Figure 1.
Wood planes of observation. The transverse (Tr) plane is perpendicular to the stem longitudinal axis; the radial longitudinal (Rd) plane is parallel to the stem longitudinal axis and oriented along the direction of a ray of the circumference described by the stem; the tangential longitudinal (Tg) plane is parallel to the stem longitudinal axis and perpendicular to the direction of a ray of the circumference described by the stem.
Figure 1.
Wood planes of observation. The transverse (Tr) plane is perpendicular to the stem longitudinal axis; the radial longitudinal (Rd) plane is parallel to the stem longitudinal axis and oriented along the direction of a ray of the circumference described by the stem; the tangential longitudinal (Tg) plane is parallel to the stem longitudinal axis and perpendicular to the direction of a ray of the circumference described by the stem.
Figure 2.
Sapwood and heartwood color. (A) heartwood color darker than sapwood color (Larix decidua); (B) heartwood and sapwood of the same color (Picea abies); (C) heartwood white (Aesculus hippocastanum); (D) heartwood brown (Tectona grandis); (E) heartwood yellow (Buxus sempervirens); (F) heartwood green (Bulnesia sarmientoi); (G) heartwood red (Pterocarpus soyauxii); (H) heartwood purple (Peltogyne paniculata); (I) heartwood black (Diospyros ebenum); (L) heartwood variegated (Microberlinia brazzavillensis).
Figure 2.
Sapwood and heartwood color. (A) heartwood color darker than sapwood color (Larix decidua); (B) heartwood and sapwood of the same color (Picea abies); (C) heartwood white (Aesculus hippocastanum); (D) heartwood brown (Tectona grandis); (E) heartwood yellow (Buxus sempervirens); (F) heartwood green (Bulnesia sarmientoi); (G) heartwood red (Pterocarpus soyauxii); (H) heartwood purple (Peltogyne paniculata); (I) heartwood black (Diospyros ebenum); (L) heartwood variegated (Microberlinia brazzavillensis).
Figure 3.
Hardwood texture (longitudinal and transverse sections). (A1,A2): fine texture (Pyrus communis); (B1,B2): medium texture (Baillonella toxisperma); (C1,C2): coarse texture (Terminalia superba).
Figure 3.
Hardwood texture (longitudinal and transverse sections). (A1,A2): fine texture (Pyrus communis); (B1,B2): medium texture (Baillonella toxisperma); (C1,C2): coarse texture (Terminalia superba).
Figure 4.
Growth rings appear as concentric circles on transverse surfaces ((A), Pinus echinata), as parallel bands on radial surfaces ((B), Pinus rigida), and as hyperbola branches, or flames, on longitudinal tangential surfaces ((C), Pinus taeda).
Figure 4.
Growth rings appear as concentric circles on transverse surfaces ((A), Pinus echinata), as parallel bands on radial surfaces ((B), Pinus rigida), and as hyperbola branches, or flames, on longitudinal tangential surfaces ((C), Pinus taeda).
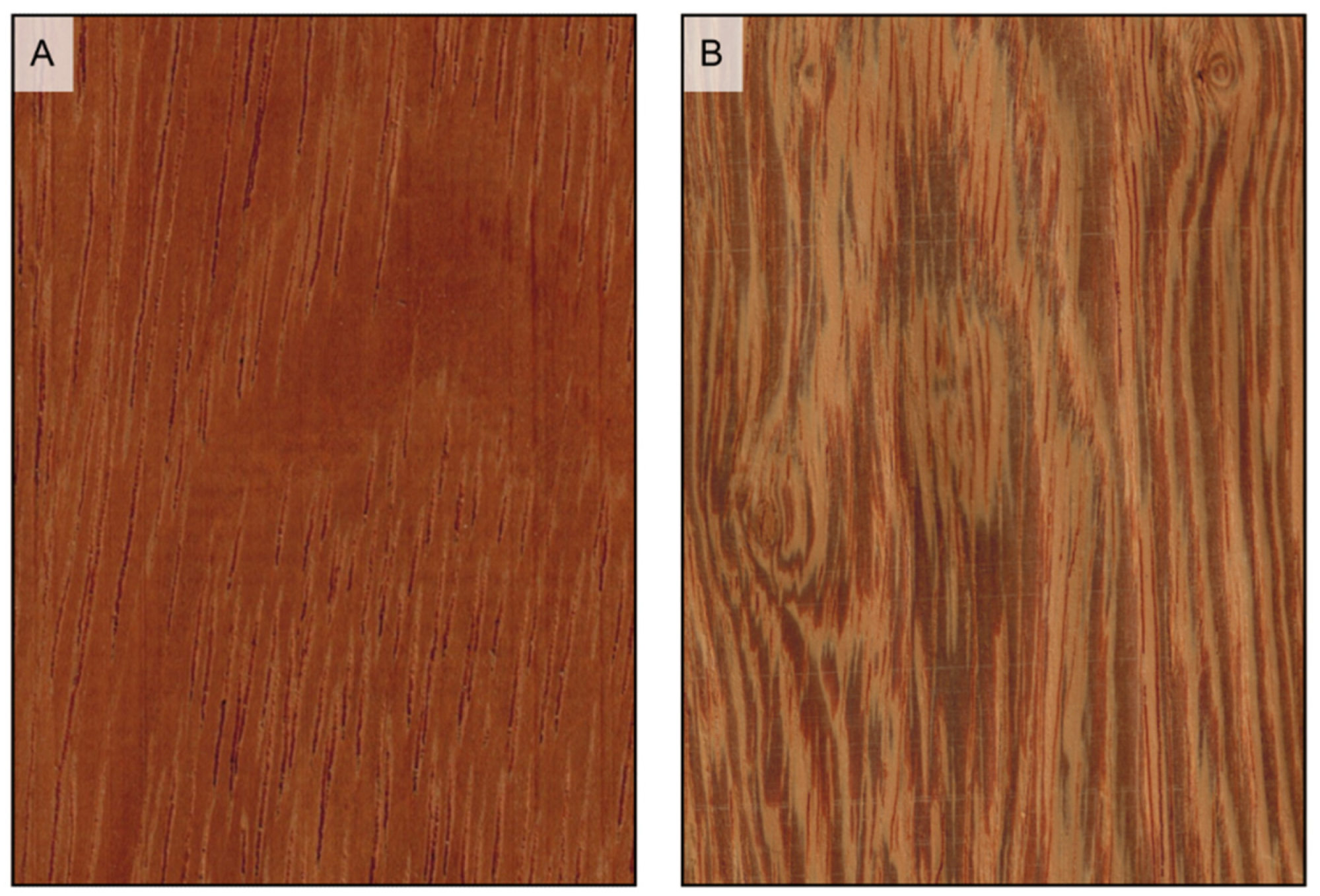
Figure 5.
Earlywood/latewood transition. Pseudotsuga menziesii is an example of abrupt transition ((A1), transverse section) and evident wood figure is ((A2), tangential section); Cupressus sempervirens is an example of gradual transition ((B1), transverse section) and faint wood figure ((B2), tangential section).
Figure 5.
Earlywood/latewood transition. Pseudotsuga menziesii is an example of abrupt transition ((A1), transverse section) and evident wood figure is ((A2), tangential section); Cupressus sempervirens is an example of gradual transition ((B1), transverse section) and faint wood figure ((B2), tangential section).
