, conservation, activity and function. J. Integr. Plant Biol. 2020, 62, 90–103. [Google Scholar] [CrossRef]
- Fankhauser, C.; Chory, J. Light control of plant development. Annu. Rev. Cell Dev. Biol. 1997, 13, 203–229. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Sánchez, J.M.; Triozzi, P.M.; Alique, D.; Geng, F.; Gao, M.; Jaeger, K.E.; Wigge, P.A.; Allona, I.; Perales, M. LHY2 integrates night-length information to determine timing of poplar photoperiodic growth. Curr. Biol. 2019, 29, 2402–2406.e4. [Google Scholar] [CrossRef]
- Meng, L.; Zhou, Y.; Gu, L.; Richardson, A.D.; Peñuelas, J.; Fu, Y.; Wang, Y.; Asrar, G.R.; De Boeck, H.J.; Mao, J.; et al. Photoperiod decelerates the advance of spring phenology of six deciduous tree species under climate warming. Glob. Chang. Biol. 2021, 27, 2914–2927. [Google Scholar] [CrossRef]
- Ream, T.S.; Woods, D.P.; Schwartz, C.J.; Sanabria, C.P.; Mahoy, J.A.; Walters, E.M.; Kaeppler, H.F.; Amasino, R.M. Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 2014, 164, 694–709. [Google Scholar] [CrossRef]
- Qin, Z.; Bai, Y.; Muhammad, S.; Wu, X.; Deng, P.; Wu, J.; An, H.; Wu, L. Divergent roles of FT-like 9 in flowering transition under different day lengths in Brachypodium distachyon. Nat. Commun. 2019, 10, 812. [Google Scholar] [CrossRef]
- Grime, J.P.; Mason, G.; Curtis, A.V.; Rodman, J.; Band, S.R. A comparative study of germination characteristics in a local flora. J. Ecol. 1981, 69, 1017–1059. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Zhou, J. Effect of exponential fertilization on growth and nutritional status in Buddhist pine (Podocarpus macrophyllus [Thunb.] D. Don) seedlings cultured in natural and prolonged photoperiods. Soil Sci. Plant Nutr. 2013, 59, 933–941. [Google Scholar] [CrossRef]
- Or’eilly, C.; Arnott, J.T.; Owens, J.N. Effect of photoperiod and moisture availability on shoot growth, seedlingmorphology, and cuticle and epicuticularwax features of container grown western hemlock seedlings. Can. J. For. Res. 1989, 19, 122–131. [Google Scholar] [CrossRef]
- Oleksyn, J.; Tjoelker, M.G.; Reich, P.B. Growth and biomass partitioning of populations of European Pinus sylvestris L. under simulated 50° and 60° N daylengths: Evidence for photoperiodic ecotypes. New Phytol. 1992, 120, 561–574. [Google Scholar] [CrossRef]
- Li, X.W.; Chen, Q.X.; Lei, H.Q.; Wang, J.W.; Yang, S.; Wei, H.X. Nutrient uptake and utilization by fragrant rosewood (Dalbergia odorifera) seedlings cultured with oligosaccharide addition under different lighting spectra. Forests 2018, 9, 29. [Google Scholar] [CrossRef]
- Wang, S.; Fang, H.; Xie, J.; Wu, Y.; Tang, Z.; Liu, Z.; Lv, J.; Yu, J. Physiological responses of cucumber seedlings to different supplemental light duration of red and blue LED. Front. Plant Sci. 2021, 12, 1403. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, M.; Jeong, B.R. Effect of supplementary lighting duration on growth and activity of antioxidant enzymes in grafted watermelon seedlings. Agronomy 2020, 10, 337. [Google Scholar] [CrossRef]
