Effects of Tree Functional Traits on Soil Respiration in Tropical Forest Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Data Collection
2.2.1. Forest Inventory
2.2.2. Soil Respiration
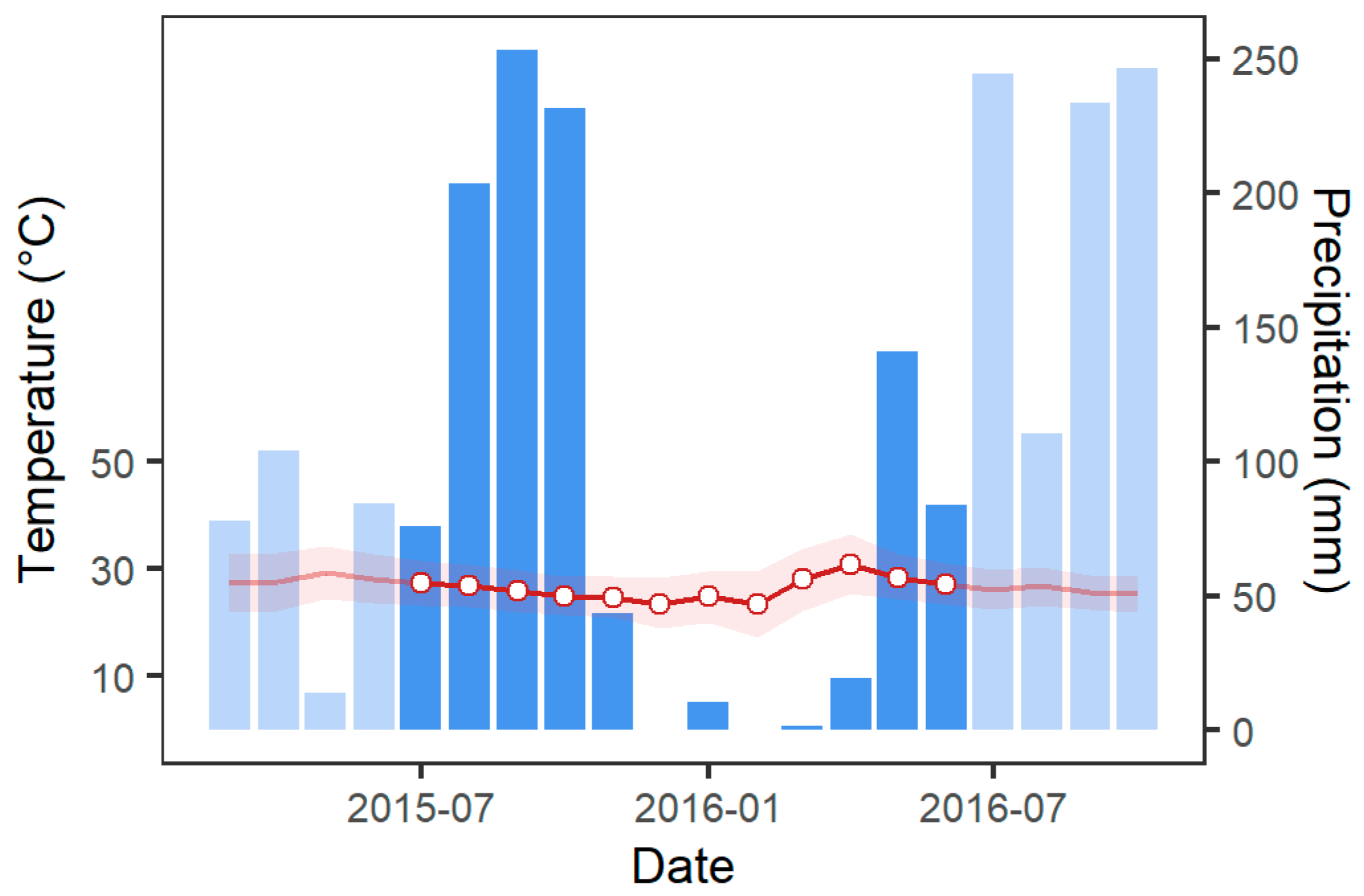
2.2.3. Climate Data
2.2.4. Soil Sampling and Analysis
2.3. Data Analyses
3. Results
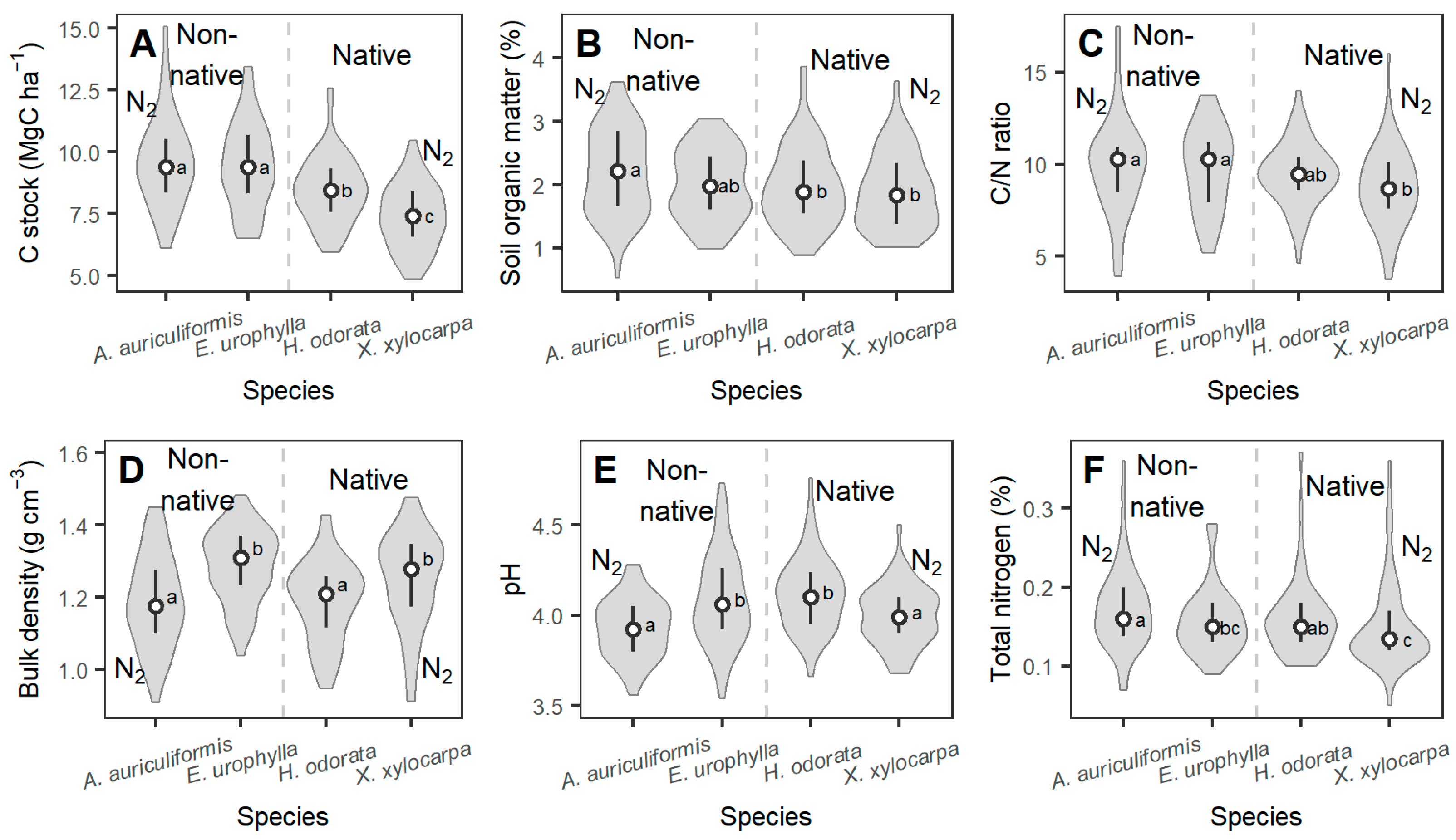
3.1. Comparison of Stand Characteristics, Soil Chemical, and Microclimatic Conditions
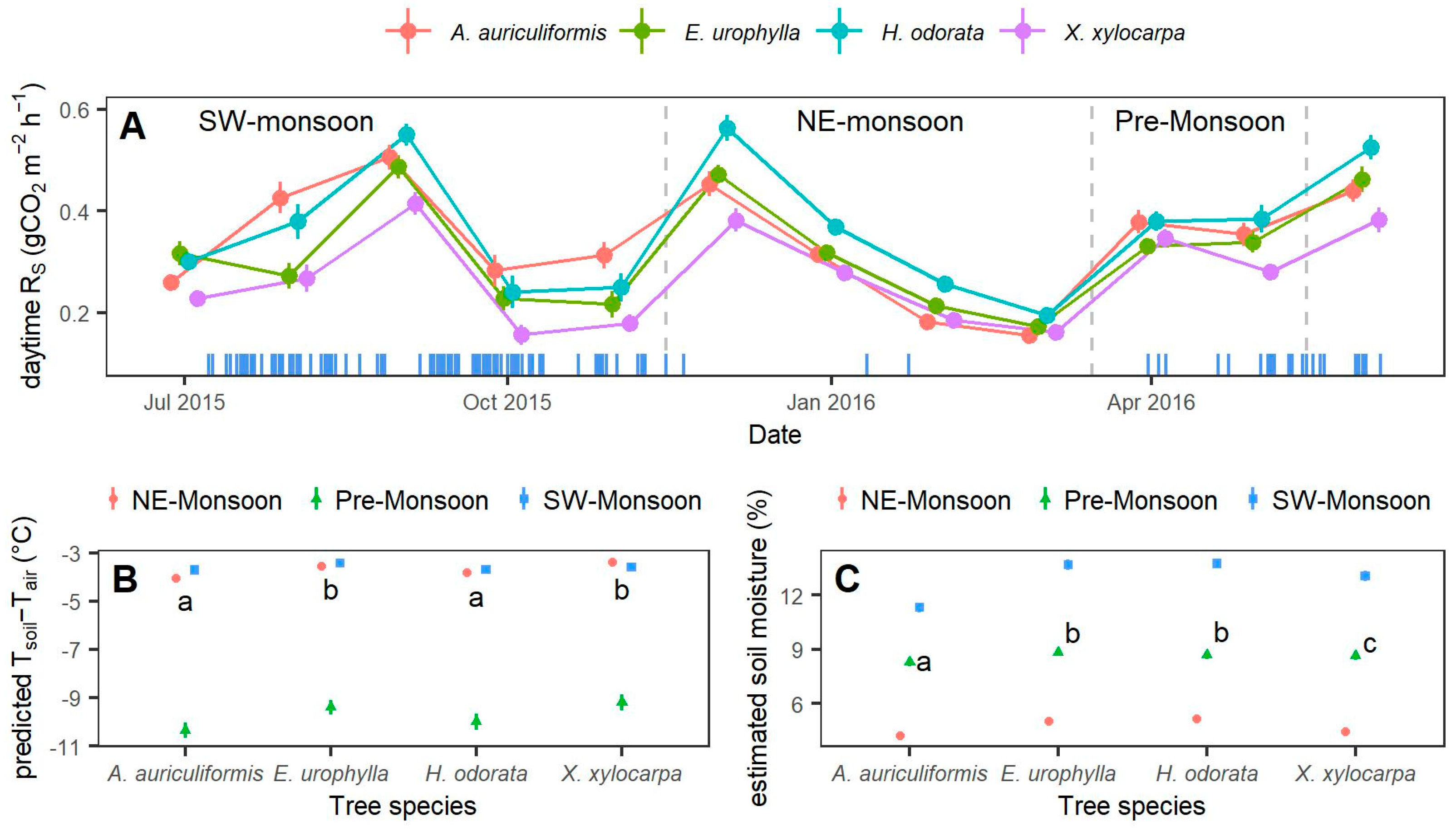
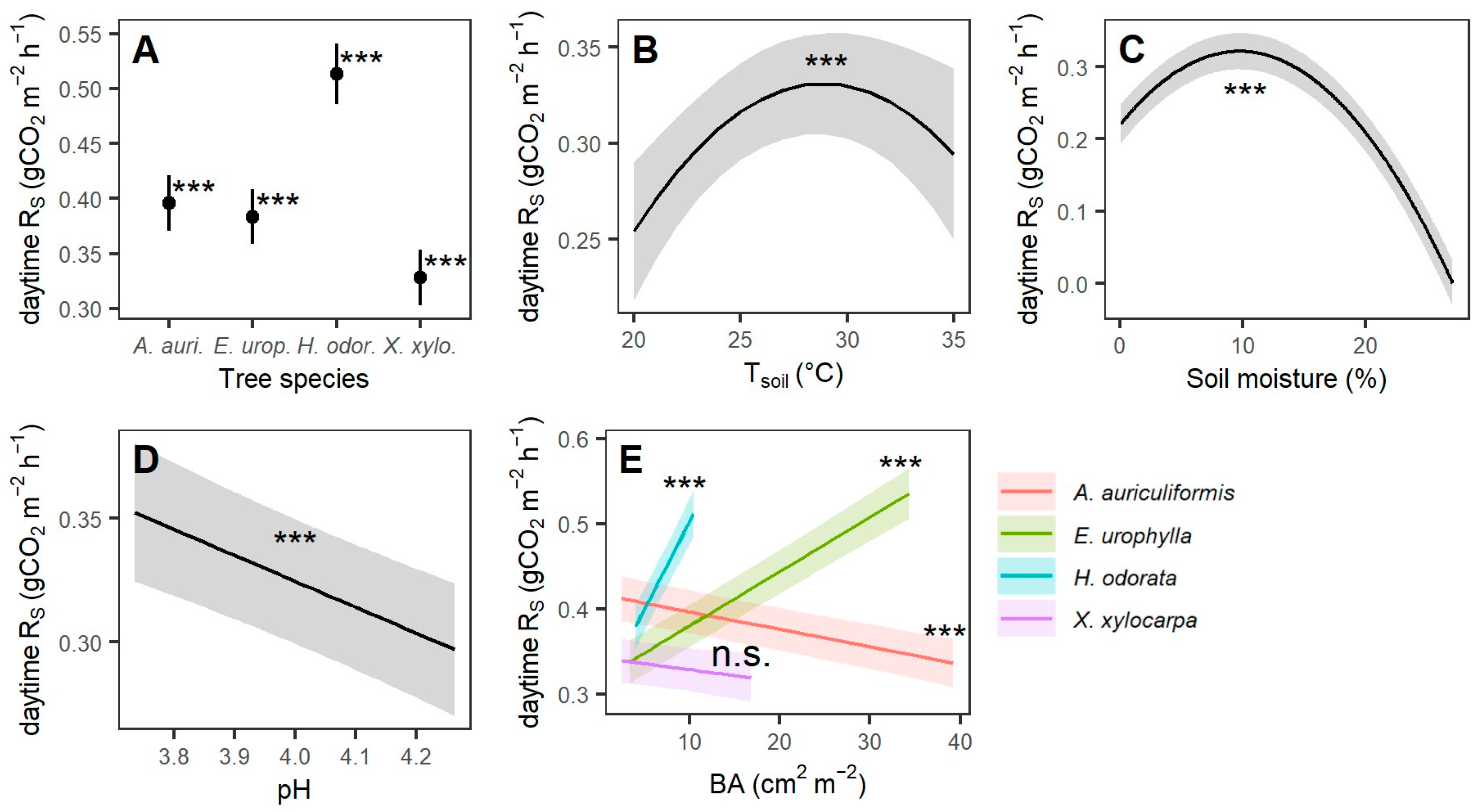
3.2. Effects of Tree Species, Soil Conditions, and Basal Area on Soil Respiration
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

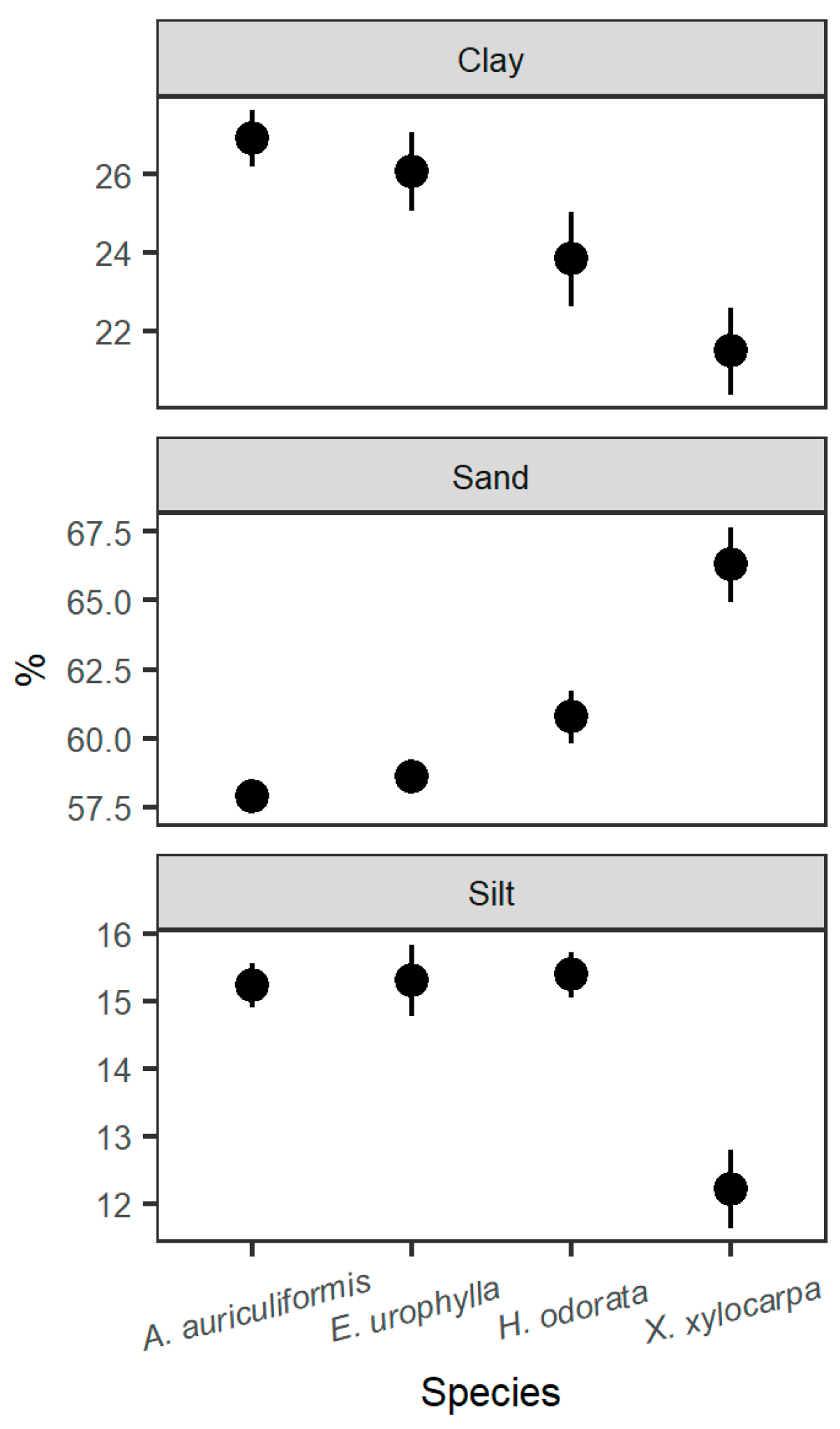
| Soil Characteristic | Depth (cm) | A. auriculiformis | E. urophylla | H. odorata | X. xylocarpa |
|---|---|---|---|---|---|
| Sand (%) | 0–15 | 58.79 (1.15) | 59.63 (0.76) | 62.04 (1.5) | 68.54 (3.46) |
| 15–30 | 56.96 (0.5) | 57.63 (1.26) | 59.51 (2.58) | 64.04 (0.5) | |
| Silt (%) | 0–15 | 15.81 (0.58) | 16.31 (0.29) | 15.64 (0.87) | 12.14 (2.00) |
| 15–30 | 14.64 (0.5) | 14.31 (1.04) | 15.14 (0.87) | 12.31 (1.04) | |
| Clay (%) | 0–15 | 25.40 (1.01) | 24.07 (0.58) | 22.32 (1.73) | 19.32 (2.00) |
| 15–30 | 28.40 (0.01) | 28.07 (1.53) | 25.35 (3.44) | 23.65 (0.58) | |
| Carbon (%) | 0–15 | 1.25 (0.12) | 1.14 (0.12) | 1.15 (0.22) | 0.86 (0.05) |
| 15–30 | 0.68 (0.09) | 0.71 (0.14) | 0.8 (0.24) | 0.59 (0.06) | |
| Nitrogen (%) | 0–15 | 0.16 (0.03) | 0.14 (0.03) | 0.14 (0.01) | 0.13 (0.01) |
| 15–30 | 0.10 (0.03) | 0.10 (0.02) | 0.11 (0.02) | 0.09 (0.01) | |
| pH | 0–15 | 3.62 (0.03) | 3.87 (0.34) | 3.85 (0.14) | 3.73 (0.07) |
| 15–30 | 3.90 (0.14) | 4.06 (0.19) | 3.97 (0.11) | 3.89 (0.07) | |
| SOM (%) | 0–15 | 1.73 (0.39) | 1.61 (0.14) | 1.83 (0.49) | 1.37 (0.35) |
| 15–30 | 1.12 (0.05) | 1.14 (0.24) | 1.13 (0.35) | 0.85 (0.28) | |
| Exch. Ca2+ (mg kg−1) | 0–15 | 59.39 (12.7) | 177.67 (137.64) | 131.49 (82.58) | 90.46 (41.21) |
| 15–30 | 17.46 (0.72) | 139.16 (104.98) | 87.74 (10.8) | 23.12 (4.52) | |
| Exch. Mg2+ (mg kg−1) | 0–15 | 24.34 (10.03) | 39.15 (27.75) | 35.48 (14.17) | 21.39 (10.02) |
| 15–30 | 14.56 (2.13) | 29.03 (21.46) | 22.19 (6.27) | 7.94 (3.58) | |
| Exch. K+ (mg kg−1) | 0–15 | 42.55 (9.62) | 60.66 (35.25) | 38.47 (6.02) | 46.45 (20.89) |
| 15–30 | 20.67 (6.04) | 29.27 (7.27) | 18.87 (4.01) | 16.62 (3.26) | |
| P (mg kg−1) | 0–15 | 4.81 (1.97) | 5.22 (1.37) | 5.09 (0.56) | 6.56 (1.80) |