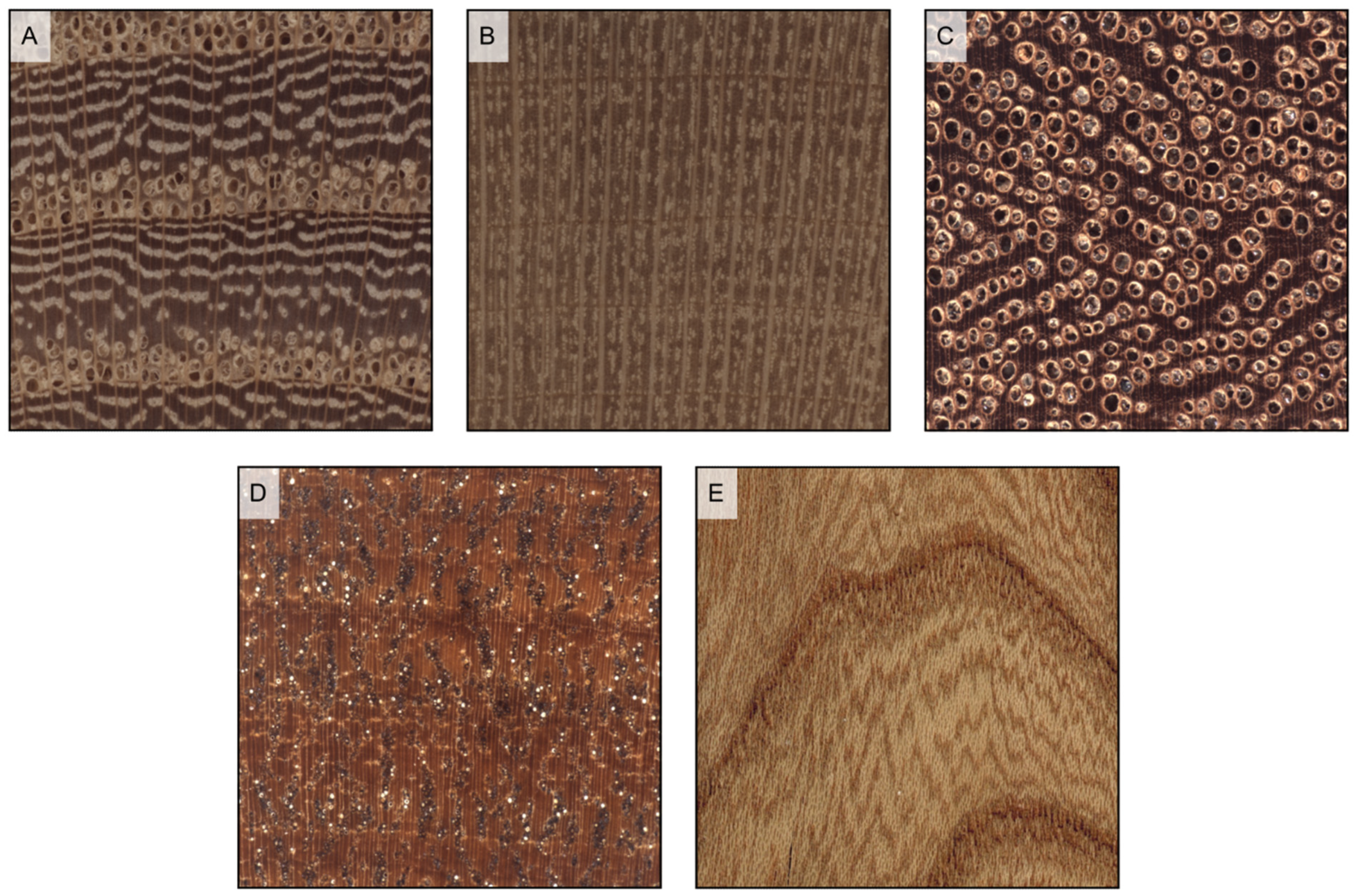
Figure 6.
Wood porosity ((A–F) transverse sections, (G–I) tangential sections). (A) ring-porous, one row only of earlywood vessels, widest tangential spacing between earlywood vessels: more than one earlywood vessel (Carya ovata); (B) ring-porous, more than one row of earlywood vessels, widest tangential spacing between earlywood vessels: one earlywood vessel at most (Gleditsia triacanthos); (C) semi-ring porous, vessels’ diameter gradually narrows from earlywood to latewood (Juglans regia); (D) semi-ring porous, vessels are of the same diameter as latewood ones but much more closely spaced (Prunus avium); (E) diffuse porous, vessels are small (Acer rubrum); (F) diffuse porous, vessels are medium (Astronium graveolens). The figure consequent to growth rings is evident in ring-porous woods ((G) Fraxinus excelsior), visible in semi-ring porous woods ((H), Juglans regia), and faint in diffuse porous woods ((I), Betula pendula).
Figure 6.
Wood porosity ((A–F) transverse sections, (G–I) tangential sections). (A) ring-porous, one row only of earlywood vessels, widest tangential spacing between earlywood vessels: more than one earlywood vessel (Carya ovata); (B) ring-porous, more than one row of earlywood vessels, widest tangential spacing between earlywood vessels: one earlywood vessel at most (Gleditsia triacanthos); (C) semi-ring porous, vessels’ diameter gradually narrows from earlywood to latewood (Juglans regia); (D) semi-ring porous, vessels are of the same diameter as latewood ones but much more closely spaced (Prunus avium); (E) diffuse porous, vessels are small (Acer rubrum); (F) diffuse porous, vessels are medium (Astronium graveolens). The figure consequent to growth rings is evident in ring-porous woods ((G) Fraxinus excelsior), visible in semi-ring porous woods ((H), Juglans regia), and faint in diffuse porous woods ((I), Betula pendula).
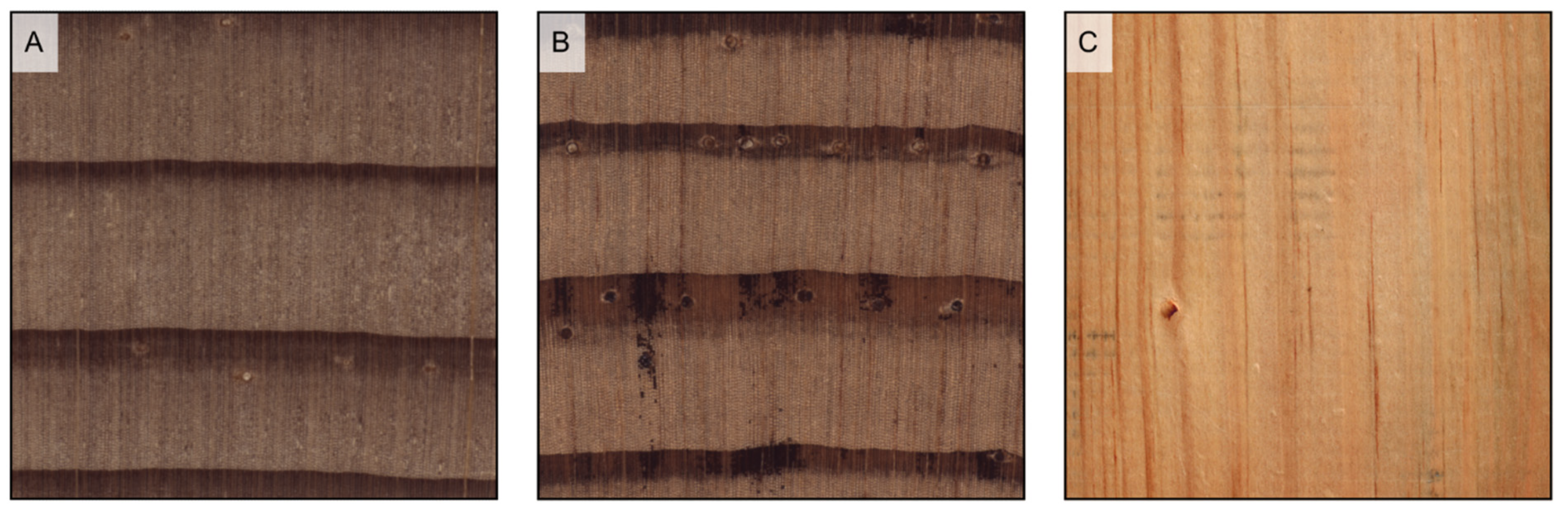
Figure 7.
