Comparative Genomics Analysis of Ganoderma Orthologs Involved in Plant-Pathogenesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrieval of Genome Assemblies and Protein Sequences
2.2. Gene Prediction from Ganoderma Species
2.3. Functional Annotation
2.4. Identification of Secondary Metabolite Gene Clusters, CAZyme and Classification of Fungal Lifestyles
2.5. Ortholog Analysis
2.6. Prediction of Effector Sequences
3. Results
3.1. Prediction of Proteins of Ganoderma Species/Strains
3.2. CAZymes and Classification of Prediction of Fungal Lifestyles
3.3. Secondary Metabolite Production Potential of Ganoderma Species
3.4. Identification of Ganoderma Orthologs
3.5. Ganoderma Orthologous Groups Associated with Pathogenesis
3.5.1. Mycotoxin and Secondary Metabolite Biosynthesis
3.5.2. Ergosterol Biosynthesis and Sterol Transport
3.5.3. Other Virulence Factors
3.5.4. Lignolytic Enzymes and Other Plant-Cell-Wall Degrading Enzymes
3.6. Putative Effectors from Ganoderma Species
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Baby, S.; Johnson, A.J.; Govindan, B. Secondary metabolites from Ganoderma. Phytochem. 2015, 114, 66–101. [Google Scholar] [CrossRef]
- Mohammed, C.L.; Rimbawanto, A.; Page, D.E. Management of basidiomycete root- and stem-rot diseases in oil palm, rubber and tropical hardwood plantation crops. For. Path. 2014, 44, 428–446. [Google Scholar] [CrossRef]
- Kandan, A.; Bhaskaran, R.; Samiyappan, R. Ganoderma—A basal stem rot disease of coconut palm in south Asia and Asia pacific regions. Arch. Phytopathol. Pflanzenschutz. 2010, 43, 1445–1449. [Google Scholar] [CrossRef]
- Loyd, A.L.; Linder, E.R.; Anger, N.A.; Richter, B.S.; Blanchette, R.A.; Smith, J.A. Pathogenicity of Ganoderma species on landscape trees in the Southeastern United States. Plant Dis. 2018, 102, 1944–1949. [Google Scholar] [CrossRef] [Green Version]
- Bishop, K.S.; Kao, C.H.; Xu, Y.; Glucina, M.P.; Paterson, R.R.; Ferguson, L.R. From 2000 years of Ganoderma lucidum to recent developments in nutraceuticals. Phytochemistry 2015, 114, 56–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Liu, J.; Xia, J.; Wang, C.; Li, X.; Deng, Y.; Bao, N.; Zhang, Z.; Qiu, M. Lanostane triterpenoids from Ganoderma hainanense J. D. Zhao. Phytochemistry 2015, 114, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Y.; Zhang, M.; Zhang, L. Ganoderma sinense polysaccharide: An adjunctive drug used for cancer treatment. Prog. Mol. Biol. Transl. Sci. 2019, 163, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Tian, D.; Liu, Y.; Li, H.; Zhu, J.; Li, M.; Xin, M.; Xia, J. Review of the molecular mechanisms of Ganoderma lucidum triterpenoids: Ganoderic acids A, C2, D, F, DM, X and Y. Eur. J. Med. Chem. 2019, 174, 130–141. [Google Scholar] [CrossRef]
- Pattanayak, S.; Das, S.; Biswal, G. Ganoderma: The wild mushroom with wonderful health benefits. J. Pharmacogn. Phytochem. 2020, 9, 313–316. [Google Scholar] [CrossRef]
- Jiang, N.; Hu, S.; Peng, B.; Li, Z.; Yuan, X.; Xiao, S.; Fu, Y. Genome of Ganoderma species provides Insights Into the evolution, conifers substrate utilization, and terpene synthesis for Ganoderma tsugae. Front. Microbiol. 2021, 12, 724451. [Google Scholar] [CrossRef]
- Tian, Y.Z.; Wang, Z.F.; Liu, Y.D.; Zhang, G.Z.; Li, G. The whole-genome sequencing and analysis of a Ganoderma lucidum strain provide insights into the genetic basis of its high triterpene content. Genomics 2021, 113, 840–849. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Lebreton, A.; Xing, J.H.; Fang, Y.X.; Si, J.; Morin, E.; Miyauchi, S.; Drula, E.; Ahrendt, S.; Cobaugh, K.; et al. Phylogenomics and Comparative Genomics Highlight Specific Genetic Features in Ganoderma Species. J. Fungi 2022, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Kües, U.; Nelson, D.R.; Liu, C.; Yu, G.J.; Zhang, J.; Li, J.; Wang, X.C.; Sun, H. Genome analysis of medicinal Ganoderma spp. with plant-pathogenic and saprotrophic life-styles. Phytochemistry 2015, 114, 18–37. [Google Scholar] [CrossRef]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.J.; Wang, M.; Huang, J.; Yin, Y.L.; Chen, Y.J.; Jiang, S.; Jin, Y.X.; Lan, X.Q.; Wong, B.H.; Liang, Y.; et al. Deep insight into the Ganoderma lucidum by comprehensive analysis of its transcriptome. PLoS ONE 2012, 7, e44031. [Google Scholar] [CrossRef]
