Influence of Vegetation Types on the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity in Karst Ecosystems
Abstract
:1. Introduction
2. Materials and Methods
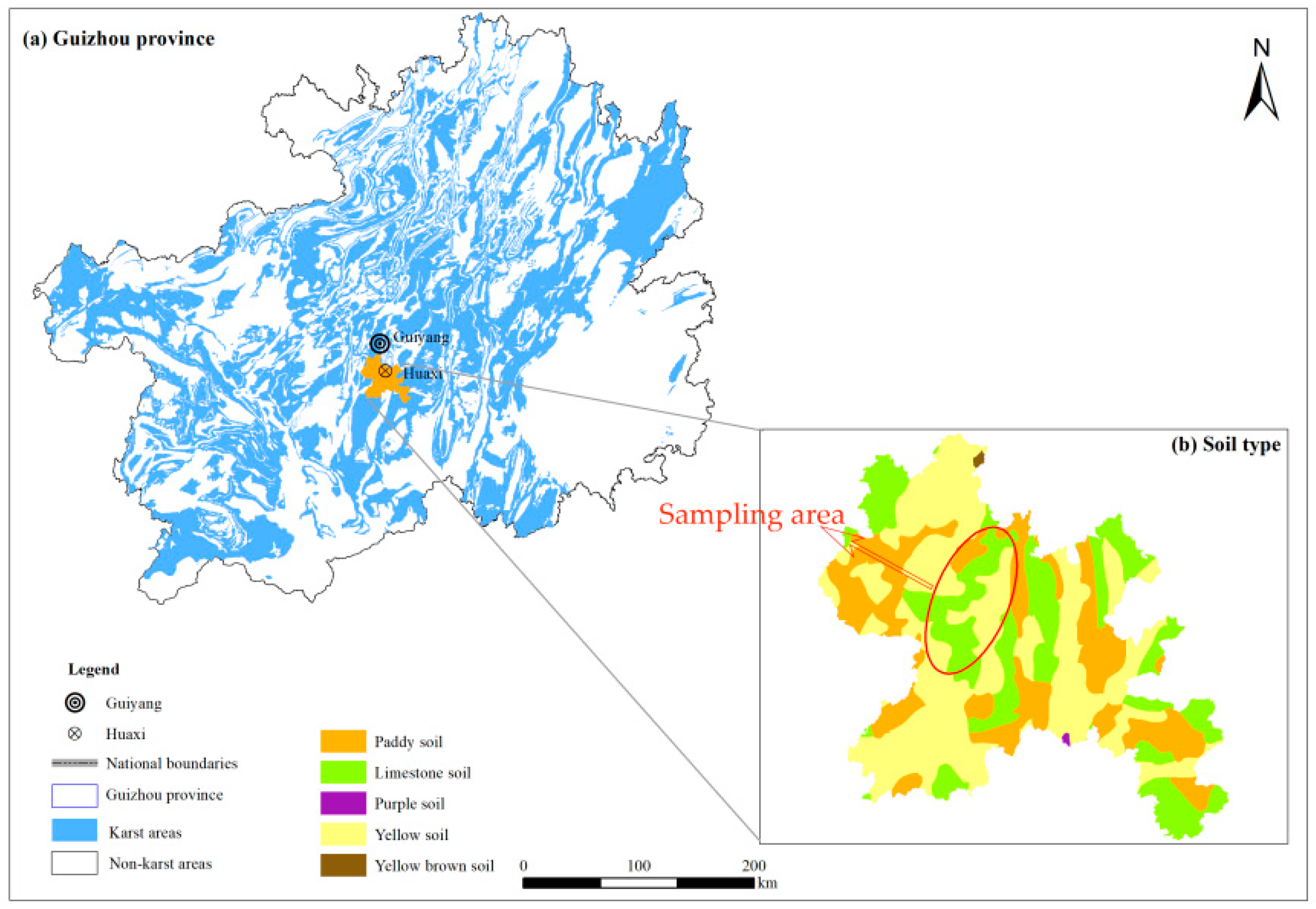
2.1. Overview of the Study Area
2.2. Sample Collection and Analysis
2.3. Data Analysis
3. Results
3.1. The C, N, and P Stoichiometric Characteristics and Soil Enzyme Activity of Litter and Soil in Different Vegetation Types
3.1.1. The C, N, and P Content Characteristics of Litter and Soil
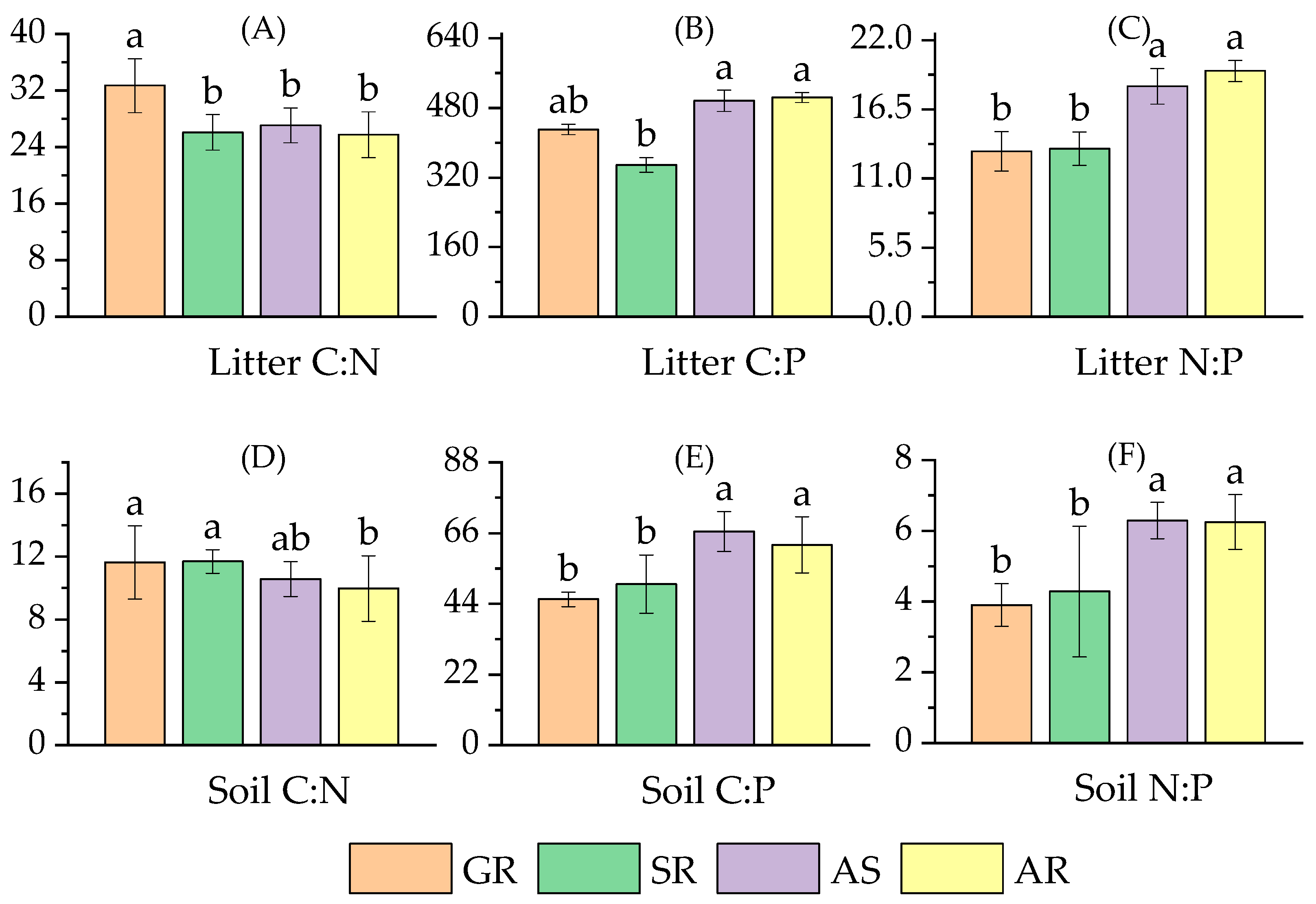
3.1.2. The Stoichiometric Characteristics of Litter and Soil
3.1.3. Characteristics of Soil Enzyme Activity
3.2. Correlation Analysis of the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity
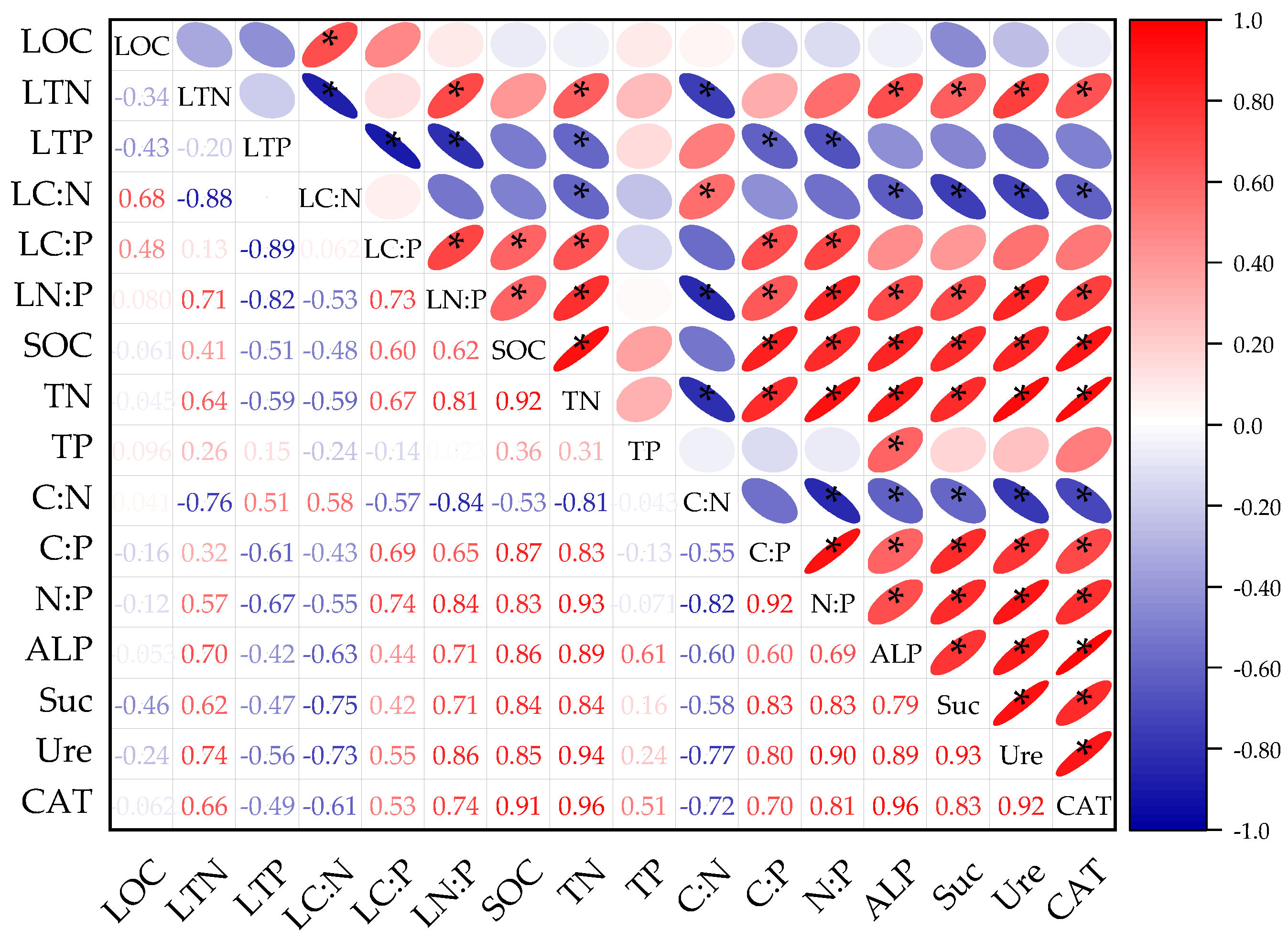
3.2.1. Correlation Analysis of Litter and Soil C, N, and P Stoichiometric Characteristics
3.2.2. Correlation Analysis of Litter C, N, and P Stoichiometric Ratio and Soil Enzymes
3.2.3. Correlation Analysis of Soil C, N, and P and Soil Enzymes
3.3. Analysis of Factors Affecting Soil Enzyme Activity
4. Discussion
4.1. The C, N, and P Stoichiometric Characteristics of Litter and Soil
4.1.1. Stoichiometric Characteristics of Litter Nutrients in Different Vegetation Types
4.1.2. Stoichiometric Characteristics of Soil Nutrients in Different Vegetation Types
4.2. Characteristics of Soil Enzyme Activity and Its Internal Correlation with the Stoichiometry of Litter and Soil
4.2.1. Characteristics of Soil Enzyme Activity in Different Vegetation Types
4.2.2. Relationships between the Nutrients, Chemometrics, and Soil Enzyme Activity of Litter and Soil