- Dueck, T.; Janse, J.; Eveleens, B.A.; Kempkes, F.L.K.; Marcelis, L.F.M. Growth of tomatoes under hybrid LED and HPS lighting. Acta Hortic. 2012, 952, 335–342. [Google Scholar] [CrossRef]
- Gómez, C.; Mitchell, C.A. In search of an optimized supplemental lighting spectrum for greenhouse tomato production with intracanopy lighting. Acta Hortic. 2016, 1134, 57–62. [Google Scholar] [CrossRef]
- Lu, N.; Maruo, T.; Johkan, M.; Hohjo, M.; Tsukagoshi, S.; Ito, Y.; Ichimura, T.; Shinohara, Y. Effect of supplemental lighting within the canopy at different developing stages on tomato yield and quality of single-truss tomato plants grown at high density. Environ. Control Biol. 2012, 50, 1–11. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Chun, I.J.; Jeong, J.H.; Kwon, S.T.; Hwang, J.M. Response of the growth characteristics and phytochemical contents of pepper (Capsicum annuum L.) seedlings with supplemental LED light in glass house. J. Bio-Environ. Control 2011, 20, 182–188. [Google Scholar]
- Kwak, M.J.; Je, S.M.; Cheng, H.C.; Seo, S.M.; Park, J.H.; Baek, S.G.; Khaine, I.; Lee, T.; Jang, J.; Li, Y.; et al. Night light-adaptation strategies for photosynthetic apparatus in yellow-poplar (Liriodendron Tulipifera L.) exposed to artificial night lighting. Forests 2018, 9, 74. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Leperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Riikonen, J. Pre-cultivation of Scots pine and Norway spruce transplant seedlings under four different light spectra did not affect their field performance. New Forest. 2016, 47, 607–619. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhang, Y.; Wang, T.; Yang, Q.; Yang, Y.; Li, Z.; Li, B.; Wen, X.; Li, W.; Yin, W.; et al. An alternative splicing variant of PtRD26 delays leaf senescence by regulating multiple NAC transcription factors in Populus. Plant Cell 2021, 33, 1594–1614. [Google Scholar] [CrossRef]
- Hao, C.; Yang, Y.; Du, J.; Deng, X.W.; Li, L. The PCY-SAG14 phytocyanin module regulated by PIFs and miR408 promotes dark-induced leaf senescence in Arabidopsis. Proc. Natl. Acad. Sci. USA 2022, 119, e2116623119. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Chen, L.; Meng, X.; Zhen, X.; Liang, Y.; Han, Y.; Li, H.; Zhang, B. Identification and function analysis of yellow-leaf mutant (YX-yl) of broomcorn millet. BMC Plant Biol. 2022, 22, 463. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Fang, S.L.; Wu, Y.F.; Chu, Y.C.; Kuo, B.J. Using sigmoid growth curves to establish growth models of tomato and eggplant stems suitable for grafting in subtropical countries. Horticulturae 2021, 7, 537. [Google Scholar] [CrossRef]