- Huang, Y.H.; Wu, H.Y.; Wu, K.M.; Liu, T.T.; Liou, R.F.; Tsai, S.F.; Shiao, M.S.; Ho, L.T.; Tzean, S.S.; Yang, U.C. Generation and analysis of the expressed sequence tags from the mycelium of Ganoderma lucidum. PLoS ONE 2013, 8, e61127. [Google Scholar] [CrossRef] [Green Version]
- Ramzi, A.B.; Che Me, M.L.; Ruslan, U.S.; Baharum, S.N.; Nor Muhammad, N.A. Insight into plant cell wall degradation and pathogenesis of Ganoderma boninense via comparative genome analysis. PeerJ 2019, 7, e8065. [Google Scholar] [CrossRef] [Green Version]
- Ho, P.Y.; Namasivayam, P.; Sundram, S.; Ho, C.L. Expression of genes encoding manganese peroxidase and laccase of Ganoderma boninense in response to nitrogen sources, hydrogen peroxide and phytohormones. Genes 2020, 11, 1263. [Google Scholar] [CrossRef]
- Ho, C.L.; Tan, Y.C.; Yeoh, K.A.; Ghazali, A.K.; Yee, W.Y.; Hoh, C.C. De novo transcriptome analyses of host-fungal interactions in oil palm (Elaeis guineensis Jacq.). BMC Genom. 2016, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.M.; Leem, Y.E.; Choi, H.T. Purification and characterization of laccase isozymes from the white-rot basidiomycete Ganoderma lucidum. Appl. Microbiol. Biotechnol. 2001, 57, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Teerapatsakul, C.; Abe, N.; Bucke, C.; Chitradon, L. Novel laccases of Ganoderma sp. KU-Alk4, regulated by different glucose concentration in alkaline media. World J. Microbiol. Biotechnol. 2007, 23, 1559–1567. [Google Scholar] [CrossRef]
- Kumar, A.; Kant, K.; Kumar., P.; Ramchiary., N. Laccase isozymes from Ganoderma lucidum MDU-7: Isolation, characterization, catalytic properties and differential role during oxidative stress. J. Mol. Catal. Enzymat. 2015, 113, 68–75. [Google Scholar] [CrossRef]
- Torres-Farradá, G.; Manzano León, A.M.; Rineau, F.; Ledo Alonso, L.L.; Sánchez-López, M.I.; Thijs, S.; Colpaert, J.; Ramos-Leal, M.; Guerra, G.; Vangronsveld, J. Diversity of ligninolytic enzymes and their genes in strains of the genus Ganoderma: Applicable for biodegradation of xenobiotic compounds? Front. Microbiol. 2017, 8, 898. [Google Scholar] [CrossRef] [Green Version]
- Si, J.; Wu, Y.; Ma, H.F.; Cao, Y.J.; Sun, Y.F.; Cui, B.K. Selection of a pH- and temperature-stable laccase from Ganoderma australe and its application for bioremediation of textile dyes. J. Environ. Manag. 2021, 299, 113619. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Guo, M.Y.; Gao, Y.H.; Bai, X.H.; Zhou, X.W. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017, 17, 19. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, R.; Ma, L.; Fan, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X.; Yang, Y. Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J. Hazard. Mater. 2011, 192, 855–873. [Google Scholar] [CrossRef]
- You, L.F.; Liu, Z.M.; Lin, J.F.; Guo, L.Q.; Huang, X.L.; Yang, H.X. Molecular cloning of a laccase gene from Ganoderma lucidum and heterologous expression in Pichia pastoris. J. Basic Microbiol. 2014, 54, S134–S141. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Deng, W.; Shen, M.; Yan, G.; Zhao, W.; Yang, Y. A laccase Gl-LAC-4 purified from white-rot fungus Ganoderma lucidum had a strong ability to degrade and detoxify the alkylphenol pollutants 4-n-octylphenol and 2-phenylphenol. J. Hazard. Mater. 2021, 408, 124775. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.Y.; Pang, C.; Tor, X.Y.; Ho, P.; Lim, Y.; Namasivayam, P.; Ho, C.L. Molecular cloning and functional analysis of a necrosis and ethylene inducing protein (NEP) from Ganoderma boninense. Physiol. Mol. Plant Pathol. 2019, 106, 42–48. [Google Scholar] [CrossRef]