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yu, M.; Tao, Y.; Liu, W.; Xing, W.; Liu, G.; Wang, L.; Ma, L. C, N, and P stoichiometry and their interaction with different plant communities and soils in subtropical riparian wetlands. Environ. Sci. Pollut. Res. 2020, 27, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, B.; An, S. Ecological stoichiometry in leaves, roots, litters and soil among different plant communities in a desertified region of Northern China. Catena 2018, 166, 328–338. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.; Zou, G.; Song, X.; Liu, F. Comparative on plant stoichiometry response to agricultural non-point source pollution in different types of ecological ditches. Environ. Sci. Pollut. Res. 2019, 26, 647–658. [Google Scholar] [CrossRef]
- Huang, D.; Wang, D.; Ren, Y. Using leaf nutrient stoichiometry as an indicator of flood tolerance and eutrophication in the riparian zone of the Lijang River. Ecol. Indic. 2019, 98, 821–829. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, S.; Song, H.; Wang, M.; Zhao, Q.; Huang, H.; Niklas, K.J. Plant type dominates fine-root C:N:P stoichiometry across China: A meta-analysis. J. Biogeogr. 2020, 47, 1019–1029. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, N.; Liu, C.; Yang, H.; Li, M.; Yu, G.; Wilcox, K.; Yu, Q.; He, N. C:N:P stoichiometry in China’s forests: From organs to ecosystems. Funct. Ecol. 2017, 32, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ma, J.; Ma, Z.; Li, L. Soil nutrient contents and stoichiometry as affected by land-use in an agro-pastoral region of northwest China. Catena 2017, 150, 146–153. [Google Scholar] [CrossRef]
- Xiao, C.; Janssens, I.A.; Zhou, Y.; Su, J.; Liang, Y.; Guenet, B. Strong stoichiometric resilience after litter manipulation experiments; a case study in a Chinese grassland. Biogeosciences 2015, 12, 757–767. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, B.; An, S.; Zeng, Q.; Zhang, H. Response of forest species to C:N:P in the plant-litter-soil system and stoichiometric homeostasis of plant tissues during afforestation on the Loess Plateau, China. Catena 2019, 183, 104186. [Google Scholar] [CrossRef]
