Genetic Differentiation of Budburst Timing in Fagus crenata Populations along a Spatial Gradient in Late Frost Timing in the Hakkoda Mountains, Northern Japan
Abstract
1. Introduction
2. Methods
2.1. Study Species
2.2. Study Area
2.3. Field Survey
2.3.1. Air Temperature
2.3.2. The Timing of Budburst in the Habitat
2.4. Common Garden Experiment
2.5. Data Analysis
2.5.1. Relationship between the Timing of Budburst of Canopy Trees and Environmental Conditions in Habitat
2.5.2. Genetic Variation in the Timing of Budburst of Saplings in the Nursery
2.5.3. Heritability of the Timing of Budburst
2.5.4. Relationship between the Timing of Budburst of Saplings in the Nursery and Environmental Conditions in the Provenances
3. Results
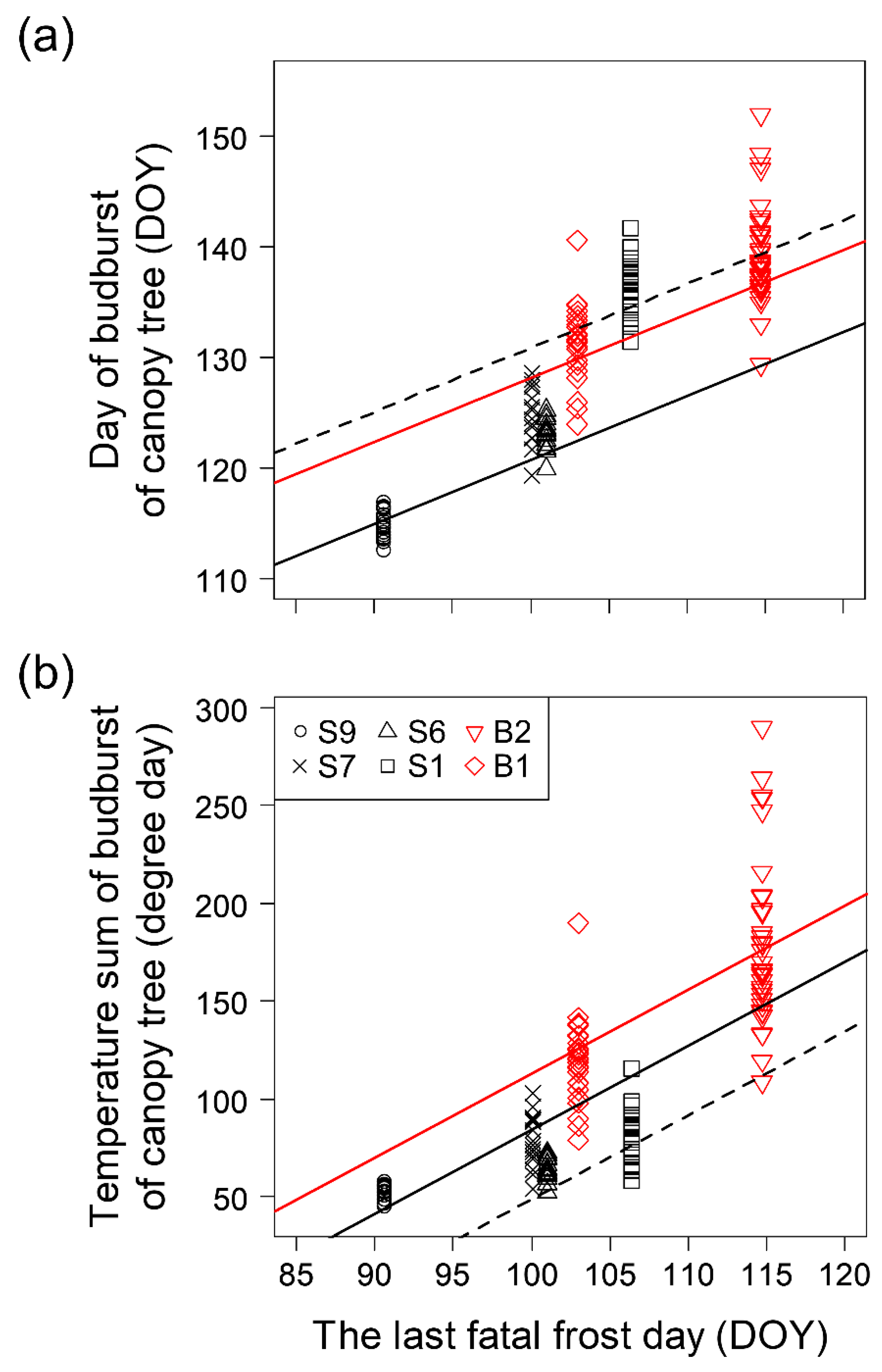
3.1. Relationships between Timing of Budburst of Canopy Trees and Environmental Conditions in the Habitat
3.2. Genetic Variations in Nursery for the Timing of Budburst
3.3. Heritability for the Timing of Budburst
3.4. Relationships between the Timing of Budburst in the Nursery and Environmental Conditions in the Provenances
4. Discussion
4.1. Interpopulation Variation in Habitats
4.2. Genetic Variation and the Heritability
4.3. Local Adaptation to Spatial Variation in Late Frost Timing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Menzel, A.; Sparks, T.H.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-kübler, K.; Bissolli, P.; Braslavská, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, L. Responses to a Warming World. Science 2001, 294, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C.; Hanley, M.E. Plants and climate change. Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef]
- Hänninen, H. Boreal and Temperature Trees in A Changing Climate; Springer Business Media: Dordrecht, The Netherlands, 2015. [Google Scholar]
- Fu, Y.H.; Zhang, X.; Piao, S.; Hao, F.; Geng, X.; Vitasse, Y.; Zohner, C.; Peñuelas, J.; Janssens, I.A. Daylength helps temperate deciduous trees to leaf-out at the optimal time. Glob. Chang. Biol. 2019, 25, 2410–2418. [Google Scholar] [CrossRef]
- Kreyling, J.; Thile, D.; Nagy, L.; Jentsch, A.; Huber, G.; Konnert, M.; Beierkuhnlein, C. Late frost sensitivity of juvenile Fagus sylvatica L. differs between southern Germany and Bulgaria and depends on preceding air temperature. Eur. J. For. Res. 2011, 131, 717–725. [Google Scholar] [CrossRef]
- Eysteinsson, T.; Karlman, L.; Fries, A.; Martinsson, O.; Skúlason, B. Variation in spring and autumn frost tolerance among provenances of Russian larches (Larix Mill.). Scand. J. For. Res. 2009, 24, 100–110. [Google Scholar] [CrossRef]