| 15–30 | 2.29 (0.46) | 2.65 (0.96) | 2.85 (1.13) | 2.95 (0.57) |
| Predictors | Estimates | CI | p |
|---|---|---|---|
| Intercept | 0.78 | 0.62–0.94 | <0.001 |
| Species [E. urophylla] | −0.10 | −0.12–0.09 | <0.001 |
| Species [H. odorata] | −0.12 | −0.15–0.10 | <0.001 |
| Species [X. xylocarpa] | −0.07 | −0.09–0.06 | <0.001 |
| Temperature [1st degree] | 0.95 | 0.43–1.46 | <0.001 |
| Temperature [2nd degree] | −0.65 | −0.97–0.32 | <0.001 |
| Moisture [1st degree] | −3.14 | −3.63–2.64 | <0.001 |
| Moisture [2nd degree] | −4.40 | −4.71–4.09 | <0.001 |
| pH | −0.10 | −0.15–0.06 | <0.001 |
| Species [A. auriculiformis] × BA | −0.00 | −0.00–0.00 | <0.001 |
| Species [E. urophylla] × BA | 0.01 | 0.01–0.01 | <0.001 |
| Species [H. odorata] × BA | 0.02 | 0.02–0.02 | <0.001 |
| Species [X. xylocarpa] × BA | −0.00 | −0.00–0.00 | 0.081 |
| Random Effects | |||
| σ2 | 0.01 | τ00 point | 0.00 |
| τ00 h:month | 0.01 | ICC | 0.38 |
| Marginal R2/Conditional R2 | 0.296/0.565 |
References
- Bonner, M.T.L.; Schmidt, S.; Shoo, L.P. A Meta-Analytical Global Comparison of Aboveground Biomass Accumulation between Tropical Secondary Forests and Monoculture Plantations. For. Ecol. Manag. 2013, 291, 73–86. [Google Scholar] [CrossRef]
- Schroeder, P. Carbon Storage Potential of Short Rotation Tropical Tree Plantations. For. Ecol. Manag. 1992, 50, 31–41. [Google Scholar] [CrossRef]
- Justine, M.; Yang, W.; Wu, F.; Tan, B.; Khan, M.; Zhao, Y. Biomass Stock and Carbon Sequestration in a Chronosequence of Pinus Massoniana Plantations in the Upper Reaches of the Yangtze River. Forests 2015, 6, 3665–3682. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T.; et al. Productivity Overshadows Temperature in Determining Soil and Ecosystem Respiration across European Forests: Respiration Across European Forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Bréchet, L.; Ponton, S.; Roy, J.; Freycon, V.; Coûteaux, M.-M.; Bonal, D.; Epron, D. Do Tree Species Characteristics Influence Soil Respiration in Tropical Forests? A Test Based on 16 Tree Species Planted in Monospecific Plots. Plant Soil 2009, 319, 235–246. [Google Scholar] [CrossRef]
- Tesfaye, M.A.; Bravo-Oviedo, A.; Bravo, F.; Kidane, B.; Bekele, K.; Sertse, D. Selection of Tree Species and Soil Management for Simultaneous Fuelwood Production and Soil Rehabilitation in the Ethiopian Central Highlands. Land Degrad. Develop. 2015, 26, 665–679. [Google Scholar] [CrossRef]