- Seitz, S.; Goebes, P.; Zumstein, P.; Assmann, T.; Kühn, P.; Niklaus, P.A.; Schuldt, A.; Scholten, T. The influence of leaf litter diversity and soil fauna on initial soil erosion in subtropical forests. Earth Surf. Proc. Land. 2015, 40, 1439–1447. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. Forest Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Bahnmann, B.; Mašínová, T.; Halvorsen, R.; Davey, M.L.; Sedlák, P.; Tomšovský, M.; Baldrian, P. Effects of oak, beech and spruce on the distribution and community structure of fungi in litter and soils across a temperate forest. Soil Biol. Biochem. 2018, 119, 162–173. [Google Scholar] [CrossRef]
- Huang, L.; Hu, H.; Bao, W.; Hu, B.; Liu, J.; Li, F. Shifting soil nutrient stoichiometry with soil of variable rock fragment contents and different vegetation types. Catena 2023, 220, 106717. [Google Scholar] [CrossRef]
- Joshi, R.K.; Garkoti, S.C. Influence of vegetation types on soil physical and chemical properties, microbial biomass and stoichiometry in the central Himalaya. Catena 2023, 222, 106835. [Google Scholar] [CrossRef]
- Zhou, Y.; Boutton, T.W.; Wu, X.B. Soil C:N:P stoichiometry responds to vegetation change from grassland to woodland. Biogeochemistry 2018, 140, 341–357. [Google Scholar] [CrossRef]
- Song, M.; Peng, W.; Du, H.; Xu, Q. Responses of Soil and Microbial C:N:P Stoichiometry to Vegetation Succession in a Karst Region of Southwest China. Forests 2019, 10, 755. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, J.; Liu, S.; Shen, J.; Huang, D.; Su, Y.; Wei, W.; Syers, J.K. Is the C:N:P stoichiometry in soil and soil microbial biomass related to the landscape and land use in southern subtropical China? Global Biogeochem. Cycles 2012, 26, 4. [Google Scholar] [CrossRef]