- Alberto, F.; Bouffier, L.; Louvet, J.M.; Lamy, J.B.; Delzon, S.; Kremer, A. Adaptive responses for seed and leaf phenology in natural populations of sessile oak along an altitudinal gradient. J. Evol. Biol. 2011, 24, 1442–1454. [Google Scholar] [CrossRef]
- Petit, R.J.; Hampe, A. Some evolutionary consequences of being a tree. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 187–214. [Google Scholar] [CrossRef]
- Savolainen, O.; Pyhäjärvi, T.; Knürr, T. Gene flow and local adaptation in trees. Annu. Rev. Ecol. Evol. Syst. 2007, 38, 595–619. [Google Scholar] [CrossRef]
- Vitasse, Y.; Delzon, S.; Bresson, C.C.; Michalet, R.; Kremer, A. Altitudinal differentiation in growth and phenology among populations of temperate-zone tree species growing in a common garden. Can. J. For. Res. 2009, 39, 1259–1269. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants; Springer: Berlin/Heidelberg, Germany, 1987. [Google Scholar]
- Vitasse, Y.; Klein, G.; Kirchner, J.W.; Rebetez, M. Intensity, frequency and spatial configuration of winter temperature inversions in the closed La Brevine valley, Switzerland. Theor. Appl. Climatol. 2017, 130, 1073–1083. [Google Scholar] [CrossRef]
- Geiger, R. The Climate Near the Ground; Harvard University Press: Cambridge, MA, USA, 1950. [Google Scholar]
- Kojima, H.; Mariko, S.; Nakamura, T.; Hayashi, I. Bud burst process and late-frost experimens on Fagus crenata and Quercus mongolica ssp. crispula. Veg. Sci. 2003, 20, 55–64. [Google Scholar] [CrossRef]
- Hashizume, H.; Lee, J.H.; Yamamoto, F. Variation in the flushing time of Fagus crenata Blume among provenances and families. J. Jpn. For. Soc. 1996, 78, 363–368, (In Japanese with English summary). [Google Scholar] [CrossRef]
- Osada, N.; Murase, K.; Tsuji, K.; Sawada, H.; Nunokawa, K.; Tsukahara, M.; Hiura, T. Genetic differentiation in the timing of budburst in Fagus crenata in relation to temperature and photoperiod. Int. J. Biometeorol. 2018, 62, 1763–1776. [Google Scholar] [CrossRef]
- Sugimoto, S.; Ishida, K. Interpopulation variation in leaf out phenology of Fagus crenata along topographic variation associated with the late frost regime in the Hakkoda Mountains, northern Japan. Ecol. Res. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of frost and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- Zohner, C.M.; Mo, L.; Renner, S.S.; Crowther, T.W. Late-spring frost risk between 1959 and 2017 decreased in North America but increased in Europe and Asia. Proc. Natl. Acad. Sci. USA 2020, 117, 12192–12200. [Google Scholar] [CrossRef]
- Nakashizuka, T. Fagus crenata. In The Trees of Japan Ι; Editorial committee of the Trees of Japan, Ed.; J-FIC: Tokyo, Japan, 2009; pp. 577–590, (In Japanese. The English title is the author’s tentative translation). [Google Scholar]