- Lim, F.-H.; Fakhrana, I.N.; Rasid, O.A.; Idris, A.S.; Ho, C.-L.; Shaharuddin, N.A.; Parveez, G.K.A. Molecular cloning and expression analysis of Ganoderma boninense cyclophilins at different growth and infection stages. Physiol. Mol. Plant Pathol. 2017, 99, 31–40. [Google Scholar] [CrossRef]
- Sulaiman, S.; Yusoff, N.; Tan, J.S.; Lee, Y.P. Deciphering the pan-genome of Ganoderma sp. to depict potential genomic components that contribute to Ganoderma boninense pathogenicity. Malays. Appl. Biol. 2018, 47, 71–80. [Google Scholar]
- Zhu, Y.; Xu, J.; Sun, C.; Zhou, S.; Xu, H.; Nelson, D.R.; Qian, J.; Song, J.; Luo, H.; Xiang, L.; et al. Chromosome-level genome map provides insights into diverse defense mechanisms in the medicinal fungus Ganoderma sinense. Sci. Rep. 2015, 5, 11087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Huang, L.; Hu, H.; Cai, M.; Liang, X.; Li, X.; Zhang, Z.; Xie, Y.; Xiao, C.; Chen, S.; et al. Whole-genome assembly of Ganoderma leucocontextum (Ganodermataceae, Fungi) discovered from the Tibetan Plateau of China. G3 2021, 11, jkab337. [Google Scholar] [CrossRef] [PubMed]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; Lopez-Giraldez, F.; Sjökvist, E.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; et al. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Gong, J.; Dai, W.; Kang, X.; Huang, Z.; Zhang, H.M.; Liu, W.; Liu, L.; Ma, J.; Xia, Z.; et al. The genome of Ganoderma lucidum provides insights into triterpenes biosynthesis and wood degradation. PLoS ONE 2012, 7, e36146. [Google Scholar] [CrossRef] [Green Version]
- Khairi, M.H.F.; Nor Muhammad, N.A.; Bunawan, H.; Abdul Murad, A.M.; Ramzi, A.B. Unveiling the core effector proteins of oil palm pathogen Ganoderma boninense via pan-secretome analysis. J. Fungi 2022, 8, 793. [Google Scholar] [CrossRef]
- Seppey, M.; Manni, M.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness. Methods Mol. Biol. 2019, 1962, 227–245. [Google Scholar] [CrossRef]
- Götz, S.; García-Gómez, J.M.; Terol, J.; Williams, T.D.; Nagaraj, S.H.; Nueda, M.J.; Robles, M.; Talón, M.; Dopazo, J.; Conesa, A. High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res. 2008, 36, 3420–3435. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef] [Green Version]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Eddy, S.R. A new generation of homology search tools based on probabilistic inference. Genome Inform. 2009, 23, 205–211. [Google Scholar]
- Yin, Y.; Mao, X.; Yang, J.; Chen, X.; Mao, F.; Xu, Y. dbCAN: A web resource for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2012, 40, W445–W451. [Google Scholar] [CrossRef] [PubMed]
- Hane, J.K.; Paxman, J.; Jones, D.A.B.; Oliver, R.P.; de Wit, P. “CATAStrophy”, a genome-informed trophic classification of filamentous plant pathogens—How many different types of filamentous plant pathogens are there? Front. Microbiol. 2020, 10, 3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Stoeckert, C.J., Jr.; Roos, D.S. OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res. 2003, 13, 2178–2189. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef] [Green Version]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. A combined transmembrane topology and signal peptide prediction method. J. Mol. Biol. 2004, 338, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Sperschneider, J.; Dodds, P.N.; Gardiner, D.M.; Singh, K.B.; Taylor, J.M. Improved prediction of fungal effector proteins from secretomes with EffectorP 2.0. Mol. Plant Path. 2018, 19, 2094–2110. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wu, S.; Su, C.; Peng, J.; Shih, Y.; Chen, L. Ganoderma multipileum, the correct name for ‘G. lucidum’ in tropical Asia. Bot. Stud. 2009, 50, 451–458. [Google Scholar]
- Kajikawa, M.; Hirai, N.; Hashimoto, T. A PIP-family protein is required for biosynthesis of tobacco alkaloids. Plant Mol. Biol. 2009, 69, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bodega, Á.; Álvarez-Álvarez, R.; Liras, P.; Martín, J.F. Silencing of a second dimethylallyltryptophan synthase of Penicillium roqueforti reveals a novel clavine alkaloid gene cluster. Appl. Microbiol. Biotechnol. 2017, 101, 6111–6121. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, H.; Lessing, F.; Winterberg, B.; Schirawski, J.; Kämper, J.; Müller, P.; Kahmann, R. A ferroxidation/permeation iron uptake system is required for virulence in Ustilago maydis. Plant Cell 2006, 18, 3332–3345. [Google Scholar] [CrossRef] [Green Version]