Axial parenchyma distribution in softwoods (transverse sections). (A) scarce diffuse (Chamaecyparis lawsoniana); (B) tangentially zonate (Cryptomeria japonica); (C) absent (Araucaria angustifolia).
Figure 7.
Axial parenchyma distribution in softwoods (transverse sections). (A) scarce diffuse (Chamaecyparis lawsoniana); (B) tangentially zonate (Cryptomeria japonica); (C) absent (Araucaria angustifolia).
Figure 8.
Axial parenchyma distribution in hardwoods (transverse sections). (A) vasicentric (Khaya ivorensis); (B) lozenge-aliform, confluent, and in marginal or seemingly marginal bands (Afzelia bipindensis); (C) winged-aliform and confluent (Gonystylus bancanus); (D) diffuse (Alstonia macrophylla); (E) diffuse-in-aggregates and vasicentric (Dalbergia retusa); (F) narrow bands and reticulate (Autranella congolensis); (G) wide bands (Millettia laurentii); (H) festooned and scalariform (Grevillea robusta); (I) absent (Salix alba).
Figure 8.
Axial parenchyma distribution in hardwoods (transverse sections). (A) vasicentric (Khaya ivorensis); (B) lozenge-aliform, confluent, and in marginal or seemingly marginal bands (Afzelia bipindensis); (C) winged-aliform and confluent (Gonystylus bancanus); (D) diffuse (Alstonia macrophylla); (E) diffuse-in-aggregates and vasicentric (Dalbergia retusa); (F) narrow bands and reticulate (Autranella congolensis); (G) wide bands (Millettia laurentii); (H) festooned and scalariform (Grevillea robusta); (I) absent (Salix alba).
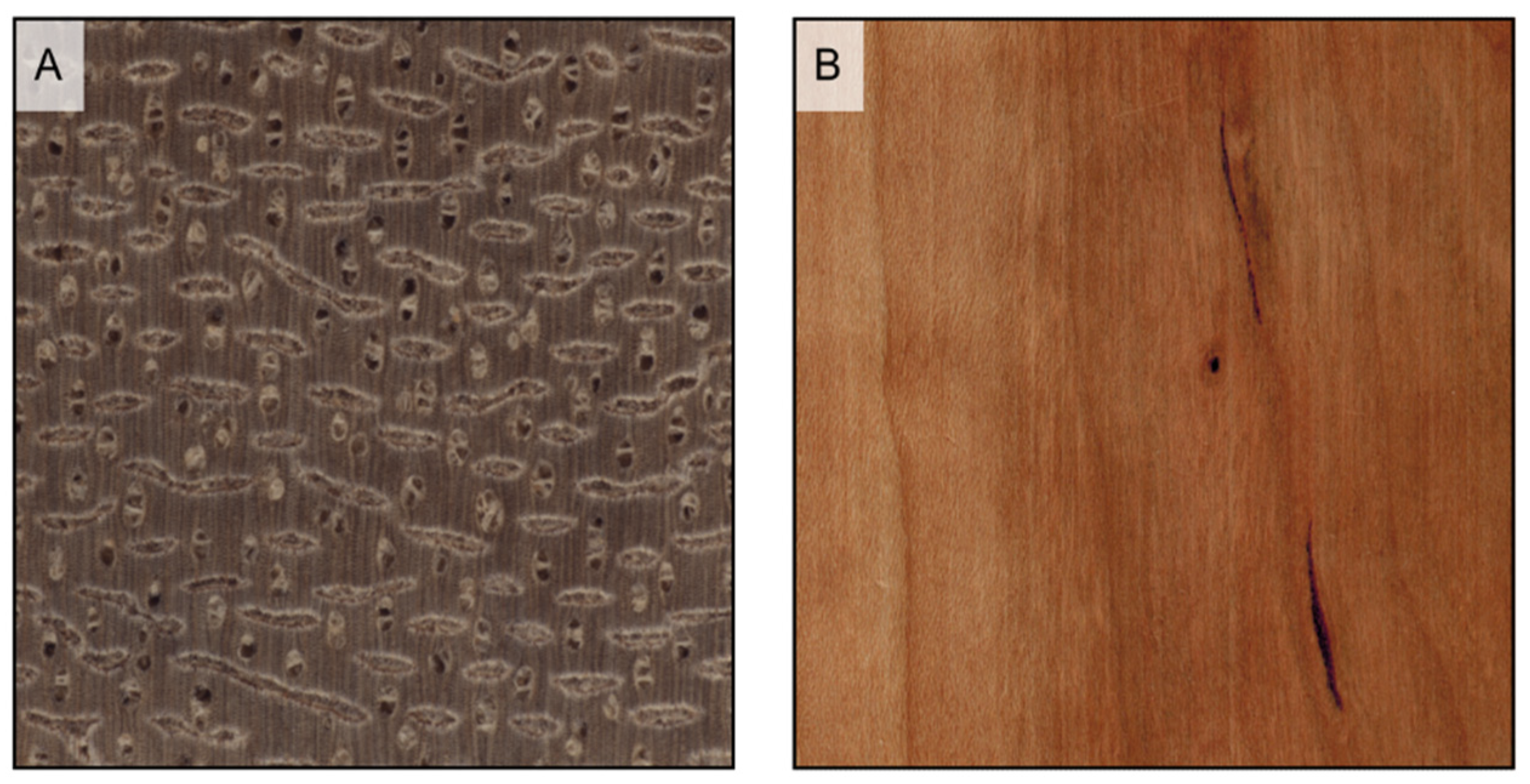
Figure 9.
If prominent enough, axial parenchyma appears on longitudinal surfaces as a light-colored band along the vessels’ grooves when paratracheal ((A) Afzelia bipindensis), and as seemingly growth rings when apotracheal in tangential bands ((B) Amphimas pterocarpoides).
Figure 9.
If prominent enough, axial parenchyma appears on longitudinal surfaces as a light-colored band along the vessels’ grooves when paratracheal ((A) Afzelia bipindensis), and as seemingly growth rings when apotracheal in tangential bands ((B) Amphimas pterocarpoides).
Figure 10.