- Zeng, Q.; Chen, Z.; Tan, W. Plant litter quality regulates soil eco-enzymatic stoichiometry and microbial nutrient limitation in a citrus orchard. Plant Soil 2021, 466, 179–191. [Google Scholar] [CrossRef]
- Burns, R.G.; DeForest, J.L.; Marxsen, J.; Sinsabaugh, R.L.; Stromberger, M.E.; Wallenstein, M.D.; Weintraub, M.N.; Zoppini, A. Soil enzymes in a changing environment: Current knowledge and future directions. Soil Biol. Biochem. 2013, 58, 216–234. [Google Scholar] [CrossRef]
- Lan, G.; Liu, C.; Wang, H.; Tang, W.; Wu, X.; Yang, H.; Tu, L.; Hu, B.X.; Cao, J.; Li, Q. The effect of land use change and soil redistribution on soil organic carbon dynamics in karst graben basin of China. J. Soils Sediments 2021, 21, 2511–2524. [Google Scholar] [CrossRef]
- Pardo-Igúzquiza, E.; Dowd, P.A.; Ruiz-Constán, A.; Martos-Rosillo, S.; Luque-Espinar, J.A.; Rodríguez-Galiano, V.; Pedrera, A. Epikarst mapping by remote sensing. Catena 2018, 165, 1–11. [Google Scholar] [CrossRef]
- Xue, F.; Long, C.; Liao, Q.; Xiong, L. An analysis of litter, soil, stoichiometry, and soil enzymes in karst forest. J. For. Environ. 2020, 40, 449–458. [Google Scholar] [CrossRef]
- Gong, J.; Hou, W.; Liu, J.; Malik, K.; Kong, X.; Wang, L.; Chen, X.; Tang, M.; Zhu, R.; Cheng, C.; et al. Effects of Different Land Use Types and Soil Depths on Soil Mineral Elements, Soil Enzyme Activity, and Fungal Community in Karst Area of Southwest China. Int. J. Environ. Res. Public Health 2022, 19, 3120. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources; FAO, ISRIC, ISSS: Rome, Italy, 2006. [Google Scholar]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000; pp. 178–200. [Google Scholar]
- Guan, S. Study Way of Soil Enzymes; China Agricultural Press: Beijing, China, 1986; p. 376. [Google Scholar]