- Winterberg, B.; Uhlmann, S.; Linne, U.; Lessing, F.; Marahiel, M.A.; Eichhorn, H.; Kahmann, R.; Schirawski, J. Elucidation of the complete ferrichrome A biosynthetic pathway in Ustilago maydis. Mol. Microbiol. 2010, 75, 1260–1271. [Google Scholar] [CrossRef]
- Yasmin, S.; Alcazar-Fuoli, L.; Gründlinger, M.; Puempel, T.; Cairns, T.; Blatzer, M.; Lopez, J.F.; Grimalt, J.O.; Bignell, E.; Haas, H. Mevalonate governs interdependency of ergosterol and siderophore biosyntheses in the fungal pathogen Aspergillus fumigatus. Proc. Natl. Acad. Sci. USA 2012, 109, E497–E504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michielse, C.B.; van Wijk, R.; Reijnen, L.; Manders, E.M.; Boas, S.; Olivain, C.; Alabouvette, C.; Rep, M. The nuclear protein Sge1 of Fusarium oxysporum is required for parasitic growth. PLoS Pathog. 2009, 5, e1000637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Gravelat, F.N.; Chiang, L.Y.; Chen, D.; Vanier, G.; Ejzykowicz, D.E.; Ibrahim, A.S.; Nierman, W.C.; Sheppard, D.C.; Filler, S.G. Aspergillus fumigatus AcuM regulates both iron acquisition and gluconeogenesis. Mol. Microbiol. 2010, 78, 1038–1054. [Google Scholar] [CrossRef] [Green Version]
- Ejzykowicz, D.E.; Solis, N.V.; Gravelat, F.N.; Chabot, J.; Li, X.; Sheppard, D.C.; Filler, S.G. Role of Aspergillus fumigatus DvrA in host cell interactions and virulence. Eukaryot Cell 2010, 9, 1432–1440. [Google Scholar] [CrossRef] [Green Version]
- Fox, D.S.; Cruz, M.C.; Sia, R.A.; Ke, H.; Cox, G.M.; Cardenas, M.E.; Heitman, J. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol. Microbiol. 2001, 39, 835–849. [Google Scholar] [CrossRef]
- De Jonge, R.; Thomma, B.P. Fungal LysM effectors: Extinguishers of host immunity? Trends Microbiol. 2009, 17, 151–157. [Google Scholar] [CrossRef]
- Krishnan, P.; Ma, X.; McDonald, B.A.; Brunner, P.C. Widespread signatures of selection for secreted peptidases in a fungal plant pathogen. BMC Evol. Biol. 2018, 18, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, Y.; Surendran, A.; Paterson, R.R.M.; Ali, A.; Ahmad, K. Current strategies and perspectives in detection and control of basal stem rot of oil palm. Saudi J. Biol. 2021, 28, 2840–2849. [Google Scholar] [CrossRef] [PubMed]
- Vinjusha, N.; Arun Kumar, T.K. Revision of Ganoderma species associated with stem rot of coconut palm. Mycologia 2022, 114, 157–174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Qiao, X.; Shao, Q.; Hassan, M.; Ma, Z. Evolution of the lignin chemical structure during the bioethanol production process and its inhibition to enzymatic hydrolysis. Energy Fuels 2020, 34, 5938–5947. [Google Scholar] [CrossRef]
- Jayasuriya, H.; Silverman, K.C.; Zink, D.L.; Jenkins, R.G.; Sanchez, M.; Pelaez, F.; Vilella, D.; Lingham, R.B.; Singh, S.B. Clavaric acid: A triterpenoid inhibitor of farnesyl-protein transferase from Clavariadelphus truncatus. J. Nat. Prod. 1998, 61, 1568–1570. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.E.; Hohn, T.M. Mycotoxins in plant pathogenesis. Mol. Plant Microbe Interact. 1997, 10, 147–152. [Google Scholar] [CrossRef] [Green Version]
- Panaccione, D.G.; Scott-Craig, J.S.; Pocard, J.A.; Walton, J.D. A cyclic peptide synthetase gene required for pathogenicity of the fungus Cochliobolus carbonum on maize. Proc. Natl. Acad. Sci. USA 1992, 89, 6590–6594. [Google Scholar] [CrossRef] [Green Version]