- Franco, A.A.; De Faria, S.M. The Contribution of N2-Fixing Tree Legumes to Land Reclamation and Sustainability in the Tropics. Soil Biol. Biochem. 1997, 29, 897–903. [Google Scholar] [CrossRef]
- Sang, P.M.; Lamb, D.; Bonner, M.; Schmidt, S. Carbon Sequestration and Soil Fertility of Tropical Tree Plantations and Secondary Forest Established on Degraded Land. Plant Soil 2013, 362, 187–200. [Google Scholar] [CrossRef]
- Epron, D.; Nouvellon, Y.; Roupsard, O.; Mouvondy, W.; Mabiala, A.; Saint-André, L.; Joffre, R.; Jourdan, C.; Bonnefond, J.-M.; Berbigier, P.; et al. Spatial and Temporal Variations of Soil Respiration in a Eucalyptus Plantation in Congo. For. Ecol. Manag. 2004, 202, 149–160. [Google Scholar] [CrossRef]
- Raich, J. Temporal Variability of Soil Respiration in Experimental Tree Plantations in Lowland Costa Rica. Forests 2017, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, S.; Tanaka, N.; Suzuki, M.; Inoue, A.; Takizawa, H.; Kosaka, I.; Tanaka, K.; Tantasirin, C.; Tangtham, N. Soil Respiration and Soil CO2 Concentration in a Tropical Forest, Thailand. J. For. Res. 2004, 9, 75–79. [Google Scholar] [CrossRef]
- Adachi, M.; Ishida, A.; Bunyavejchewin, S.; Okuda, T.; Koizumi, H. Spatial and Temporal Variation in Soil Respiration in a Seasonally Dry Tropical Forest, Thailand. J. Trop. Ecol. 2009, 25, 531–539. [Google Scholar] [CrossRef]
- Takahashi, M.; Hirai, K.; Limtong, P.; Leaungvutivirog, C.; Suksawang, S.; Panuthai, S.; Anusontpornperm, S.; Marod, D. Soil Respiration in Different Ages of Teak Plantations in Thailand. JARQ 2009, 43, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, M.; Hirai, K.; Limtong, P.; Leaungvutivirog, C.; Panuthai, S.; Suksawang, S.; Anusontpornperm, S.; Marod, D. Topographic Variation in Heterotrophic and Autotrophic Soil Respiration in a Tropical Seasonal Forest in Thailand. Soil Sci. Plant Nutr. 2011, 57, 452–465. [Google Scholar] [CrossRef]
- Hanpattanakit, P.; Leclerc, M.Y.; Mcmillan, A.M.S.; Limtong, P.; Maeght, J.-L.; Panuthai, S.; Inubushi, K.; Chidthaisong, A. Multiple Timescale Variations and Controls of Soil Respiration in a Tropical Dry Dipterocarp Forest, Western Thailand. Plant Soil 2015, 390, 167–181. [Google Scholar] [CrossRef]