- Yanghua, Y.; Xinping, Z.; Hong, L. Ecological stoichiometry of Zanthoxylum planispinum var. dintanensis plantation at different altitudes in rocky desertification area of central Guizhou. Acta Ecol. Sin. 2019, 39, 5536–5545. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, X.; Lu, X.; Xue, Z. Ecological stoichiometry of carbon, nitrogen, and phosphorus in estuarine wetland soils: Influences of vegetation coverage, plant communities, geomorphology, and seawalls. J. Soils Sediments 2013, 13, 1043–1051. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Wang, J.; Song, Y. Leaf-litter-soil stoichiometry and carbon and nitrogen isotopes of plant communities in dolomite district in Guizhou Province. Acta Ecol. Sin. 2022, 42, 3356–3365. [Google Scholar] [CrossRef]
- Tian, J.; Sheng, M.; Wang, P.; Wen, P. Influence of Land Use Change on Litter and Soil C, N, P Stoichiometric Characteristics and Soil Enzyme Activity in Karst Ecosystem, Southwest China. Environ. Sci. 2019, 40, 4278–4286. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Li, R.; Yan, J.; Sha, L.; Han, S. C:N:P stoichiometric characteristics of four forest types’ dominant tree species in China. Chin. J. Plant Ecol. 2011, 35, 587–595. [Google Scholar]
- Sardans, J.; Janssens, I.A.; Alonso, R.; Veresoglou, S.D.; Rillig, M.C.; Sanders, T.G.; Carnicer, J.; Filella, I.; Farré-Armengol, G.; Peñuelas, J. Foliar elemental composition of European forest tree species associated with evolutionary traits and present environmental and competitive conditions. Glob. Ecol. Biogeogr. 2015, 24, 240–255. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, M.; Xu, L.; Zhu, J.; Dai, G.; He, N. C:N:P stoichiometry in terrestrial ecosystems in China. Sci. Total. Environ. 2021, 795, 148849. [Google Scholar] [CrossRef]
- Kang, H.; Xin, Z.; Berg, B.; Burgess, P.J.; Liu, Q.; Liu, Z.; Li, Z.; Liu, C. Global pattern of leaf litter nitrogen and phosphorus in woody plants. Ann. For. Sci. 2010, 67, 811. [Google Scholar] [CrossRef]