- Baker, S.E.; Kroken, S.; Inderbitzin, P.; Asvarak, T.; Li, B.Y.; Shi, L.; Yoder, O.C.; Turgeon, B.G. Two polyketide synthase-encoding genes are required for biosynthesis of the polyketide virulence factor, T-toxin, by Cochliobolus heterostrophus. Mol. Plant Microbe Interact. 2006, 19, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koczyk, G.; Pawłowska, J.; Muszewska, A. Terpenoid biosynthesis dominates among secondary metabolite clusters in Mucoromycotina genomes. J. Fungi 2021, 7, 285. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, N.; Ho, C.L.; Zainudin, N.A.I.M.; Wahab, M.A.B.A.; Wong, M.Y. Identification of non-ribosomal peptide synthetase in Ganoderma boninense Pat. that was expressed during the interaction with oil palm. Sci. Rep. 2021, 11, 16330. [Google Scholar] [CrossRef]
- Kröber, A.; Scherlach, K.; Hortschansky, P.; Shelest, E.; Staib, P.; Kniemeyer, O.; Brakhage, A.A. HapX mediates iron homeostasis in the pathogenic dermatophyte Arthroderma benhamiae but is dispensable for virulence. PLoS ONE 2016, 11, e0150701. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.L.; Christenson, J.K.; Wackett, L.P. Biosynthesis and chemical diversity of β-lactone natural products. Nat. Prod. Rep. 2019, 36, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.X.; Yan, X.; Fu, W.H.; Yi, L.Q.; Tang, B.W.; Yu, L.B.; Fang, M.J.; Wu, Z.; Qiu, Y.K. New β-lactone with tea pathogenic fungus inhibitory effect from marine-derived fungus MCCC3A00957. J. Agric. Food Chem. 2019, 67, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Lees, N.D.; Skaggs, B.; Kirsch, D.R.; Bard, M. Cloning of the late genes in the ergosterol biosynthetic pathway of Saccharomyces cerevisiae—A review. Lipids 1995, 30, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Aaron, K.E.; Pierson, C.A.; Lees, N.D.; Bard, M. The Candida albicans ERG26 gene encoding the C-3 sterol dehydrogenase (C-4 decarboxylase) is essential for growth. FEMS Yeast Res. 2001, 1, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.A.; Johnson, T.A.; Lees, N.D.; Barbuch, R.; Eckstein, J.A.; Bard, M. Cloning and sequencing of the Candida albicans C-4 sterol methyl oxidase gene (ERG25) and expression of an ERG25 conditional lethal mutation in Saccharomyces cerevisiae. Lipids 2000, 35, 257–262. [Google Scholar] [CrossRef]
- Pierson, C.A.; Jia, N.; Mo, C.; Lees, N.D.; Sturm, A.M.; Eckstein, J.; Barbuct, R.; Bard, M. Isolation, characterization, and regulation of the Candida albicans ERG27 gene encoding the sterol 3-keto reductase. Med. Mycol. 2004, 42, 461–473. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Jian, Y.; Chen, Y.; Kistler, H.C.; He, P.; Ma, Z.; Yin, Y. A phosphorylated transcription factor regulates sterol biosynthesis in Fusarium graminearum. Nat. Commun. 2019, 10, 1228. [Google Scholar] [CrossRef] [Green Version]
- Schneiter, R.; Di Pietro, A. The CAP protein superfamily: Function in sterol export and fungal virulence. Biomol. Concepts 2013, 4, 519–525. [Google Scholar] [CrossRef]
- Yin, Q.Y.; de Groot, P.W.; Dekker, H.L.; de Jong, L.; Klis, F.M.; de Koster, C.G. Comprehensive proteomic analysis of Saccharomyces cerevisiae cell walls: Identification of proteins covalently attached via glycosylphosphatidylinositol remnants or mild alkali-sensitive linkages. J. Biol. Chem. 2005, 280, 20894–20901. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, V.; Schneiter, R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 16882–16887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, R.; Köffel, R.; Schneiter, R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J. 2007, 26, 5109–5119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravichandra, N.G. Frontiers in Phytopathology; I.K. International Publishing House Pvt Ltd.: New Delhi, India, 2016. [Google Scholar]
- Bishop, A.C.; Sun, T.; Johnson, M.E.; Bruno, V.M.; Patton-Vogt, J. Robust utilization of phospholipase-generated metabolites, glycerophosphodiesters, by Candida albicans: Role of the CaGit1 permease. Eukaryot. Cell 2011, 10, 1618–1627. [Google Scholar] [CrossRef] [Green Version]