- Boonriam, W.; Suwanwaree, P.; Hasin, S.; Archawakom, T.; Chanonmuang, P.; Yamada, A. Seasonal Changes in Spatial Variation of Soil Respiration in Dry Evergreen Forest, Sakaerat Biosphere Reserve, Thailand. ScienceAsia 2021, 47S, 112. [Google Scholar] [CrossRef]
- Rodtassana, C.; Unawong, W.; Yaemphum, S.; Chanthorn, W.; Chawchai, S.; Nathalang, A.; Brockelman, W.Y.; Tor-ngern, P. Different Responses of Soil Respiration to Environmental Factors across Forest Stages in a Southeast Asian Forest. Ecol. Evol. 2021, 11, 15430–15443. [Google Scholar] [CrossRef] [PubMed]
- Vesterdal, L.; Elberling, B.; Christiansen, J.R.; Callesen, I.; Schmidt, I.K. Soil Respiration and Rates of Soil Carbon Turnover Differ among Six Common European Tree Species. For. Ecol. Manag. 2012, 264, 185–196. [Google Scholar] [CrossRef]
- Russell, A.E.; Raich, J.W.; Arrieta, R.B.; Valverde-Barrantes, O.; González, E. Impacts of Individual Tree Species on Carbon Dynamics in a Moist Tropical Forest Environment. Ecol. Appl. 2010, 20, 1087–1100. [Google Scholar] [CrossRef] [Green Version]
- Raich, J.W.; Tufekciogul, A. Vegetation and Soil Respiration: Correlations and Controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand Age-Related Effects on Soil Respiration in a First Rotation Sitka Spruce Chronosequence in Central Ireland: Stand Age-Related Effects on Soil Respiration. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Meier, I.C.; Finzi, A.C.; Phillips, R.P. Root Exudates Increase N Availability by Stimulating Microbial Turnover of Fast-Cycling N Pools. Soil Biol. Biochem. 2017, 106, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Zwetsloot, M.J.; Kessler, A.; Bauerle, T.L. Phenolic Root Exudate and Tissue Compounds Vary Widely among Temperate Forest Tree Species and Have Contrasting Effects on Soil Microbial Respiration. New Phytol. 2018, 218, 530–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bréchet, L.; Le Dantec, V.; Ponton, S.; Goret, J.-Y.; Sayer, E.; Bonal, D.; Freycon, V.; Roy, J.; Epron, D. Short- and Long-Term Influence of Litter Quality and Quantity on Simulated Heterotrophic Soil Respiration in a Lowland Tropical Forest. Ecosystems 2017, 20, 1190–1204. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Cheng, R.; Shi, Z.; Wang, W.; Xu, G.; Liu, S. Impacts of Nitrogen-Fixing and Non-Nitrogen-Fixing Tree Species on Soil Respiration and Microbial Community Composition during Forest Management in Subtropical China. Ecol. Res. 2016, 31, 683–693. [Google Scholar] [CrossRef]