- Sariyildiz, T. Interactions between litter quality, decomposition and soil fertility: A laboratory study. Soil Biol. Biochem. 2003, 35, 391–399. [Google Scholar] [CrossRef]
- Sun, J.; Gao, P.; Li, C.; Wang, R.; Niu, X.; Wang, B. Ecological stoichiometry characteristics of the leaf–litter–soil continuum of Quercus acutissima Carr. and Pinus densiflora Sieb. in Northern China. Environ. Earth Sci. 2019, 78, 20. [Google Scholar] [CrossRef]
- Ma, L.; Huang, W.; Guo, C.; Wang, R.; Xiao, C. Soil Microbial Properties and Plant Growth Responses to Carbon and Water Addition in a Temperate Steppe: The Importance of Nutrient Availability. PLoS ONE 2012, 7, e35165. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Guenet, B.; Zhou, Y.; Su, J.; Janssens, I.A. Priming of soil organic matter decomposition scales linearly with microbial biomass response to litter input in steppe vegetation. Oikos 2015, 124, 649–657. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Zhao, Y.; Xu, X.; Lei, J.; Hill, R.L. Litter decomposition and nutrient dynamics of three woody halophytes in the Taklimakan Desert Highway Shelterbelt. Arid. Land Res. Manag. 2017, 31, 335–351. [Google Scholar] [CrossRef]
- Pan, F.; Zhang, W.; Wang, K.; He, X.; Liang, S.; Wei, G. Litter C:N:P ecological stoichiometry character of plant communities in typical Karst Peak Cluster Depression. Acta Ecol. Sin. 2011, 31, 335–343. [Google Scholar]
- Tian, H.; Chen, G.; Zhang, C.; Melillo, J.M.; Hall, C.A.S. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Zeng, Z.; Wang, K.; Liu, X.; Zeng, F.; Song, T.; Peng, W.; Zhang, H.; Du, H. Stoichiometric characteristics of plants, litter and soils in karst plant communities of North west Guangxi. Chin. J. Plant Ecol. 2015, 39, 682–693. [Google Scholar] [CrossRef]