- Bishop, A.C.; Ganguly, S.; Solis, N.V.; Cooley, B.M.; Jensen-Seaman, M.I.; Filler, S.G.; Mitchell, A.P.; Patton-Vogt, J. Glycerophosphocholine utilization by Candida albicans: Role of the Git3 transporter in virulence. J. Biol. Chem. 2013, 288, 33939–33952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Vallet, A.; Tian, H.; Rodriguez-Moreno, L.; Valkenburg, D.J.; Saleem-Batcha, R.; Wawra, S.; Kombrink, A.; Verhage, L.; de Jonge, R.; van Esse, H.P.; et al. A secreted LysM effector protects fungal hyphae through chitin-dependent homodimer polymerization. PLoS Pathog. 2020, 16, e1008652. [Google Scholar] [CrossRef]
- Pazzagli, L.; Cappugi, G.; Manao, G.; Camici, G.; Santini, A.; Scala, A. Purification, characterization, and amino acid sequence of cerato-platanin, a new phytotoxic protein from Ceratocystis fimbriata f. sp. platani. J. Biol. Chem. 1999, 274, 24959–24964. [Google Scholar] [CrossRef] [Green Version]
- Sbrana, F.; Bongini, L.; Cappugi, G.; Fanelli, D.; Guarino, A.; Pazzagli, L.; Scala, A.; Vassalli, M.; Zoppi, C.; Tiribilli, B. Atomic force microscopy images suggest aggregation mechanism in cerato-platanin. Eur. Biophys. J. 2007, 36, 727–732. [Google Scholar] [CrossRef] [Green Version]
- Cai, F.; Gao, R.; Zhao, Z.; Ding, M.; Jiang, S.; Yagtu, C.; Zhu, H.; Zhang, J.; Ebner, T.; Mayrhofer-Reinhartshuber, M.; et al. Evolutionary compromises in fungal fitness: Hydrophobins can hinder the adverse dispersal of conidiospores and challenge their survival. ISME J. 2000, 14, 2610–2624. [Google Scholar] [CrossRef]
- Sakamoto, Y.; Watanabe, H.; Nagai, M.; Nakade, K.; Takahashi, M.; Sato, T. Lentinula edodes tlg1 encodes a thaumatin-like protein that is involved in lentinan degradation and fruiting body senescence. Plant Physiol. 2006, 141, 793–801. [Google Scholar] [CrossRef] [Green Version]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric beta-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef]
- Fujimoto, H.; Aoyama, H.; Noguchi-Yachide, T.; Hashimoto, Y.; Kobayashi, H. Fusarielin A as an anti-angiogenic and anti-proliferative agent: Basic biological characterization. Chem. Pharm. Bull. 2008, 56, 298–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]

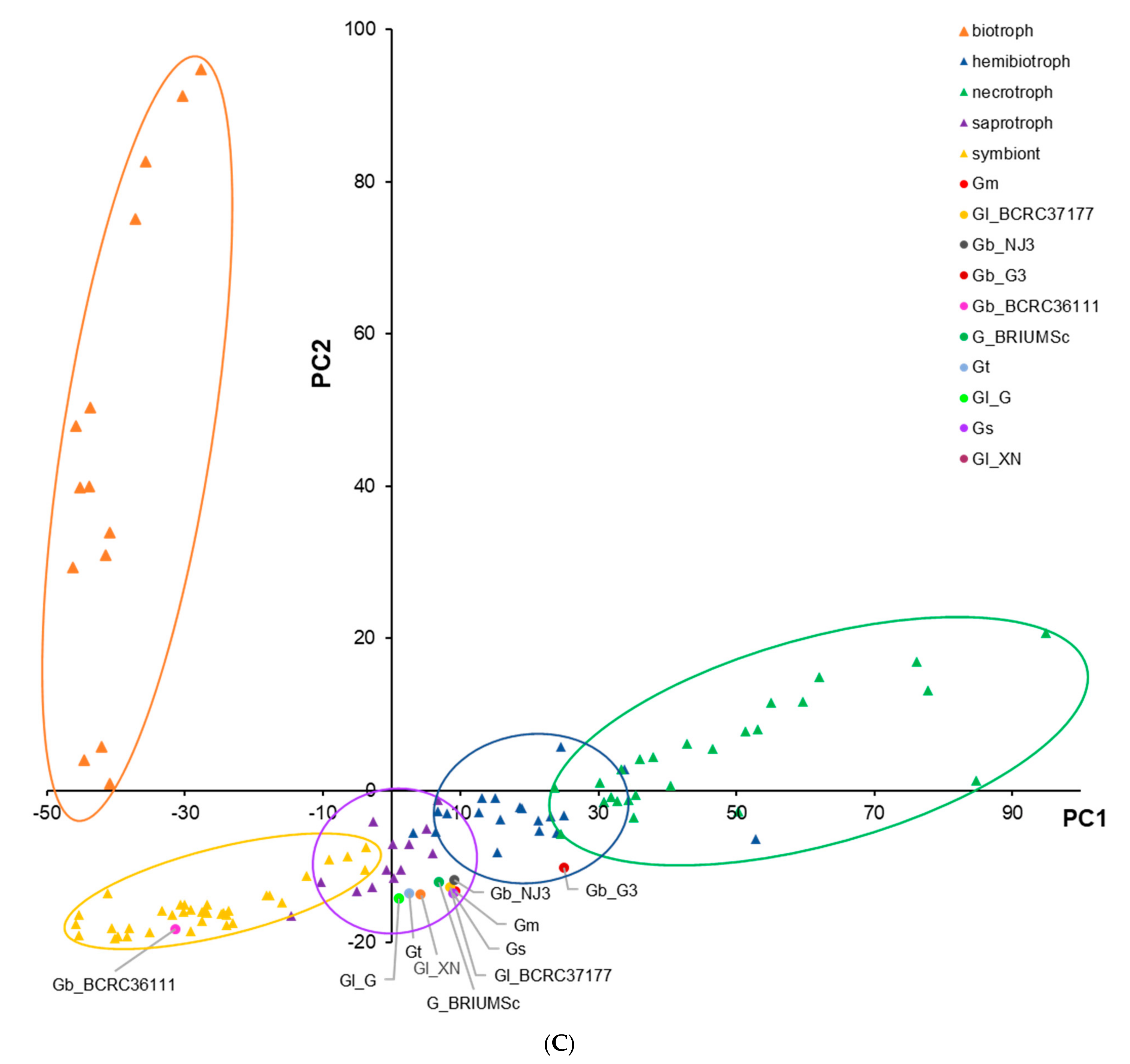
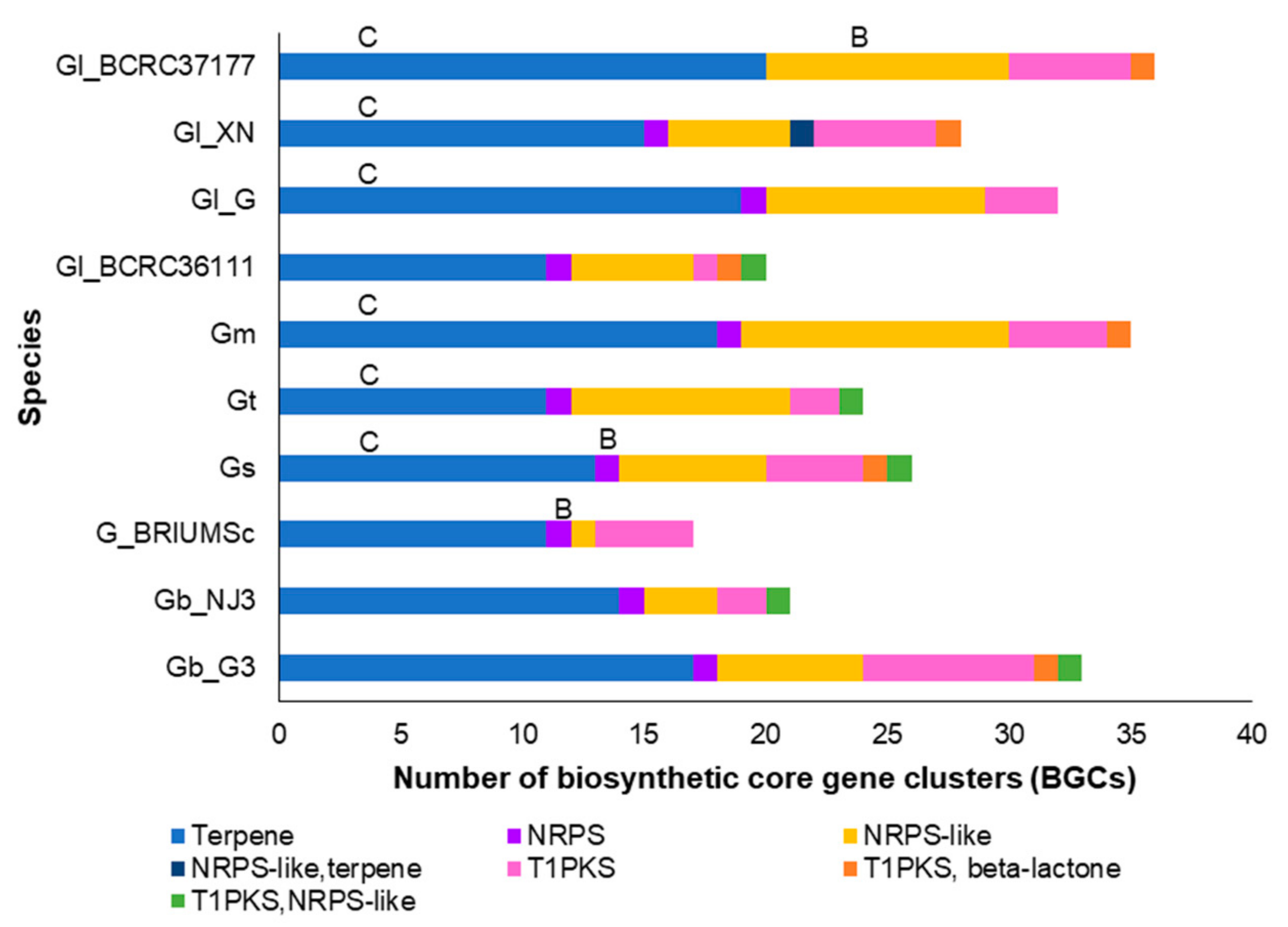
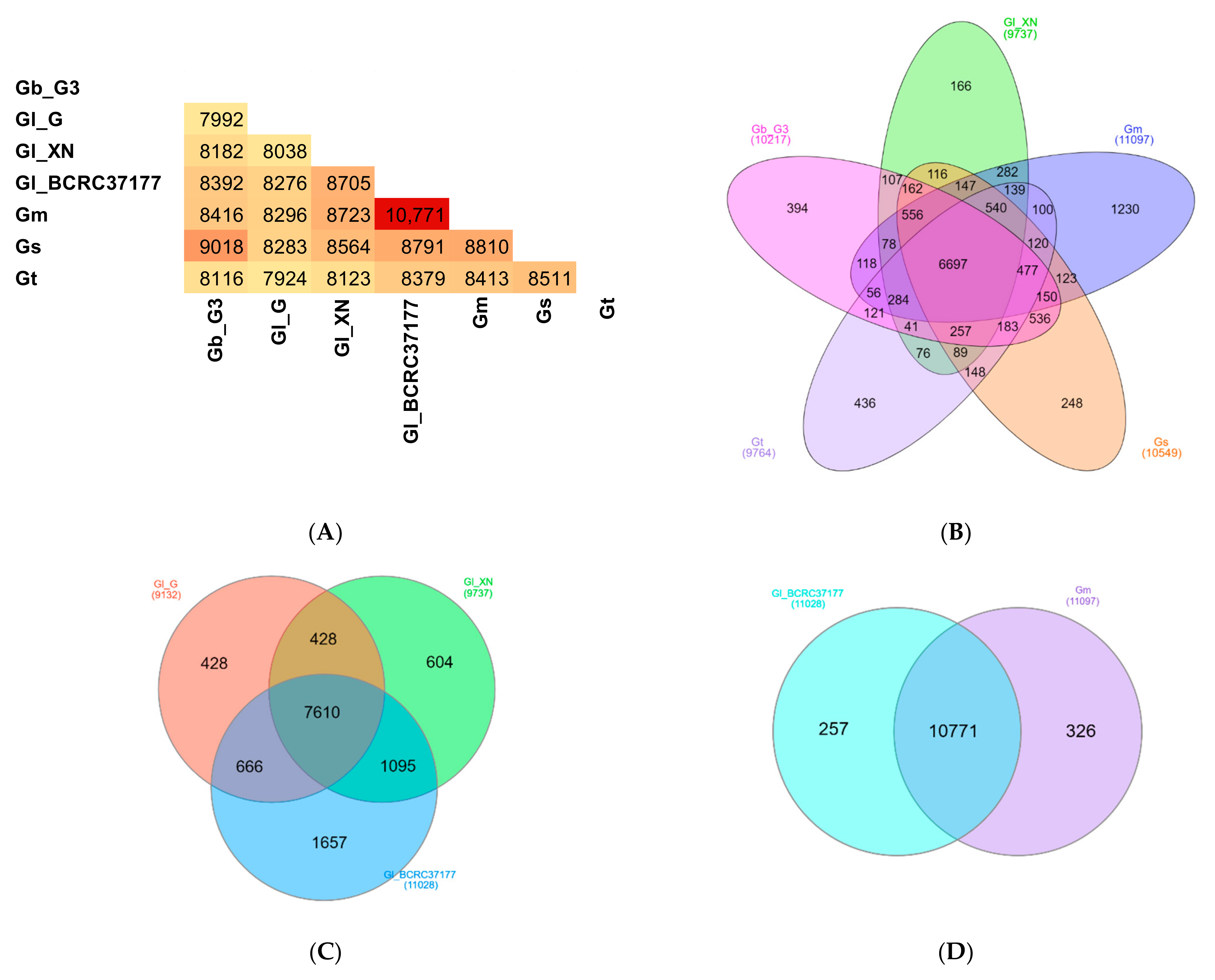


| Species | Number of Predicted Proteins in the Genome | Completeness of Proteome Predicted by BUSCO | Functional Annotations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| % Complete BUSCOs (C) | % Complete and Single-Copy BUSCOs (S) | % Complete and Duplicated BUSCOs (D) | % Fragmented BUSCOs (F) | % Missing BUSCOs (M) | % Genes with BlastP Matches | % Genes with BlastP Matches That are Mapped to GO | % Genes with KO Annotation | ||
| G. boninense G3 | 20,564 | 82.6 | 66.7 | 15.9 | 5.3 | 12.1 | 47.95 | 47.93 | 28.08 |
| G. boninense NJ3 | 21,487 | 66.7 | 63.6 | 3.1 | 10.9 | 22.4 | 39.47 | 39.47 | 24.46 |
| G. lucidum G260125-1 | 11,040 | 87.7 | 86.9 | 0.8 | 3.6 | 8.7 | 57.48 | 57.45 | 34.02 |
| G. lucidum Xiangnong | 12,342 | 89.4 | 88.6 | 0.8 | 1.9 | 8.7 | 53.82 | 53.80 | 30.46 |
| G. lucidum BCRC36111 | 12,843 | 75 | 71.1 | 3.9 | 7.8 | 17.2 | 52.93 | 52.90 | 32.04 |
| G. lucidum BCRC37177 | 12,533 | 90.5 | 89.8 | 0.7 | 2.9 | 6.6 | 54.62 | 54.61 | 31.86 |
| G. multipileum | 13,358 | 90.6 | 89.8 | 0.8 | 2.9 | 6.5 | 53.18 | 53.17 | 30.87 |
| G. sinense ZZ0214-1 | 15,478 | 90.1 | 89.0 | 1.1 | 5.0 | 4.9 | 47.83 | 47.70 | 27.08 |
| G. tsugae | 15,426 | 83.5 | 79.8 | 3.7 | 5.6 | 10.9 | 48.17 | 48.15 | 28.90 |
| Ganoderma sp. BRIUMSc | 18,612 | 68.8 | 64.3 | 4.5 | 12.7 | 18.5 | 47.67 | 47.65 | 29.76 |
| GO IDs | GO Annotation | Putative Identity of Protein | Number of Ortholog Group(s) | Number of Proteins | No Proteins in Each Ganoderma Species/Strains | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gb_G3 | Gl_G | Gl_XN | Gl_BCRC37177 | Gm | Gs | Gt | |||||
| Mycotoxin metabolism | |||||||||||
| GO:0009407 | P:toxin catabolic process | Glutathione S-transferase U9 | 1 | 6 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| Glutathione S-transferase U6 | 1 | 8 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | ||