- Tang, C.; Yu, Q. Impact of Chemical Composition of Legume Residues and Initial Soil PH on PH Change of a Soil after Residue Incorporation. Plant Soil 1999, 215, 29–38. [Google Scholar] [CrossRef]
- Dakora, F.D.; Phillips, D.A. Root Exudates as Mediators of Mineral Acquisition in Low-Nutrient Environments. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 201–213. ISBN 978-90-481-6013-6. [Google Scholar]
- Fujii, K. Soil Acidification and Adaptations of Plants and Microorganisms in Bornean Tropical Forests. Ecol. Res. 2014, 29, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Janssens, I.A.; Dieleman, W.; Luyssaert, S.; Subke, J.-A.; Reichstein, M.; Ceulemans, R.; Ciais, P.; Dolman, A.J.; Grace, J.; Matteucci, G.; et al. Reduction of Forest Soil Respiration in Response to Nitrogen Deposition. Nat. Geosci. 2010, 3, 315–322. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L. On the Success and Failure of Mixed-Species Tree Plantations: Lessons Learned from a Model System of Eucalyptus Globulus and Acacia Mearnsii. For. Ecol. Manag. 2005, 209, 147–155. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The Sensitivity of Soil Respiration to Soil Temperature, Moisture, and Carbon Supply at the Global Scale. Glob. Chang. Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef]
- Wood, T.E.; Detto, M.; Silver, W.L. Sensitivity of Soil Respiration to Variability in Soil Moisture and Temperature in a Humid Tropical Forest. PLoS ONE 2013, 8, e80965. [Google Scholar] [CrossRef]
- Burgess, S.S.O.; Adams, M.A.; Turner, N.C.; Ong, C.K. The Redistribution of Soil Water by Tree Root Systems. Oecologia 1998, 115, 306–311. [Google Scholar] [CrossRef]
- Stahl, C.; Hérault, B.; Rossi, V.; Burban, B.; Bréchet, C.; Bonal, D. Depth of Soil Water Uptake by Tropical Rainforest Trees during Dry Periods: Does Tree Dimension Matter? Oecologia 2013, 173, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Virapongse, A. Smallholders and Forest Landscape Restoration in Upland Northern Thailand. Int. Forest. Rev. 2017, 19, 102–119. [Google Scholar] [CrossRef]
- Elliott, S.; Kuaraksa, C. Producing Framework Tree Species for Restoring Forest Ecosystems in Northern Thailand. Small-Scale For. 2008, 7, 403–415. [Google Scholar] [CrossRef]
- Staporn, D.; Marod, D.; Wongprom, J.; Diloksumpun, S. Drivers of Native Species Regeneration in the Process of Restoring a Dry Evergreen Forest from Exotic Tree Plantations in Northeastern Thailand. Forests 2022, 13, 1321. [Google Scholar] [CrossRef]