- Robbins, C.J.; Matthaeus, W.J.; Cook, S.C.; Housley, L.M.; Robison, S.E.; Garbarino, M.A.; LeBrun, E.S.; Raut, S.; Tseng, C.Y.; King, R.S. Leaf litter identity alters the timing of lotic nutrient dynamics. Freshw. Biol. 2019, 64, 2247–2259. [Google Scholar] [CrossRef]
- Liu, R.; Wang, D. C:N:P stoichiometric characteristics and seasonal dynamics of leaf-root-litter-soil in plantations on the loess plateau. Ecol. Indic. 2021, 127, 107772. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in soil carbon fractions and enzyme activities under different vegetation types of the northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Zheng, L.; Long, C. Eco-stoichiometric characteristics of soil in different topographical sites of Maolan karst forest. J. Southen Agric. 2020, 51, 545–551. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Ananbeh, H.; Stojanović, M.; Pompeiano, A.; Voběrková, S.; Trasar-Cepeda, C. Use of soil enzyme activities to assess the recovery of soil functions in abandoned coppice forest systems. Sci. Total. Environ. 2019, 694, 133692. [Google Scholar] [CrossRef]
- Song, X.; Yang, J.; Hussain, Q.; Liu, X.; Zhang, J.; Cui, D. Stable isotopes reveal the formation diversity of humic substances derived from different cotton straw-based materials. Sci. Total. Environ. 2020, 740, 140202. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Maspons, J.; Molowny Horas, R.; Fernández Martínez, M.; Janssens, I.A.; Richter, A.; Ciais, P.; Obersteiner, M.; Peñuelas, J. The effect of global change on soil phosphatase activity. Glob. Chang. Biol. 2021, 27, 5989–6003. [Google Scholar] [CrossRef]
- Yao, L.; Rashti, M.R.; Brough, D.M.; Burford, M.A.; Liu, W.; Liu, G.; Chen, C. Stoichiometric control on riparian wetland carbon and nutrient dynamics under different land uses. Sci. Total. Environ. 2019, 697, 134127. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Y.; Sun, D.; Li, H.; Dong, Y.; Wang, Z.; Wang, S. Soil nutrients and nutrient ratios influence the ratios of soil microbial biomass and metabolic nutrient limitations in mountain peatlands. Catena 2022, 218, 106528. [Google Scholar] [CrossRef]