| Glutathione S-transferase U23 | 1 | 6 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| GO:0043386 | P:mycotoxin biosynthetic process | Oxidase ustYa | 2 | 12 | 3 | 1 | 2 | 1 | 1 | 2 | 2 |
| GO:0045122 | P:aflatoxin biosynthetic process | Norsolorinic acid ketoreductase | 1 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| O-methylsterigmatocystin oxidoreductase | 2 | 8 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| FAD-binding monooxygenase aflW | 2 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Secondary metabolism | |||||||||||
| GO:0009820 | P:alkaloid metabolic process | Tryprostatin B 6-hydroxylase | 4 | 25 | 6 | 3 | 3 | 3 | 3 | 5 | 2 |
| Probable inactive reductase easA | 2 | 16 | 4 | 2 | 2 | 2 | 2 | 2 | 2 | ||
| GO:0035835 | P:indole alkaloid biosynthetic process | Chanoclavine-I aldehyde reductase fgaOx3 | 9 | 6 | 1 | 0 | 1 | 1 | 1 | 1 | 1 |
| GO:0019748 | P:secondary metabolic process | Dehydrogenase orsE | 2 | 14 | 2 | 2 | 1 | 2 | 2 | 3 | 2 |
| Isoflavone reductase (IFR) homolog A622-like | 1 | 10 | 2 | 2 | 2 | 1 | 1 | 1 | 1 | ||
| GO:0016114 | P:terpenoid biosynthetic process | Cytochrome P450 monooxygenase andK | 4 | 17 | 4 | 2 | 2 | 2 | 2 | 3 | 2 |
| Cytochrome P450 monooxygenase mpaDE | 6 | 28 | 3 | 3 | 4 | 4 | 4 | 5 | 5 | ||
| Cytochrome P450 monooxygenase yanC | 3 | 18 | 2 | 2 | 2 | 2 | 2 | 6 | 2 | ||
| Δ (6)-protoilludene synthase | 10 | 60 | 10 | 10 | 5 | 10 | 9 | 9 | 7 | ||
| FAD-dependent monooxygenase yanF | 3 | 6 | 0 | 0 | 0 | 0 | 2 | 0 | 4 | ||
| Methyltransferase ausD | 2 | 6 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | ||
| Methyltransferase trt5 | 1 | 6 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | ||
| Multifunctional cytochrome P450 | 1 | 9 | 2 | 0 | 1 | 1 | 2 | 2 | 1 | ||
| N-Methyltransferase vrtF | 2 | 4 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | ||
| O-Mevalon transferase yanI | 2 | 6 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Pathogenesis | |||||||||||
| GO:0009405 | P:pathogenesis | Acyl-CoA ligase sidI | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Aldo-keto reductase AFTS1 | 5 | 17 | 3 | 3 | 1 | 2 | 3 | 3 | 2 | ||
| Cytochrome P450 monooxygenase BOA3 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Cytochrome P450 monooxygenase BOA4 | 3 | 16 | 2 | 3 | 2 | 3 | 2 | 2 | 2 | ||
| Cytochrome P450 monooxygenase CLM2 | 5 | 22 | 3 | 2 | 4 | 3 | 3 | 3 | 4 | ||
| Enoyl-CoA hydratase AFT3-1 | 3 | 21 | 1 | 3 | 3 | 3 | 3 | 4 | 4 | ||
| Enoyl-CoA isomerase/hydratase fer4 | 1 | 8 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| FAD-dependent monooxygenase OpS4 | 8 | 44 | 4 | 6 | 6 | 8 | 8 | 7 | 5 | ||
| Hydroxymethylglutaryl (HMG)-CoA synthase | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Longiborneol synthase CLM1 | 7 | 39 | 6 | 3 | 7 | 8 | 7 | 5 | 3 | ||
| Orsellinic acid synthase ArmB | 1 | 9 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Oxidoreductase BOA17 | 8 | 41 | 10 | 5 | 4 | 6 | 6 | 6 | 4 | ||
| Reducing polyketide synthase BOA9 | 1 | 5 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| Fe-regulated protein 8 | 2 | 8 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | ||
| Ferric/cupric reductase transmembrane | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| β-1,2-xylosyltransferase 1 | 1 | 8 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| bZIP transcription factor hapX | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| C2H2 finger domain transcription factor dvrA | 1 | 8 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Calcineurin subunit B | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Global transcription regulator sge1 | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Glycerophosphoinositol permease 1 | 1 | 10 | 2 | 1 | 1 | 1 | 1 | 2 | 2 | ||
| LysM domain-containing protein ARB_00327 | 1 | 7 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| Metallocarboxypeptidase A-like protein | 1 | 10 | 1 | 2 | 1 | 1 | 2 | 2 | 1 | ||
| Probable aspartic-type endopeptidase CTSD | 1 | 8 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Leucine aminopeptidase | 2 | 19 | 3 | 3 | 2 | 2 | 2 | 3 | 4 | ||