- Maruyama, K. Comparative studies on the phenological sequences among different tree species and layer communities—Ecological studies on natural beech forest (33). Bull. Niigata Univ. For. 1979, 12, 19–41, (In Japanese with English summary). [Google Scholar]
- Tomita, M.; Seiwa, K. Influence of canopy tree phenology on understorey populations of Fagus crenata. J. Veg. Sci. 2004, 15, 379–388. [Google Scholar] [CrossRef]
- Takahashi, K. On the snow scale for measuring maximum snow depth. Seppyou 1968, 30, 11–14, (In Japanese with English summary). [Google Scholar] [CrossRef]
- R Development Core Team. R Version 4.0.1; A language and environment for statical computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H.B. Lmer test package: Tests in linear mixed effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. R Package Version 1.43.17. 2020. Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 30 May 2022).
- Schuetzenmeister, A. VCA: Variance Component Analysis. R Package Version 1.4.5. 2022. Available online: https://cran.r-project.org/web/packages/VCA/ (accessed on 30 May 2022).
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; LongmanGroup Ltd.: Essex, UK, 1996. [Google Scholar]
- Osada, N.; Hiura, T. Intraspecific differences in spring leaf phenology in relation to tree size in temperature deciduous trees. Tree Physiol. 2019, 39, 782–791. [Google Scholar] [CrossRef]
- Vitasse, Y. Ontogenic changes rather than difference in temperature cause understory trees to leaf out earlier. New Phytol. 2013, 198, 149–155. [Google Scholar] [CrossRef]
- Howe, G.T.; Aitken, S.N.; Neale, D.B.; Jermstad, K.D.; Wheeler, N.C.; Chen, T.H. From genotype to phenotype: Unraveling the complexities of cold adaptation in forest trees. Can. J. Bot. 2003, 81, 1247–1266. [Google Scholar] [CrossRef]
- Cornelius, J. Heritabilities and additive genetic coefficients of variation in forest trees. Can. J. For. Res. 1994, 24, 2. [Google Scholar] [CrossRef]
- Vitasse, Y.; Baumgarten, F.; Zohner, C.M.; Kaewthongrach, R.; Fu, Y.H.; Walde, M.G.; Moser, B. Impact of microclimatic conditions and resource availability on spring and autumn phenology of temperate tree seedlings. New Phytol. 2021, 232, 537–550. [Google Scholar] [CrossRef]
- Cooper, H.F.; Grady, K.C.; Cowan, J.A.; Best, R.J.; Allan, G.J.; Whitham, T.G. Genotypic variation in phenological plasticity: Reciprocal common gardens reveal adaptive responses to warmer springs but not fall frost. Glob. Chang. Biol. 2018, 25, 187–200. [Google Scholar] [CrossRef]