- Hirose, T.; Oikawa, S. Mean residence time of leaf number, area, mass, and nitrogen in canopy photosynthesis. Oecologia 2012, 169, 927–937. [Google Scholar] [CrossRef]
- Li, D.; Chen, J.M.; Yu, W.; Zheng, H.; Yao, X.; Cao, W.; Wei, D.; Xiao, C.; Zhu, Y.; Cheng, T. Assessing a soil-removed semi-empirical model for estimating leaf chlorophyll content. Remote Sens. Environ. 2022, 282, 113284. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhang, L.C.; Hong, S.S.; Shen, Y.G. Responses of gas exchange and chlorophyll fluorescence to different low temperatures in Satsuma mandarin (Citrus unshiu Marc.). Acta Phytophysiol. Sin. 2000, 26, 88–94. (In Chinese) [Google Scholar]
- Fang, S.; Xue, J.; Tang, L. Biomass production and carbon sequestration potential in poplar plantations with different management patterns. J. Environ. Manag. 2007, 85, 672–679. [Google Scholar] [CrossRef]
- Shi, Q.; Tian, D.; Wang, J.; Chen, A.; Miao, Y.; Chen, Y.; Li, J.; Wu, X.; Zheng, B.; Guo, W.; et al. Overexpression of miR390b promotes stem elongation and height growth in Populus. Hortic. Res. 2022, 10, uhac258. [Google Scholar] [CrossRef] [PubMed]
- Fang, S. Silviculture of poplar plantation in China: A review. J. Appl. Ecol. 2008, 19, 2308–2316. [Google Scholar]
- Zlatković, M.; Tenorio-Baigorria, I.; Lakatos, T.; Tóth, T.; Koltay, A.; Pap, P.; Marković, M.; Orlović, S. Bacterial canker disease on Populus × euramericana Caused by Lonsdalea populi in Serbia. Forests 2020, 11, 1080. [Google Scholar] [CrossRef]
- Pallardy, S.G.; Gibbins, D.E.; Rhoads, J.L. Biomass production by two-year-old poplar clones on floodplain sites in the Lower Midwest, USA. Agroforest. Syst. 2003, 59, 21–26. [Google Scholar] [CrossRef]
- Böhlenius, H.; Huang, T.; Charbonnel-Campaa, L.; Brunner, A.M.; Jansson, S.; Strauss, S.H.; Nilsson, O. CO/FT regulatory module controls timing of flowering and seasonal growth cessation in trees. Science 2006, 312, 1040–1043. [Google Scholar] [CrossRef]
- Meyer, M.; Morgenstern, K.; Heilig, D.; Heil, B.; Kovács, G.; Leibing, C.; Krabel, D. Biomass allocation and root characteristics of early-stage poplars (Populus spp.) for assessing their water-deficit response during SRC establishment. BioEnerg. Res. 2021, 14, 385–398. [Google Scholar] [CrossRef]
- Zhang, M.; Suren, H.; Holliday, J.A. Phenotypic and genomic local adaptation across latitude and altitude in Populus trichocarpa. Genome Biol. Evol. 2019, 11, 2256–2272. [Google Scholar] [CrossRef]
- Ceulemans, R.; Deraedt, W. Production physiology and growth potential of poplars under short-rotation forestry culture. Forest Ecol. Manag. 1999, 121, 9–23. [Google Scholar] [CrossRef]
- Yu, Z.M.; Kang, W.J.; Tu, S.P. Simulation of growth curve based on nonlinear models of logistic and gompertz for Euscaphis konishii seedling. Acta Agric. Univ. Jiangxiensis 2017, 39, 1187–1195. (In Chinese) [Google Scholar]