| Tripeptidyl-peptidase SED2 | 7 | 34 | 5 | 4 | 7 | 4 | 4 | 4 | 6 | ||
| Tripeptidyl-peptidase SED3 | 2 | 15 | 2 | 3 | 2 | 2 | 2 | 2 | 2 | ||
| Tripeptidyl-peptidase SED4 | 2 | 8 | 3 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| Sterol biosynthesis and transport | |||||||||||
| GO:0006696 | P:ergosterol biosynthetic process | 3-keto-steroid reductase (EC 1.1.1.270) | 4 | 18 | 3 | 2 | 1 | 1 | 5 | 3 | 3 |
| C-8 sterol isomerase (EC 5.-.-.-) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Ergosterol biosynthetic protein 28 | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| NADH-cytochrome b5 reductase 2 (EC 1.6.2.2) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| NADPH-cytochrome P450 reductase (EC 1.6.2.4) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Sterol 24-C-methyltransferase erg6 (EC 2.1.1.-) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Sterol-4-α-carboxylate 3-dehydrogenase (EC 1.1.1.170) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| GO:0032443 | P:regulation of ergosterol biosynthetic process | Damage response protein 1 | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| GO:0015248 | F:sterol transporter activity | Oxysterol-binding protein-related protein 3 (ORP-3) | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Protein PRY2 (Pathogenesis-related protein 2) | 6 | 43 | 8 | 6 | 5 | 5 | 5 | 8 | 6 | ||
| Cell wall protein PRY3 (Pathogenesis-related protein 3) | 2 | 12 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | ||
| Cell wall degradation | |||||||||||
| GO:0046274 | P: lignin catabolic process | 3-O-methyltransferase | 2 | 10 | 1 | 2 | 1 | 2 | 2 | 1 | 1 |
| 4-O-methyl-glucuronoyl methylesterase | 6 | 40 | 7 | 6 | 5 | 5 | 5 | 6 | 6 | ||
| 4-O-methyltransferase | 13 | 60 | 11 | 5 | 5 | 10 | 11 | 10 | 8 | ||
| Laccase | 24 | 103 | 15 | 14 | 14 | 16 | 16 | 16 | 12 | ||
| GO:0006979 | P:response to oxidative stress | Ligninases | 3 | 16 | 3 | 2 | 2 | 2 | 2 | 2 | 3 |
| Manganese peroxidase | 3 | 19 | 4 | 2 | 2 | 2 | 2 | 4 | 3 | ||
| Versatile peroxidase | 3 | 19 | 3 | 3 | 3 | 3 | 3 | 3 | 1 | ||
| Number of Proteins with Signal Peptide | Number of Predicted Effector Proteins (% of Number of Proteins with Signal Peptide) | Number of Predicted Effector Proteins with Annotation | Over-Represented GO Terms of Effectors | |
|---|---|---|---|---|
| G. boninense G3 | 2327 | 175 (7.5) | 80 | GO:0005576 (extracellular region) |
| G. lucidum G260125-1 | 1407 | 102 (7.2) | 59 | GO:0010466 (negative regulation of peptidase activity) GO:0004867 (serine-type endopeptidase inhibitor activity) GO:0043086 (negative regulation of catalytic activity) |
| G. lucidum Xiangnong | 1565 | 107 (6.8) | 52 | - |
| G. lucidum BCRC37177 | 1592 | 123 (7.7) | 66 | GO:0005576 (extracellular region) GO:0005615 (extracellular space) |
| G. multipileum | 1625 | 118 (7.3) | 63 | GO:0010466 (negative regulation of peptidase activity) GO:0004867 (serine-type endopeptidase inhibitor activity) |
| G. sinense ZZ0214-1 | 1908 | 153 (8.0) | 71 | GO:0005576 (extracellular region) |
| G. tsugae | 1648 | 144 (8.7) | 85 | GO:0005576 (extracellular region) GO:0030234 (enzyme regulator activity) GO:0016798 (hydrolase activity, acting on glycosyl bonds) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, C.-L. Comparative Genomics Analysis of Ganoderma Orthologs Involved in Plant-Pathogenesis. Forests 2023, 14, 653. https://doi.org/10.3390/f14030653
Ho C-L. Comparative Genomics Analysis of Ganoderma Orthologs Involved in Plant-Pathogenesis. Forests. 2023; 14(3):653. https://doi.org/10.3390/f14030653
Chicago/Turabian StyleHo, Chai-Ling. 2023. "Comparative Genomics Analysis of Ganoderma Orthologs Involved in Plant-Pathogenesis" Forests 14, no. 3: 653. https://doi.org/10.3390/f14030653
APA StyleHo, C.-L. (2023). Comparative Genomics Analysis of Ganoderma Orthologs Involved in Plant-Pathogenesis. Forests, 14(3), 653. https://doi.org/10.3390/f14030653