Rays’ visibility to the naked eye. In Quercus petraea, some of the rays are clearly more evident than the others on the transverse surface ((A1) transverse section), are visible on the tangential surface and over 5 mm high ((A2) longitudinal tangential surface), and visible on the radial surface ((A3) longitudinal radial surface); in Platanus occidentalis, rays are visible on the transverse surface and noded ((B1) transverse section), they are visible on the tangential surface and less than 5 mm high ((B2) longitudinal tangential surface), and visible on the radial surface ((B3) longitudinal radial surface); in Populus tremula, rays are not visible on either the transverse surface ((C1) transverse section) and the tangential surface ((C2) longitudinal tangential surface), while barely noticeable on the radial surface ((C3) longitudinal radial surface); in Pinus sylvestris, rays are not visible on either the transverse surface ((D1) transverse surface) and the tangential surface ((D2) tangential surface), while barely noticeable on the radial surface ((D3) longitudinal radial surface).
Figure 10.
Rays’ visibility to the naked eye. In Quercus petraea, some of the rays are clearly more evident than the others on the transverse surface ((A1) transverse section), are visible on the tangential surface and over 5 mm high ((A2) longitudinal tangential surface), and visible on the radial surface ((A3) longitudinal radial surface); in Platanus occidentalis, rays are visible on the transverse surface and noded ((B1) transverse section), they are visible on the tangential surface and less than 5 mm high ((B2) longitudinal tangential surface), and visible on the radial surface ((B3) longitudinal radial surface); in Populus tremula, rays are not visible on either the transverse surface ((C1) transverse section) and the tangential surface ((C2) longitudinal tangential surface), while barely noticeable on the radial surface ((C3) longitudinal radial surface); in Pinus sylvestris, rays are not visible on either the transverse surface ((D1) transverse surface) and the tangential surface ((D2) tangential surface), while barely noticeable on the radial surface ((D3) longitudinal radial surface).
![Forests 14 00644 g010a]()
![Forests 14 00644 g010b]()
Figure 11.
Ripple marks determined by storied rays on a tangential section of Swietenia macrophylla.
Figure 11.
Ripple marks determined by storied rays on a tangential section of Swietenia macrophylla.
Figure 12.
Vessels’ arrangement (all transverse sections except (E)). (A) tangential bands (latewood vessels only) (Ulmus glabra); (B) radial pattern (Ilex aquifolium); (C) diagonal pattern (Eucalyptus deglupta); (D) dendritic pattern (Bulnesia sarmientoi); (E) jagged pattern on longitudinal tangential surface determined by wavy tangential bands of latewood vessels (Ulmus glabra).
Figure 12.
Vessels’ arrangement (all transverse sections except (E)). (A) tangential bands (latewood vessels only) (Ulmus glabra); (B) radial pattern (Ilex aquifolium); (C) diagonal pattern (Eucalyptus deglupta); (D) dendritic pattern (Bulnesia sarmientoi); (E) jagged pattern on longitudinal tangential surface determined by wavy tangential bands of latewood vessels (Ulmus glabra).
Figure 13.
Vessels’ groupings (transverse sections). (A) in radial multiples of 2–3 vessels (Hevea brasiliensis); (B) exclusively solitary (Quercus ilex); (C) radial multiples of 4 or more common (Dyera costulata); (D) clusters common (Celtis occidentalis).
Figure 13.
Vessels’ groupings (transverse sections). (A) in radial multiples of 2–3 vessels (Hevea brasiliensis); (B) exclusively solitary (Quercus ilex); (C) radial multiples of 4 or more common (Dyera costulata); (D) clusters common (Celtis occidentalis).
Figure 14.
Decorative grain deviations. (A) interlocked grain (Entandrophragma candollei); (B) wavy grain (Acer sp.); (C) quilt (Pterocaprus sp.); (D) burl (Pterocarpus indicus); (E) burl (Ulmus campestris); (F) crotch (Juglans regia).
Figure 14.
Decorative grain deviations. (A) interlocked grain (Entandrophragma candollei); (B) wavy grain (Acer sp.); (C) quilt (Pterocaprus sp.); (D) burl (Pterocarpus indicus); (E) burl (Ulmus campestris); (F) crotch (Juglans regia).
Figure 15.
(A) spiral grain is visible in the tree in the foreground, while it is not present in that in the background (Fagus sp.); (B) spiral grain in a softwood pole. Grain deviates around knots ((C) small knots on Larix decidua; (D) pair of knots on Castanea sativa).
Figure 15.
(A) spiral grain is visible in the tree in the foreground, while it is not present in that in the background (Fagus sp.); (B) spiral grain in a softwood pole. Grain deviates around knots ((C) small knots on Larix decidua; (D) pair of knots on Castanea sativa).
Figure 16.
Vessel deposits (all transverse sections except (E), longitudinal section). Tyloses in the vessels of Robinia pseudoacacia (A) and Morus mesozygia (B); gums in the vessels of Swietenia humilis (C) and Lophira alata (D,E).
Figure 16.
Vessel deposits (all transverse sections except (E), longitudinal section). Tyloses in the vessels of Robinia pseudoacacia (A) and Morus mesozygia (B); gums in the vessels of Swietenia humilis (C) and Lophira alata (D,E).
Figure 17.
Axial canals in softwoods (all transverse sections except (C)). (A) axial canals small (Picea engelmannii); (B) axial canals large (Pinus rigida); (C) vertical streaks on a longitudinal surface determined by axial canals (Pinus chihuahuana).
Figure 17.
Axial canals in softwoods (all transverse sections except (C)). (A) axial canals small (Picea engelmannii); (B) axial canals large (Pinus rigida); (C) vertical streaks on a longitudinal surface determined by axial canals (Pinus chihuahuana).
Figure 18.
Axial canals in hardwoods (all transverse sections except (D)). (A) axial canals diffuse (Daniellia ogea); (B) axial canals in short tangential lines (Dipterocarpus alatus); (C) axial canals in long tangential lines (Shorea acuminata); (D) streaks on a longitudinal surface determined by axial canals (Prioria sp.).
Figure 18.
Axial canals in hardwoods (all transverse sections except (D)). (A) axial canals diffuse (Daniellia ogea); (B) axial canals in short tangential lines (Dipterocarpus alatus); (C) axial canals in long tangential lines (Shorea acuminata); (D) streaks on a longitudinal surface determined by axial canals (Prioria sp.).
Figure 19.
Traumatic canals as they appear on the transverse section of Scleronema micranthum (A) and Cedrus atlantica (B), and on the longitudinal section of Cariniana micrantha (C) and Eucalyptus obliqua (D).
Figure 19.
Traumatic canals as they appear on the transverse section of Scleronema micranthum (A) and Cedrus atlantica (B), and on the longitudinal section of Cariniana micrantha (C) and Eucalyptus obliqua (D).
Figure 20.
Diffuse included phloem on Aquilaria malaccensis ((A) transverse section) and pith flecks on Prunus serotina ((B) longitudinal section).
Figure 20.
Diffuse included phloem on Aquilaria malaccensis ((A) transverse section) and pith flecks on Prunus serotina ((B) longitudinal section).
Table 1.
List of macroscopic features for hardwood and softwood identification according to [
4]. MFN = macroscopic feature number, P = present, A = absent, V = variable, NA = not applicable, TR = Transverse section, TLS = Tangential longitudinal section, RLS = Radial longitudinal section.