| Sites | Topography | Altitude (m) | Snow Depth a (cm) | Dominant Species |
|---|---|---|---|---|
| S1 | Hillside slope | 900 | 379 | Fagus crenata and Abies mariesii |
| S6 | Hillside slope | 620 | 262 | Fagus crenata and Magnolia obovate |
| S7 | Hillside slope | 600 | 287 | Fagus crenata |
| S9 | Hillside slope | 450 | 179 | Fagus crenata and Quercus crispula |
| B1 | Basin | 570 | 247 | Fagus crenata and Quercus crispula |
| B2 | Basin | 560 | 248 | Fagus crenata and Quercus crispula |
| Provenance | Number of Saplings | Number of Mother Trees | Height (cm) Mean (Range) | Diameter at the Stem Base (mm) Mean (Range) |
|---|---|---|---|---|
| Hillside slope | ||||
| S1 | 11 | 6 | 76.4 (39.8−116.5) | 22.3 (18.4−32.2) |
| S6 | 3 | 1 | 140.5 (72.3−229.7) | 24.6 (17.9−30.8) |
| S7 | 13 | 8 | 115.6 (75.8−164.2) | 29.2 (18.9−41.5) |
| S9 | 6 | 3 | 151.7 (92.7−225.0) | 24.3 (19.6−31.0) |
| Basin | ||||
| B1 | 11 | 8 | 110.1 (59.3−147.3) | 24.6 (18.0−31.2) |
| Number of Mother Trees | Number of Saplings per Mother Tree | The Day of Budburst | Temperature Sum of Budburst | |||||
|---|---|---|---|---|---|---|---|---|
| Provenance | (DOY) | (Degree Days) | ||||||
| (Topography) a | Estimate | Std. Error | p Value | Estimate | Std. Error | p Value | ||
| B1 (b) | 8 | 1–2 | 127.6 | 2.1 | – | 200.3 | 17.0 | – |
| S1 (s) | 6 | 1–5 | 125.4 | 1.8 | 0.24 | 183.3 | 16.3 | 0.31 |
| S6 (s) | 1 | 3 | 118.8 | 3.3 | <0.05 | 134.0 | 29.4 | <0.05 |
| S7 (s) | 8 | 1–4 | 125.2 | 1.7 | 0.19 | 183.4 | 15.1 | 0.28 |
| S9 (s) | 3 | 1–3 | 119.4 | 2.2 | <0.01 | 137.4 | 19.9 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugimoto, S.; Ishida, K. Genetic Differentiation of Budburst Timing in Fagus crenata Populations along a Spatial Gradient in Late Frost Timing in the Hakkoda Mountains, Northern Japan. Forests 2023, 14, 659. https://doi.org/10.3390/f14040659
Sugimoto S, Ishida K. Genetic Differentiation of Budburst Timing in Fagus crenata Populations along a Spatial Gradient in Late Frost Timing in the Hakkoda Mountains, Northern Japan. Forests. 2023; 14(4):659. https://doi.org/10.3390/f14040659
Chicago/Turabian StyleSugimoto, Saki, and Kiyoshi Ishida. 2023. "Genetic Differentiation of Budburst Timing in Fagus crenata Populations along a Spatial Gradient in Late Frost Timing in the Hakkoda Mountains, Northern Japan" Forests 14, no. 4: 659. https://doi.org/10.3390/f14040659
APA StyleSugimoto, S., & Ishida, K. (2023). Genetic Differentiation of Budburst Timing in Fagus crenata Populations along a Spatial Gradient in Late Frost Timing in the Hakkoda Mountains, Northern Japan. Forests, 14(4), 659. https://doi.org/10.3390/f14040659