- Yazdan, I.; Majid, N.; Gholamhassan, R. Investigation on germination and seedling growth of three Salicornia species in response to different levels of salinity stress originated from sodium chloride using gompertz function. Environ. Stre. Crop Sci. 2022, 15, 231–246. [Google Scholar]
- Johnsen, K.H.; Seiler, J.R. Growth, shoot phenology and physiologyof diverse seed sources of black spruce: I. Seedling responses to varied atmospheric CO2 concentrations and photoperiods. Tree Physiol. 1996, 16, 367–373. [Google Scholar] [CrossRef]
- Deng, X.W.; Quail, P.H. Signalling in light-controlled development. Semin. Cell Dev. Biol. 1999, 10, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Arnott, T.A. Photoperiod control of container seedlings. In Proceedings of the Western Forest Nursery Council and Intermountain Nurseryman’s Association, Ogden, Utah, 14–16 August 1984; pp. 9–13. [Google Scholar]
- Yin, J.; Lin, F.; De Lombaerde, E.; Mao, Z.; Liu, S.; Ye, J.; Fang, S.; Wang, X. The effects of light, conspecific density and soil fungi on seedling growth of temperate tree species. Forest Ecol. Manag. 2023, 529, 120683. [Google Scholar] [CrossRef]
- Huo, C. Effects of light and nitrogen on growth, carbon and nitrogen metabolism of Fraxinus mandshurica seedlings. J. Northeast For. Univ. 2009, 45, 38–44. (In Chinese) [Google Scholar]
- Xiao, F.; Chen, C.; Gong, W.; Xiong, Y.; Zhou, Y.; Guo, W.; Li, B.; Wang, Y. Trade-off between shade tolerance and chemical resistance of invasive Phytolacca americana under different light levels compared with its native and exotic non-invasive congeners. Environ. Exp. Bot. 2022, 196, 104809. [Google Scholar] [CrossRef]
- Beuker, E. Adaptation to climatic changes of the timing of bud burst in populations of Pinus sylvestris L. and Picea abies (L.) Karst. Tree Physiol. 1994, 14, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, M.Y.; Liu, N.; Ding, C.; Gu, B.; Chen, C.; Ning, K.; Su, X.; Huang, Q. Effects of different irradiation duration on growth and photosynthetic characteristics of Populus × euramericana seedlings. Sci. Silv. Sin. 2018, 54, 33–41. (In Chinese) [Google Scholar]
- Murray, M.B.; Cannell, M.G.R.; Smith, R.I. Date of budburst of fifteen tree species in Britain following climatic warming. J. Appl. Ecol. 1989, 26, 693–700. [Google Scholar] [CrossRef]
- Howe, G.T.; Hackett, W.P.; Furnier, G.R.; Klevorn, R.E. Photoperiodic responses of a northern and southern ecotype of black cottonwood. Physiol. Plant 1995, 93, 695–708. [Google Scholar] [CrossRef]
- Ekmekci, Y.; Terzoğlu, S. Interactive effects of vernalization, day length and light intensity on the number of leaves and flag leaf area in some wheat cultivars. Turk. J. Bot. 1998, 22, 303–312. [Google Scholar]