Table 1.
List of macroscopic features for hardwood and softwood identification according to [
4]. MFN = macroscopic feature number, P = present, A = absent, V = variable, NA = not applicable, TR = Transverse section, TLS = Tangential longitudinal section, RLS = Radial longitudinal section.
| | Structure | Property | MFN | Character | Character States |
|---|
| Anato-mical features | Hardwood | Growth rings | Growth rings | 1 | Growth rings distinct | P/A/V |
| 2 | Growth rings per cm | Numerical value/NA |
| Vessels | Porosity | 3 | Diffuse porous | P/A/V |
| 4 | Semi-ring porous | P/A/V |
| 5 | Ring-porous | P/A/V |
| 6 | Number of rows of earlywood pores | One row/More than one row/V/NA |
| 7 | Widest tangential spacing between earlywood vessels | One earlywood vessel at most/More than one earlywood vessel |
| Arrangement | 8 | Vessels in tangential bands | P/A/V |
| 9 | Vessels in radial pattern | P/A/V |
| 10 | Vessels in diagonal pattern (echelon) | P/A/V |
| 11 | Vessels in dendritic pattern (flame-like) | P/A/V |
| Groupings | 12 | Solitary and in radial multiples of 2–3 vessels | P/A/V |
| 13 | Exclusively solitary (90% or more) | P/A/V |
| 14 | Radial multiples of 4 or more common | P/A/V |
| 15 | Clusters common | P/A/V |
| Frequency | 16 | ≤5 vessels per square mm | P/A/V |
| 17 | 6–20 vessels per square mm | P/A/V |
| 18 | >20 vessels per square mm | P/A/V |
| Vessel diameter/Pore visibility | 19 | Small (not visible to the naked eye, less than 80 μm) | P/A/V |
| 20 | Medium (just visible to the naked eye, 80–130 μm) | P/A/V |
| 21 | Large (commonly visible to the naked eye, larger than 130 μm) | P/A/V |
| Latewood pore visibility | 22 | Latewood pores large, individually distinct, and few enough that they can be readily counted | P/A/V/NA |
| Vesselless bands | 23 | Vesselless tangential bands | P/A |
| Tyloses | 24 | Tyloses common (TR, TLS, RLS) | P/A/V |
| Vessel deposits | 25 | Gums and other deposits in heartwood vessels (TR, TLS, RLS) | P/A/V |
| 26 | Deposits white (TR, TLS, RLS) | P/A |
| 27 | Deposits yellow (TR, TLS, RLS) | P/A |
| 28 | Deposits dark (TR, TLS, RLS) | P/A |
| Axial parenchyma | Distribution | 29 | Diffuse | P/A/V |
| 30 | Diffuse-in-aggregates | P/A/V |
| 31 | Vasicentric | P/A/V/Unilateral |
| 32 | Lozenge-aliform | P/A/V/Unilateral |
| 33 | Winged-aliform | P/A/V/Unilateral |
| 34 | Confluent | P/A/V/Unilateral |
| 35 | Banded | Majority wide/Majority narrow/V/A |
| 36 | Banded parenchyma distribution | Throughout the ring/In latewood only/In earlywood only/NA |
| 37 | Parenchyma bands wider than rays | P/A/V |
| 38 | Parenchyma in marginal or seemingly marginal bands | P/A/V |
| 39 | Reticulate | P/A/V |
| 40 | Scalariform | P/A/V |
| 41 | Festooned | P/A/V |
| 42 | Predominant parenchyma pattern within the body of the ‘growth ring’ | A/Diffuse/Diffuse-in-aggregates/Vasicentric/Lozenge-aliform/Winged-aliform/Confluent/Ban-ded/In marginal or seemingly marginal bands/Reticulate/Scalariform/Festooned |
| Rays | Width | 43 | Ray visibility to the naked eye on the transverse section | Rays not visible/Rays visible/Some of the rays clearly more evident than the others |
| 44 | Ray visibility with the naked eye on the tangential surface (TLS) | Rays not visible/Rays visible |
| 45 | Ratio of ray width to pore diameter | Larger rays narrower than wider pores/Larger rays as wide or wider than wider pores |
| 46 | Noded rays | P/A/V |
| Storying | 47 | Ray storying (TLS) | Not storied (absent)/Regular coarse storying/Regular fine storying/Irregular coarse storying/Irregular fine storying |
| Height | 48 | Ray height (TLS) | Highest rays less than 5 mm high/Highest rays more than 5 mm high |
| Rays per mm | 49 | Rays per mm | ≤4 mm/5–12 mm/>12 mm/NA |
| Wood rayless | 50 | | |
| Fibers | Arrangement | 51 | Fibers in radial arrangement | P/A |
| Canals | Intercellular canals | 52 | Axial canals | A/Diffuse/In short tangential lines/In long tangential lines/V |
| 53 | Traumatic canals | P |
| 54 | Radial canals | P/A |
| Phloem | Phloem | 55 | Included phloem | A/Diffuse/Concentric |
| Softwood | Growth rings | Earlywood/
Latewood transition | 56 | Earlywood/Latewood transition | Abrupt transition from earlywood to latewood/Gradual transition from earlywood to latewood/V |
| Axial canals | Axial canals | 57 | Axial canals | Large/Small/A |
| 57b | Traumatic canals | P |
| Axial parenchyma | Visibility | 58 | Axial parenchyma visible with hand lens | Scarce diffuse/Tangentially zonate/A |
| Non- anato-mical features | Hardwood + Softwood | Heartwood | Color | 59 | Heartwood color darker than sapwood color | P/A |
| 60 | Heartwood basically brown or shades of brown | P/A |
| 61 | Heartwood basically red or shades of red | P/A |
| 62 | Heartwood basically yellow or shades of yellow | P/A |
| 63 | Heartwood basically white to gray | P/A |
| 64 | Heartwood with streaks | P/A |
| Density | 65 | Density | Density low: <0.40 g/cm3/Density medium: 0.40–0.75 g/cm3/Density high: >0.75 g/cm3 |
| Odor | 66 | Odor | A/Distinctly present and pleasant (sweet, spicy, floral)/Distinctly present and unpleasant (sour, bitter, fetid) |
| Oily surface | 67 | Oily surface | P/A |
| | Habit | 68 | Tree | P/A/V |
| 69 | Shrub | P/A/V |
| 70 | Vine/Liana/Climber | P/A/V |
| Geographical distribution | 71 | Europe and temperate Asia (Brazier and Franklin region 74) | P/A |
| 72 | Europe, excluding Mediterranean | P/A |
| 73 | Mediterranean including Northern Africa and Middle East | P/A |
| 74 | Temperate Asia (China), Japan, USSR | P/A |
| 75 | Central South Asia (Brazier and Franklin region 75) | P/A |
| 76 | India, Pakistan, Sri Lanka | P/A |
| 77 | Burma | P/A |
| 78 | Southeast Asia and the Pacific (Brazier and Franklin region 76) | P/A |
| 79 | Thailand, Laos, Vietnam, Cambodia (Indochina) | P/A |
| 80 | Indomalesia: Indonesia, Philippines, Malaysia, Brunei, Singapore, Papua New Guinea, and Solomon Islands | P/A |
| 81 | Pacific Islands (including New Caledonia, Samoa, Hawaii, and Fiji) | P/A |
| 82 | Australia and New Zealand (Brazier and Franklin region 77) | P/A |
| 83 | Australia | P/A |
| 84 | New Zealand | P/A |
| 85 | Tropical mainland Africa and adjacent islands (Brazier and Franklin region 78) | P/A |
| 86 | Tropical Africa | P/A |
| 87 | Madagascar & Mauritius, Reunion & Comores | P/A |
| 88 | Southern Africa (south of the Tropic of Capricorn) (Brazier and Franklin region 79) | P/A |
| 89 | North America, north of Mexico (Brazier and Franklin region 80) | P/A |