- Peng, S.L.; Liu, J.; Lu, H.F. Characteristics and Role of Acacia Auriculiformis on Vegetation Restoration in Lower Subtropics of China. J. Trop. For. Sci. 2005, 17, 508–525. [Google Scholar]
- Morris, J.; Ningnan, Z.; Zengjiang, Y.; Collopy, J.; Daping, X. Water Use by Fast-Growing Eucalyptus Urophylla Plantations in Southern China. Tree Physiol. 2004, 24, 1035–1044. [Google Scholar] [CrossRef] [Green Version]
- Oldfield, S.; Lusty, C.; MacKinven, A. The World List of Threatened Trees; World Conservation Press: Cambridge, UK, 1998. [Google Scholar]
- Podong, C.; Poolsiri, R.; Katzensteiner, K.; Pengthamkeerati, P.; Thongdeenok, P. Species Diversity and Litter Dynamics in Secondary Mixed Deciduous Forest, Thung Salaeng Lung National Park, Northern, Thailand. Folia For. Pol. Seria A-For. 2013, 55, 196–204. [Google Scholar] [CrossRef]
- Moormann, F.R.; Rojanasoonthon, S. The Soils of the Kingdom of Thailand; Soil Survey Report SSR.; Soil Survey Division, Department of Land Development: Bangkok, Thailand, 1972. [Google Scholar]
- Ando, U.; Iwasa, M. Report on Implementation Results on Sakaerat Field Station, Phase I (1981–1986). R. For. Dep. Bangk. Thail. 1987, 433. [Google Scholar]
- Sakai, A.; Visaratana, T.; Vacharangkura, T.; Thai-ngam, R.; Tanaka, N.; Ishizuka, M.; Nakamura, S. Effect of Species and Spacing of Fast-Growing Nurse Trees on Growth of an Indigenous Tree, Hopea Odorata Roxb., in Northeast Thailand. For. Ecol. Manag. 2009, 257, 644–652. [Google Scholar] [CrossRef]
- Sakai, A.; Visaratana, T.; Vacharangkura, T.; Thai-Ngam, R.; Nakamura, S. Growth Performance of Four Dipterocarp Species Planted in a Leucaena Leucocephala Plantation and in an Open Site on Degraded Land under a Tropical Monsoon Climate. JARQ 2014, 48, 95–104. [Google Scholar] [CrossRef] [Green Version]
- Geng, Y.; Wang, Y.; Yang, K.; Wang, S.; Zeng, H.; Baumann, F.; Kuehn, P.; Scholten, T.; He, J.-S. Soil Respiration in Tibetan Alpine Grasslands: Belowground Biomass and Soil Moisture, but Not Soil Temperature, Best Explain the Large-Scale Patterns. PLoS ONE 2012, 7, e34968. [Google Scholar] [CrossRef] [Green Version]
- Page-Dumroese, D.S.; Brown, R.E.; Jurgensen, M.F.; Mroz, G.D. Comparison of Methods for Determining Bulk Densities of Rocky Forest Soils. Soil Sci. Soc. Am. J. 1999, 63, 379–383. [Google Scholar] [CrossRef]