- Szulc, P.; Bocianowski, J.; Nowosad, K.; Zielewicz, W.; Kobus-Cisowska, J. SPAD leaf greenness index: Green mass yield indicator of maize (Zea mays L.), genetic and agriculture practice relationship. Plants 2021, 10, 830. [Google Scholar] [CrossRef] [PubMed]
- Lim, P.O.; Kim, H.J.; Hong, G.N. Leaf senescence. Annu. Rev. Plant Biol. 2007, 58, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Bergstand, K.-J.; Schüssler, H.K. Growth, development and photosynthesis of some horticultural plants as affected by different supplementary lighting technologies. Europ. J. Hort. Sci. 2013, 78, 119–125. [Google Scholar]
- Yue, X.; Hu, Y.; Zhang, H.; Schmidhalter, S. Evaluation of both SPAD reading and SPAD index on estimating the plant nitrogen status of winter wheat. Int. J. Plant Prod. 2020, 14, 67–75. [Google Scholar] [CrossRef]
- Du, X.; Gao, Z.; Sun, X.; Bian, D.; Ren, J.; Yan, P.; Cui, Y. Increasing temperature during early spring increases winter wheat grain yield by advancing phenology and mitigating leaf senescence. Sci. Total Environ. 2022, 812, 152557. [Google Scholar] [CrossRef]


| Function Type | Function Name | Equation | Ranges of C |
|---|---|---|---|
| Quasi-linear | Generalized Single Index | y = a + b ∗ exp(−c ∗ t) | 0–50 |
| Quasi-power | Richards | y = a ∗ (1 − exp(−c ∗ t))^b | 0–2 |
| Quasi-hyperbolic | Logistic | y = a/(1 + b ∗ exp(−c ∗ t)) | 0.1–9 |
| Quasi-power | Levakovic | y = a ∗ (t^2/(c + t^2))^b | 1–50 |
| Quasi-exponential | Gompertz | y = a ∗ exp(−b ∗ exp(−c ∗ t)) | 0–2 |
| Quasi-linear | Log-Linear | y = a + b ∗ log(t + c) | 0–50 |
| Trait | Days | 11 h | 12 h | 13 h | 14 h | 15 h | CK |
|---|---|---|---|---|---|---|---|
| H | 150 | 60.80 ± 10.07 | 61.66 ± 8.02 | 64.66 ± 15.87 | 101.51 ± 36.69 | 99.53 ± 31.33 | 102.19 ± 42.49 |
| 135 | 60.80 ± 10.06 | 61.66 ± 8.02 | 64.66 ± 15.87 | 99.04 ± 35.93 | 95.92 ± 30.54 | 100.51 ± 42.73 | |
| 120 | 60.80 ± 10.05 | 61.66 ± 8.02 | 64.66 ± 15.87 | 96.07 ± 34.10 | 92.85 ± 27.70 | 100.10 ± 42.50 | |
| 105 | 60.80 ± 10.04 | 61.66 ± 8.02 | 64.66 ± 15.87 | 93.13 ± 31.57 | 88.80 ± 25.12 | 99.17 ± 41.36 | |
| 90 | 59.14 ± 10.22 | 60.18 ± 8.14 | 63.88 ± 15.27 | 89.27 ± 28.04 | 83.33 ± 20.97 | 95.51 ± 36.67 | |
| 75 | 58.32 ± 10.28 | 59.81 ± 8.27 | 62.31 ± 13.30 | 81.08 ± 19.71 | 75.63 ± 15.96 | 83.83 ± 28.18 | |
| 60 | 57.35 ± 10.23 | 59.08 ± 8.00 | 59.75 ± 10.22 | 72.48 ± 13.16 | 65.76 ± 10.09 | 68.69 ± 16.04 | |
| 45 | 54.83 ± 9.59 | 55.59 ± 6.84 | 55.53 ± 8.98 | 65.12 ± 11.32 | 58.58 ± 7.86 | 57.45 ± 11.58 | |
| 30 | 37.98 ± 5.11 | 37.32 ± 4.47 | 36.39 ± 4.84 | 41.25 ± 6.22 | 39.75 ± 5.38 | 39.37 ± 7.10 | |
| 0 | 23.67 ± 4.01 | 23.62 ± 3.90 | 23.69 ± 4.05 | 23.71 ± 4.10 | 23.71 ± 4.10 | 23.69 ± 4.08 | |
| GD | 150 | 6.60 ± 0.88 | 6.46 ± 0.70 | 7.11 ± 1.30 | 7.76 ± 1.75 | 7.40 ± 1.62 | 9.49 ± 2.56 |
| 135 | 6.29 ± 0.75 | 6.20 ± 0.68 | 6.57 ± 1.09 | 7.25 ± 1.48 | 6.92 ± 1.34 | 8.94 ± 2.41 | |