| 90 | Neotropics and temperate Brazil (Brazier and Franklin region 81) | P/A |
| 91 | Mexico and Central America | P/A |
| 92 | Carribbean | P/A |
| 93 | Tropical South America | P/A |
| 94 | Southern Brazil | P/A |
| 95 | Temperate South America including Argentina, Chile, Uruguay, and S. Paraguay (Brazier and Franklin region 82) | P/A |
| Hardwood | Heartwood | Fluorescence | 96 | Surface fluorescence color | A/Basically yellow/Basically green/Other colors/V |
| 97 | Surface fluorescence intensity | Weakly fluorescent/Strongly fluorescent/V/NA |
| Extractives | 98 | Water extract fluorescence | A/Basically blue/Basically green/Bluish-green/V |
| 99 | Ethanol extract fluorescence | A/Basically blue/Basically green/Bluish-green/V |
| 100 | Water extract color | Colorless/Brown or shades of brown/Red or shades of red/Yellow or shades of yellow/Other shades |
| 101 | Ethanol extract color | Colorless/Brown or shades of brown/Red or shades of red/Yellow or shades of yellow/Other shades |
| Froth test | 102 | Froth after shaking in water | Positive/Weakly positive/A |
| Burning splinter test | 103 | Splinter burns to: | Charcoal/Partial ash/Full ash (white)/Full ash (yellow-brown)/Full ash (other) |
| | Chrome Azurol-S test | 104 | Chrome Azurol-S test | Positive/Negative |
| Grain | 105 | Interlocked grain | P |
| Surface marks | 106 | Surface marks | Pith flecks/Gum deposits/Kino veins/Pitch pockets/Latex traces/Resin veins |
Table 2.
List of recent references on macroscopic wood identification. The identification method is reported as traditional when performed by humans, and as machine vision when on an automated basis.
Table 2.
List of recent references on macroscopic wood identification. The identification method is reported as traditional when performed by humans, and as machine vision when on an automated basis.
| Name | Year | Area | Taxa (n) | Method (Traditional/Machine Vision) | References |
|---|
| Identification of Central American, Mexican, and Caribbean Woods | 2022 | Mexico, Central America, Caribbean | 138 species | Traditional | [16] |
| UTForest—UTFPR Classificador | 2021 | Brazil | 44 species | Traditional | [17] |
| Macroscopic wood identification key for Atlantic Forest species | 2021 | Brazil | 102 species | Traditional | [18] |
| Macroscopic wood identification key for Brazilian endangered species | 2021 | Brazil | 29 species | Traditional | [19] |
| Identification of Tree Species from the Peruvian Tropical Amazon “Selva Central” Forests According to Wood Anatomy | 2021 | Peru | 20 species | Traditional | [20] |
| Macroscopic wood identification key for Itatiaia National Park, RJ, Brazil | 2020 | Brazil | 41 species | Traditional | [21] |
| Field identification manual for Ghanaian timbers | 2020 | Ghana | 102 species | Traditional | [22] |
| Atlas of macroscopic wood identification: with a special focus on timbers used in Europe and CITES-listed species | 2019 | Global | 292 genera
335 species | Traditional | [3] |
| Forest Species Classifier | 2018 | Brazil | 112 species | Traditional | [23] |
| MacroHOLZdata | 2016 | Global | 150 species | Traditional | [24] |
Forest Species
Database—Macroscopic | 2014 | Brazil | 41 species | Traditional | [25] |
| Brazilian commercial timbers | 2010 | Brazil | 275 species | Traditional | [26] |
| CITESwoodID | 2005 | Various world regions | 75 species | Traditional | [13] |
| Towards Sustainable North American Wood Product Value Chains, Part I: Computer Vision Identification of Diffuse Porous Hardwoods | 2022 | North America | 24 genera
105 species | Machine vision | [27] |
| Towards sustainable North American wood product value chains, Part 2: computer vision identification of ring-porous hardwoods | 2022 | North America | 15 genera
68 species | Machine vision | [28] |
| Wood identification based on longitudinal section images by using deep learning | 2021 | North America | 11 species | Machine vision | [29] |
| Wood species automatic identification from wood core images with a residual convolutional neural network | 2021 | Europe | 14 species | Machine vision | [30] |
| Machine vision for field-level wood identification | 2020 | Amazonia Atlantic region | 21 species | Machine vision | [31] |
| Rapid field identification of CITES timber species by deep learning | 2020 | Suriname | 14 species | Machine vision | [32] |
| Software for forest species recognition based on digital images of wood | 2018 | Brazil | 41 species | Machine vision | [33] |
| Forest species recognition using deep convolutional neural networks | 2014 | Brazil | 41 species | Machine vision | [34] |
Table 3.
List of macroscopic features of softwoods and hardwoods.
Table 3.
List of macroscopic features of softwoods and hardwoods.
| Macroscopic Feature | Softwoods | Hardwoods |
|---|
| Axial tracheids | Present | Rare (fibre tracheids s.l.) |
| Rays | Not visible to the naked eye on transverse and tangential planes | In several species visible to the naked eye on all three planes |
| Vessels | Absent | Present. In some species big enough to be seen by the naked eye |
| Fibers | Absent | Present |
| Canals | Present in some species | Present in some species |
| Appearance of growth rings | Distinct in most species | Variable |
Table 4.
Examples of species for different heartwood colors.
Table 4.
Examples of species for different heartwood colors.
| Heartwood Color | Hardwoods | Softwoods |
|---|
| White | Acer spp., Carpinus betulus, Dyera costulata, Ilex aquifolium, Tilia cordata | Abies spp., Picea spp. |
| Brown | Carya ovata, Castanea sativa, Cordia dodecandra, Quercus spp., Tectona grandis | Araucaria spp., Pinus spp. |
| Yellow | Berberis vulgaris, Buxus sempervirens, Chloroxylon swietenia, Zanthoxylum flavum | Agathis spp., Cupressus spp. |
| Green | Cholorocardum rodiei, Guaiacum officinale, Liriodendron tulipifera, Handroanthus spp., Pistacia spp. | - |
| Red | Brosimum rubescens, Paubrasilia echinata, Pterocarpus soyauxii | Larix decidua, Taxus baccata |
| Orange | Centrolobium spp., Dalbergia retusa, Pterocarpus dalbergioides | - |
| Pink | Berchemya zeyheri, Dalbergia decipularis | - |
| Purple | Dalbergia cearensis, Peltogyne spp. | - |
| Black | Dalbergia melanoxylon, Diospyros crassiflora, Diospyros ebenum | - |
| Variegated | Astronium graveolens, Cordia dodecandra, Dalbergia nigra, Diospyros malabarica, Microberlinia brazzavillensis, Olea europaea, Zygia racemosa | - |
Table 5.