- Burt, R. Soil Survey Investigations Report, No. 45, Version 2.0; Natural Resources Conservation Service: Washington, DC, USA, 2011. [Google Scholar]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1149–1178. ISBN 978-0-89118-204-7. [Google Scholar]
- Luo, Y.; Zhou, X. Soil Respiration and the Environment; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 978-0-08-046397-1. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. R Package Version 0.7. 0. Computer Software. 2021. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 5 March 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Soft. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Lüdecke, D. ggeffects: Tidy Data Frames of Marginal Effects from Regression Models. J. Open Source Softw. 2018, 3, 772. [Google Scholar] [CrossRef] [Green Version]
- Mohan Kumar, B.; Jacob George, S.; Jamaludheen, V.; Suresh, T.K. Comparison of Biomass Production, Tree Allometry and Nutrient Use Efficiency of Multipurpose Trees Grown in Woodlot and Silvopastoral Experiments in Kerala, India. For. Ecol. Manag. 1998, 112, 145–163. [Google Scholar] [CrossRef]
- Chae, N. Annual Variation of Soil Respiration and Precipitation in a Temperate Forest (Quercus Serrata and Carpinus Laxiflora) Under East Asian Monsoon Climate. J. Plant Biol. 2011, 54, 101–111. [Google Scholar] [CrossRef]
- Tomar, U.; Baishya, R. Seasonality and Moisture Regime Control Soil Respiration, Enzyme Activities, and Soil Microbial Biomass Carbon in a Semi-Arid Forest of Delhi, India. Ecol. Process 2020, 9, 50. [Google Scholar] [CrossRef]
- Swarnalatha, B.; Reddy, M.V. Leaf Litter Breakdown and Nutrient Release in Three Tree Plantations Compared with a Natural Degraded Forest on the Coromandel Coast (Puducherry, India). Ecotropica 2011, 17, 39–51. [Google Scholar]
- Bernhard-Reversat, F. The Leaching of Eucalyptus Hybrids and Acacia Auriculiformis Leaf Litter: Laboratory Experiments on Early Decomposition and Ecological Implications in Congolese Tree Plantations. Appl. Soil Ecol. 1999, 12, 251–261. [Google Scholar] [CrossRef]
- Supawong, P.; Poolsiri, R.; Piboon, C. Leaf Litter Decomposition of Indigenous Tree Plantations at Thong Pha Phum Plantation, Kanchanaburi Province. Thai. J. For. 2016, 35, 74–85. Available online: https://li01.tci-thaijo.org/index.php/tjf/article/view/248154 (accessed on 5 March 2023).