| 120 | 6.11 ± 0.70 | 6.04 ± 0.66 | 6.35 ± 1.00 | 6.86 ± 1.29 | 6.54 ± 1.12 | 8.58 ± 2.31 | |
| 105 | 5.98 ± 0.67 | 5.90 ± 0.63 | 6.07 ± 0.73 | 6.55 ± 0.99 | 6.22 ± 0.89 | 8.10 ± 1.96 | |
| 90 | 5.86 ± 0.66 | 5.75 ± 0.60 | 5.90 ± 0.66 | 6.34 ± 0.88 | 5.96 ± 0.78 | 7.65 ± 1.72 | |
| 75 | 5.67 ± 0.63 | 5.56 ± 0.59 | 5.64 ± 0.64 | 6.03 ± 0.70 | 5.67 ± 0.62 | 6.98 ± 1.41 | |
| 60 | 5.47 ± 0.63 | 5.34 ± 0.55 | 5.24 ± 0.52 | 5.70 ± 0.66 | 5.32 ± 0.50 | 5.89 ± 0.80 | |
| 45 | 5.25 ± 0.59 | 5.10 ± 0.53 | 4.96 ± 0.48 | 5.36 ± 0.59 | 5.08 ± 0.44 | 5.39 ± 0.64 | |
| 30 | 4.67 ± 0.56 | 4.40 ± 0.52 | 4.33 ± 0.49 | 4.58 ± 0.56 | 4.56 ± 0.45 | 4.59 ± 0.56 | |
| 0 | 3.66 ± 0.56 | 3.61 ± 0.55 | 3.62 ± 0.51 | 3.65 ± 0.56 | 3.65 ± 0.54 | 3.64 ± 0.56 |
| Figure | Ranges of R2 | |
|---|---|---|
| H | GD | |
| Generalized Single Index | 0.7756–0.9802 | 0.8583–0.9751 |
| Richards | 0.5535–0.7746 | 0.4132–0.6666 |
| Logistic | 0.5516–0.7732 | 0.7640–0.9566 |
| levakovic | 0.5524–0.7738 | 0.4121–0.6655 |
| Gompertz | 0.8947–0.9871 | 0.9104–0.9793 |
| Log-Linear | 0.7676–0.9755 | 0.8321–0.9694 |
| Trait | Treatment | a | b | c | t1 | tmax | t2 | Expected Duration | R2 |
|---|---|---|---|---|---|---|---|---|---|
| H | 11 h | 62.736 | 0.992 | 0.033 | 12 | 46 | 80 | 68 | 0.9484 |
| 12 h | 63.913 | 1.017 | 0.032 | 12 | 48 | 83 | 71 | 0.9547 | |
| 13 h | 68.032 | 1.083 | 0.029 | 14 | 56 | 98 | 84 | 0.9153 | |
| 14 h | 110.867 | 1.572 | 0.021 | 29 | 114 | 199 | 170 | 0.9386 | |
| 15 h | 112.989 | 1.587 | 0.018 | 34 | 135 | 235 | 201 | 0.9871 | |
| CK | 119.668 | 1.667 | 0.018 | 35 | 139 | 242 | 207 | 0.8947 | |
| GD | 11 h | 6.650 | 0.594 | 0.018 | 12 | 49 | 85 | 73 | 0.9664 |
| 12 h | 6.706 | 0.623 | 0.016 | 15 | 59 | 103 | 88 | 0.9758 | |
| 13 h | 8.607 | 0.869 | 0.009 | 36 | 141 | 246 | 210 | 0.9634 | |
| 14 h | 8.997 | 0.900 | 0.011 | 32 | 125 | 219 | 187 | 0.9793 | |
| 15 h | 9.527 | 0.946 | 0.008 | 45 | 175 | 305 | 261 | 0.9663 | |
| CK | 15.030 | 1.443 | 0.008 | 71 | 279 | 488 | 417 | 0.9104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Liu, N.; Ding, C.; Liu, F.; Su, X.; Huang, Q. Growth of Populus × euramericana Plantlet under Different Light Durations. Forests 2023, 14, 579. https://doi.org/10.3390/f14030579
Liu C, Liu N, Ding C, Liu F, Su X, Huang Q. Growth of Populus × euramericana Plantlet under Different Light Durations. Forests. 2023; 14(3):579. https://doi.org/10.3390/f14030579
Chicago/Turabian StyleLiu, Chenggong, Ning Liu, Changjun Ding, Fenfen Liu, Xiaohua Su, and Qinjun Huang. 2023. "Growth of Populus × euramericana Plantlet under Different Light Durations" Forests 14, no. 3: 579. https://doi.org/10.3390/f14030579
APA StyleLiu, C., Liu, N., Ding, C., Liu, F., Su, X., & Huang, Q. (2023). Growth of Populus × euramericana Plantlet under Different Light Durations. Forests, 14(3), 579. https://doi.org/10.3390/f14030579