Examples of species for fine, medium, and coarse textures (these texture types are commonly reported in literature, for instance in [
41]).
Table 5.
Examples of species for fine, medium, and coarse textures (these texture types are commonly reported in literature, for instance in [
41]).
| Texture | Hardwood Species |
|---|
| Fine | Buxus sempervirens, Fagus sylvatica, Machaerium scleroxylon, Paubrasilia chinate, Pericospsis elata, Pyrus communis, Santalum album |
| Medium | Astromiun graveolens, Bowdichia nitida, Cordia dodecandra, Dipteryx odorata, Gonystylus bancanus, Mansonia altissima, Morus mesozygia |
| Coarse | Afzelia spp., Aucoumea klaineana, Castanea sativa, Dalbergia stevensonii, Hevea brasiliensis, Hymenaea courbaril, Terminalia superba |
Table 6.
Examples of species for different growth rings’ occurrence and visibility.
Table 6.
Examples of species for different growth rings’ occurrence and visibility.
| Growth Rings | Hardwood Species |
|---|
| Distinct and clearly visible | Carya ovata, Cedrela odorata, Fagus sylvatica, Fraxinus excelsior, Juglans regia, Prunus avium, Quercus petraea, Tectona grandis, Ulmus glabra |
| Distinct but difficult to see | Eucalyptus camaldulensis, Malus sylvestris, Olea europaea, Platanus orientalis, Pyrus communis |
| Indistinct | Acacia koa, Brosimum alicastrum, Intsia bijuga, Khaya spp., Metopium brownei, Milicia spp., Millettia spp., Swietenia macrophylla |
| Variable | Aniba rosodora, Cordia dodecandra, Dalbergia cearensis, Dalbergia nigra, Santalum album, Tessmannia africana |
Table 7.
Examples of species for different types of transition in softwoods.
Table 7.
Examples of species for different types of transition in softwoods.
| Transition | Softwood Species |
|---|
| Abrupt | Larix decidua, Pinus spp. (hard pines group), Pseudotsuga menziesii, Tsuga heterophylla |
| Gradual | Abies alba, Agathis australis, Araucaria angustifolia, Calocedrus decurrens, Cedrus atlantica, Chamaecyparis lawsoniana, Cryptomeria japonica, Cupressus sempervirens, Pinus spp. (soft pines group), Taxus baccata |
| Variable | Fitzroya cupressoides, Picea abies, Sequoia sempervirens, Taxodium distichum, Thuja plicata |
Table 8.
Examples of species for different types of porosity in hardwoods.
Table 8.
Examples of species for different types of porosity in hardwoods.
| Porosity | Hardwood Species |
|---|
| Ring-porous | Carya ovata, Castanea sativa, Gleditsia triacanthos, Paulownia tomentosa, Quercus rubra, Robinia pseudoacacia, Ulmus minor |
| Semi-ring porous | Dalbergia decipularis, Juglans nigra, Juglans regia, Prunus avium, Prunus serotina, Pterocarpus indicus |
| Diffuse porous | Acer pseudoplatanus, Amphimas pterocarpoides, Betula spp., Handroanthus spp., Intsia bijuga, Liriodednron tulipifera, Magnolia ovata, Pyrus communis |
Table 9.
Examples of species for different types of axial parenchyma distribution in softwoods.
Table 9.
Examples of species for different types of axial parenchyma distribution in softwoods.
| Axial Parenchyma Distribution | Softwood Species |
|---|
| Scarce diffuse | Cedrus atlantica, Chamaecyparis lawsoniana |
| Tangentially zonate | Calocedrus decurrens, Cryptomeria japonica, Cupressus sempervirens, Fitzroya cupressoides, Podocarpus neriifolius, Thuja plicata |
| Absent | Abies alba, Araucaria angustifolia, Larix decidua, Picea abies, Pinus spp., Taxus baccata |
Table 10.
Examples of species for different types of axial parenchyma distribution in hardwoods.
Table 10.
Examples of species for different types of axial parenchyma distribution in hardwoods.
| Axial Parenchyma Distribution | Hardwood Species |
|---|
| Absent | Acer pseudoplatanus, Alnus glutinosa, Buxus sempervirens, Casearia praecox, Euxylophora paraensis, Nothofagus pumilio, Populus spp. |
| Diffuse | Alstonia macrophylla, Aspidosperma polyneuron, Dillenia indica, Testulea gabonensis, Tetramerista glabra |
| Diffuse-in-aggregates | Carpinus betulus, Caryocar glabrum, Coula edulis, Dalbergia nigra, Heritiera utilis, Mammea Africana, Tilia cordata |
| Vasicentric | Acacia mangium, Antiaris toxicaria, Cordia dodecandra, Khaya ivorensis, Myroxylon balsamum, Newtonia leucocarpa |
| Lozenge-aliform | Afzelia spp., Berlinia bracteosa, Dipteryx odorata, Hymenaea courbaril, Koompassia malaccensis, Mangifera indica |
| Winged-aliform | Brosimum spp., Gonystylus spp., Jacaranda copaia, Poga oleosa, Simarouba amara, Tessmannia africana, Vochysia tetraphylla |
| Confluent | Andira coriacea, Dicorynia guianensis, Hymenolobium flavum, Leplaea cedrata, Milicia spp., Pterocaprus spp., Zygia racemosa |
| Narrow bands | Alstonia scholaris, Autranella congolensis, Cariniana legalis, Carya ovata, Diospyros spp., Dyera costulata, Hevea brasiliensis |
| Wide bands | Amphimas pterocarpoides, Clarisia racemosa, Ficus spp., Lophira alata, Millettia laurentii, Morus mesozygia, Swartzia cubensis |
| Marginal or seemingly marginal bands | Carapa guianensis, Chloroxylon swietenia, Gluta renghas, Swietenia macrophylla, Swintonia floribunda, Zanthoxylum flavum |
| Reticulate | Alstonia scholaris, Autranella congolensis, Cariniana legalis, Chrysophyllum africanum, Diospyros spp., Lophira alata |
| Scalariform | Ceiba pentandra, Dyera costulata, Grevillea robusta, Hexalobus crispiflorus, Roupala montana, Sterculia rhinopetala |
| Festooned | Grevillea robusta, Roupala montana |
Table 11.
Examples of species for different ray features in hardwoods.
Table 11.
Examples of species for different ray features in hardwoods.
| Ray Features | Hardwood Species |
|---|
| Rays not visible on the transverse surface | Autranella congolensis, Baikiaea plurijuga, Betula pendula, Dalbergia spp., Populus spp., Pterocarpus spp. |
| Rays visible on the transverse surface | Aucoumea klaineana, Celtis adolfi-friderici, Entandrophragma spp., Ilex aquifolium, Prunus avium |
| Some of the rays clearly more evident than the others on the transverse surface | Alnus glutinosa, Carpinus betulus, Fagus sylvatica, Gleditsia triacanthos, Quercus spp., Roupala montana |
| Rays not visible on the tangential surface | Afzelia spp., Dalbergia spp., Dyera costulata, Gluta renghas, Juglans regia, Salix spp. |
| Rays visible on the tangential surface, less than 5 mm high | Acer pseudoplatanus, Entandrophragma spp., Fagus sylvatica, Khaya spp., Platanus spp. |
| Rays visible on the tangential surface, over 5 mm high | Alnus glutinosa, Carpinus betulus, Quercus spp., Scaphium macropodum |
| Rays storied, fine | Dalbergia spp., Guaiacum officinale, Handroanthus spp., Machaerium scleroxylon, Pterocarpus spp. |
| Rays storied, coarse | Andira coriacea, Dipteryx odorata, Mansonia altissima, Millettia laurentii, Swietenia macrophylla |
Table 12.