- Bréchet, L.M.; Lopez-Sangil, L.; George, C.; Birkett, A.J.; Baxendale, C.; Castro Trujillo, B.; Sayer, E.J. Distinct Responses of Soil Respiration to Experimental Litter Manipulation in Temperate Woodland and Tropical Forest. Ecol. Evol. 2018, 8, 3787–3796. [Google Scholar] [CrossRef]
- Kume, T.; Tanaka, N.; Yoshifuji, N.; Chatchai, T.; Igarashi, Y.; Suzuki, M.; Hashimoto, S. Soil Respiration in Response to Year-to-Year Variations in Rainfall in a Tropical Seasonal Forest in Northern Thailand: Soil Respiration and Rainfall in Tropical Seasonal Forest. Ecohydrology 2013, 6, 134–141. [Google Scholar] [CrossRef]
- Dong, T.L.; Beadle, C.; Eyles, A.; Forrester, D.I.; Doyle, R.; Worledge, D.; Churchill, K.; Khanh, D.C. Growth and Physiology of Hopea Odorata Planted within Gaps in an Acacia Plantation Acting as a Nurse Crop. Plant Ecol. Divers. 2016, 9, 549–562. [Google Scholar] [CrossRef]
- Bauhus, J.; van Winden, A.P.; Nicotra, A.B. Aboveground Interactions and Productivity in Mixed-Species Plantations of Acacia Mearnsii and Eucalyptus Globulus. Can. J. For. Res. 2004, 34, 686–694. [Google Scholar] [CrossRef]
- Forrester, D.I.; Bauhus, J.; Cowie, A.L.; Vanclay, J.K. Mixed-Species Plantations of Eucalyptus with Nitrogen-Fixing Trees: A Review. For. Ecol. Manag. 2006, 233, 211–230. [Google Scholar] [CrossRef] [Green Version]
- Marron, N.; Epron, D. Are Mixed-Tree Plantations Including a Nitrogen-Fixing Species More Productive than Monocultures? For. Ecol. Manag. 2019, 441, 242–252. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis: Advanced Course; UW-Madison Libraries Parallel Press: Madison, WI, USA, 2005. [Google Scholar]
- Sumner, M.E.; Miller, W.P. Cation Exchange Capacity and Exchange Coefficients. In Methods of Soil Analysis: Part 3 Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; SSSA Book Series; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 2018; pp. 1201–1229. ISBN 978-0-89118-866-7. [Google Scholar]
- Bray, R.H.; Kurtz, L.T. Determination of Total, Organic, and Available forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–46. [Google Scholar] [CrossRef]

| Tree Species | Density (Trees ha−1) | BA (m2 ha−1) | QMD (cm) | MAIDBH (cm yr−1) | HDom (m) |
|---|---|---|---|---|---|
| A. auriculiformis | 1012 | 13.8 | 13.2 | 1.2 | 18.6 |
| E. urophylla | 812 | 11.6 | 13.5 | 1.23 | 21.8 |
| H. odorata | 812 | 6.8 | 10.3 | 0.94 | 11.2 |
| X. xylocarpa | 964 | 7.1 | 9.7 | 0.88 | 12.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ontong, N.; Poolsiri, R.; Diloksumpun, S.; Staporn, D.; Jenke, M. Effects of Tree Functional Traits on Soil Respiration in Tropical Forest Plantations. Forests 2023, 14, 715. https://doi.org/10.3390/f14040715
Ontong N, Poolsiri R, Diloksumpun S, Staporn D, Jenke M. Effects of Tree Functional Traits on Soil Respiration in Tropical Forest Plantations. Forests. 2023; 14(4):715. https://doi.org/10.3390/f14040715
Chicago/Turabian StyleOntong, Natthapong, Roongreang Poolsiri, Sapit Diloksumpun, Duriya Staporn, and Michael Jenke. 2023. "Effects of Tree Functional Traits on Soil Respiration in Tropical Forest Plantations" Forests 14, no. 4: 715. https://doi.org/10.3390/f14040715