Examples of species for different types of vessels’ arrangement.
Table 12.
Examples of species for different types of vessels’ arrangement.
| Vessels’ Arrangement | Hardwood Species |
|---|
| Tangential bands | Catalpa speciosa, Celtis australis, Gleditsia triacanthos, Grevillea robusta, Morus rubra, Roupala montana, Ulmus spp. |
| Radial pattern | Autranella congolensis, Baillonella toxisperma, Ilex aquifolium, Madhuca utilis, Manilkara bidentata, Palaquium obovatum |
| Diagonal pattern (echelon) | Bulnesia sarmientoi, Calophyllum canum, Eucalyptus spp., Mammea africana, Tieghemella heckelii |
| Dendritic pattern (flame-like) | Autranella congolensis, Bulnesia sarmientoi, Calophyllum canum, Castanea sativa, Quercus spp. |
Table 13.
Examples of species for different types of vessels’ groupings.
Table 13.
Examples of species for different types of vessels’ groupings.
| Vessels’ Groupings | Hardwood Species |
|---|
| In radial multiples of 2–3 vessels | Acer pseudoplatanus, Amburana cearensis, Dalbergia spp., Erythrophleum ivorense, Fagus sylvatica, Hevea brasiliensis, Myroxylon balsamum |
| Exclusively solitary (90% or more) | Buxus sempervirens, Eucalyptus camaldulensis, Guaiacum officinale, Malus sylvestris, Quercus ilex, Santalum album |
| Radial multiples of 4 or more common | Alnus glutinosa, Aquilaria malaccensis, Baillonella toxisperma, Dyera costulata, Manilkara bidentata, Parahancornia fasciculata |
| Clusters common | Catalpa speciosa, Gleditsia triacanthos, Gymnocladus dioicus, Morus rubra, Robinia pseudoacacia, Ulmus spp. |
Table 14.
Examples of species for different vessel frequencies.
Table 14.
Examples of species for different vessel frequencies.
| Vessels’ Frequency | Hardwood Species |
|---|
| ≤5 vessels per square mm | Berlinia bracteosa, Dalbergia nigra, Hevea brasiliensis, Lophira alata, Ochroma pyramidale, Pterocarpus soyauxii |
| 6–20 vessels/square mm | Carapa guianensis, Entandrophragma cylindricum, Leplaea cedrata, Juglans regia, Swietenia macrophylla |
| >20 vessels/square mm | Acer spp., Betula spp., Dalbergia cearensis, Hopea ferrea, Liriodendron tulipifera, Prunus avium |
Table 15.
Examples of species that may present decorative figures due to grain deviations.
Table 15.
Examples of species that may present decorative figures due to grain deviations.
| Decorative Figure | Species |
|---|
| Interlocked | Chloroxylon swietenia, Entandrophragma cylindricum, Khaya spp., Lovoa trichilioides, Myroxylon balsamum, Turraeanthus africanus |
| Curly/Fiddleback | Acer spp., Chloroxylon swietenia, Entandrophragma cylindricum., Fraxinus spp., Guibourtia spp., Juglans regia |
| Quilt | Acer spp., Afzelia xylocarpa, Guibourtia tessmannii, Pterocarpus spp., Entandrophragma cylindricum. |
| Burl | Eucalyptus spp., Juglans regia, Pterocarpus indicus, Tetraclinis articulata, Ulmus spp. |
| Crotch | Amburana cearensis, Juglans spp., Swietenia spp. |
Table 16.
Examples of species for different types of vessel deposits.
Table 16.
Examples of species for different types of vessel deposits.
| Vessel Deposits | Hardwood Species |
|---|
| Tyloses | Bagassa guianensis, Carya ovata, Eucalyptus spp., Gluta renghas, Morus mesozygia, Quercus petraea, Robinia psuedoacacia |
| Gums | Afzelia spp., Dalbergia spp., Intsia spp., Khaya spp., Lophira alata, Platymiscium spp., Pterocarpus spp., Swartzia spp. |
Table 17.
Examples of species for different types of axial canals’ distribution.
Table 17.
Examples of species for different types of axial canals’ distribution.
| Axial Canals’ Distribution | Hardwood Species |
|---|
| Diffuse | Anisoptera spp., Daniellia spp., Detarium spp., Dipterocarpus spp., Prioria spp., Tessmannia spp., Vatica spp. |
| In short tangential lines | Daniellia spp., Detarium spp., Dipterocarpus spp., Sindoropsis letestui, Tessmannia spp. |
| In long tangential lines | Copaifera spp., Eperua spp., Hopea spp., Neobalanocarpus heimii, Shorea spp., Sindora spp. |
Table 18.
Examples of species for different wood density classes.
Table 18.
Examples of species for different wood density classes.
| Wood Density | Species |
|---|
| <400 kg/m3 | Aquilaria malaccensis., Bombax spp., Calocedrus decurrens, Ceiba pentandra, Ochroma pyramidale, Paulownia tomentosa, Thuja plicata |
| 400–750 kg/m3 | Abies alba, Castanea sativa, Fagus sylvatica, Gmelina arborea, Juglans regia, Pinus spp., Quercus rubra, Tectona grandis |
| 760–1000 kg/m3 | Afzelia spp., Baikiaea plurijuga, Buxus sempervirens, Dalbergia nigra, Koompassia malaccensis, Olea europaea, Peltogyne spp. |
| >1000 kg/m3 | Brosimum guianense, Dalbergia melanoxylon, Guaiacum officinale, Handroanthus spp., Manilkara bidentata, Zollernia paraensis |
Table 19.
Examples of species for different wood odors.
Table 19.
Examples of species for different wood odors.
| Wood Odor | Species |
|---|
| Present and pleasant (sweet, spicy, floral) | Aniba rosodora, Cedrela odorata, Cedrus spp., Cupressus spp., Juniperus spp., Myroxylon balsamum, Santalum album |
| Present and unpleasant (sour, bitter, fetid) | Alstonia congensis, Dinizia excelsa, Gonystylus spp., Microberlinia brazzavillensis, Piptadeniastrum africanum |
Table 20.
Examples of species with different heartwood fluorescence.
Table 20.
Examples of species with different heartwood fluorescence.
| Heartwood Fluorescence | Hardwood Species |
|---|
| Yellow, strong | Acacia mangium, Albizia ferruginea, Cedrelinga cateniformis, Dipteryx odorata, Robinia pseudoacacia |
| Yellow, weak | Brachystegia zenkeri, Guibourtia tessmannii, Mangifera indica, Pentaclethra macrophylla |
| Green, strong | Balfouridendron riedelianium, Dimorphandra polyandra |
| Green, weak | Centrolobium robustum, Dinizia excelsa |