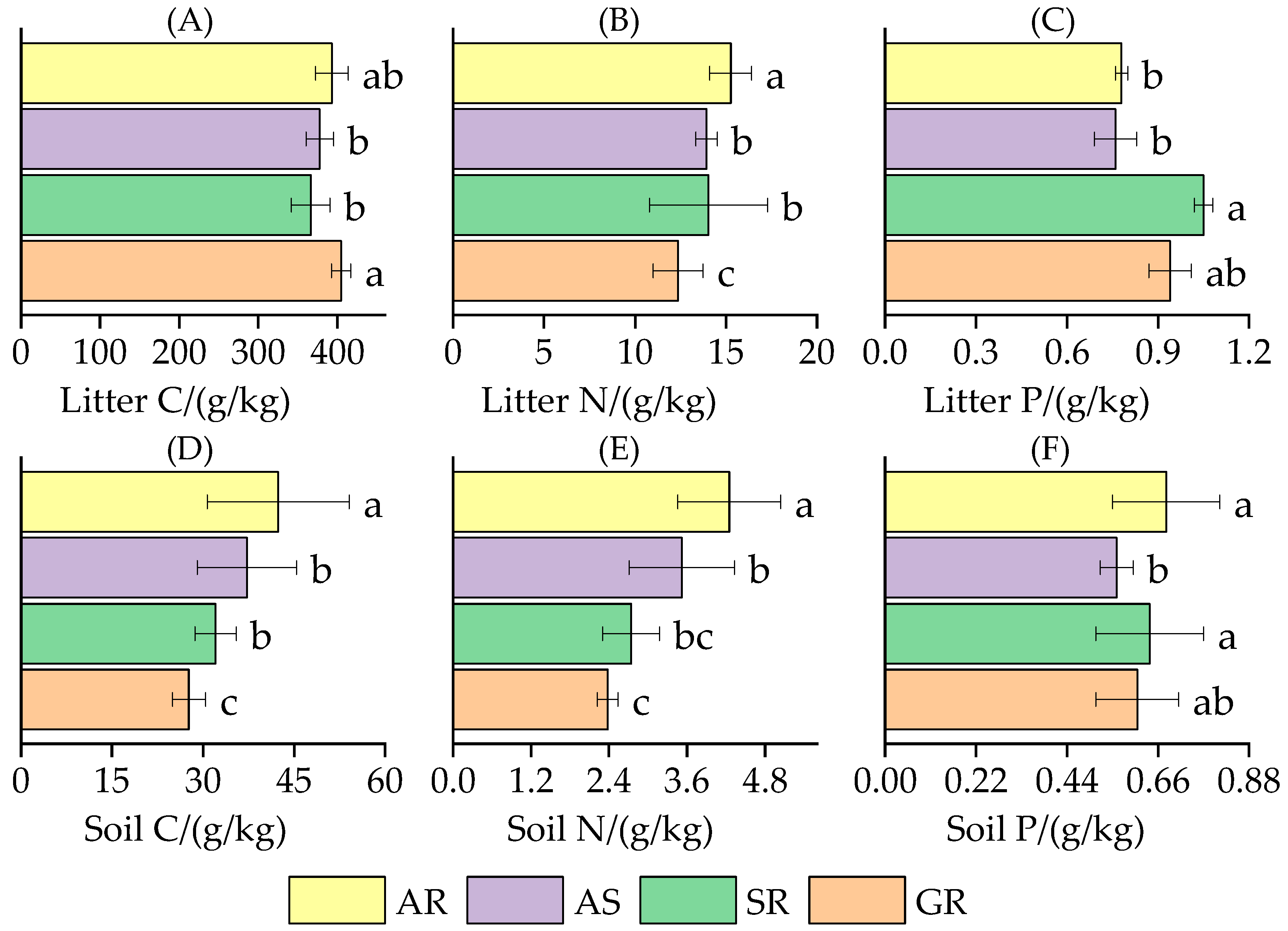

| Vegetation Type | Slope (°) | Altitude (m) | Aspect | Community Characteristics | Dominant Species |
|---|---|---|---|---|---|
| Grassland | 30–40 | 1206 | NW | The community level was singular, consisting of herbs and no or few shrubs that were about 1 m high, with a coverage of more than 90%, accompanied by a small number of thorny shrubs; the litter was about 3–5 cm thick. | Imperata cylindrica, Conyza canadensis, Rubus parvifolius |
| Shrubland | 30–40 | 1184 | NW | The vertical structure of the community was simple, mainly dominated by a shrub layer, with or without a small number of trees; the shrub layer coverage rate was more than 70%, and the shrub layer height was 1.5–2 m, with a few rattan thorns; the litter was about 1–3 cm thick. | Rubus parvifolius, Coriaria nepalensis, Viburnum rhytidophyllum, Rubus coreanus |
| Arbor and Shrub Compound Forest | 30–40 | 1214 | NW | The community hierarchy was differentiated, with a height of about 3~12 m; the coverage of woody plants was more than 80%, while the coverage of herbaceous plants under the forest was low, with a small number of Masson’s pines; and the litter was about 3~7 cm thick. | Toricellia angulata, Paulownia, Quercus glauca |
| Arbor Forest | 40–50 | 1222 | NW | The forest level differentiation was obvious and the tree layer and shrub layers were relatively developed; the tree layer was 10–20 m high, with a coverage rate of up to 80%, and the shrub layer accounted for about 10–20%; there was a small amount of exposed bedrock exposed in the community, with epiphytic lichens; and the litter was about 2–5 cm thick. | Cinnamomum camphora, Populus, Quercus glauca, Celtis sinensis |
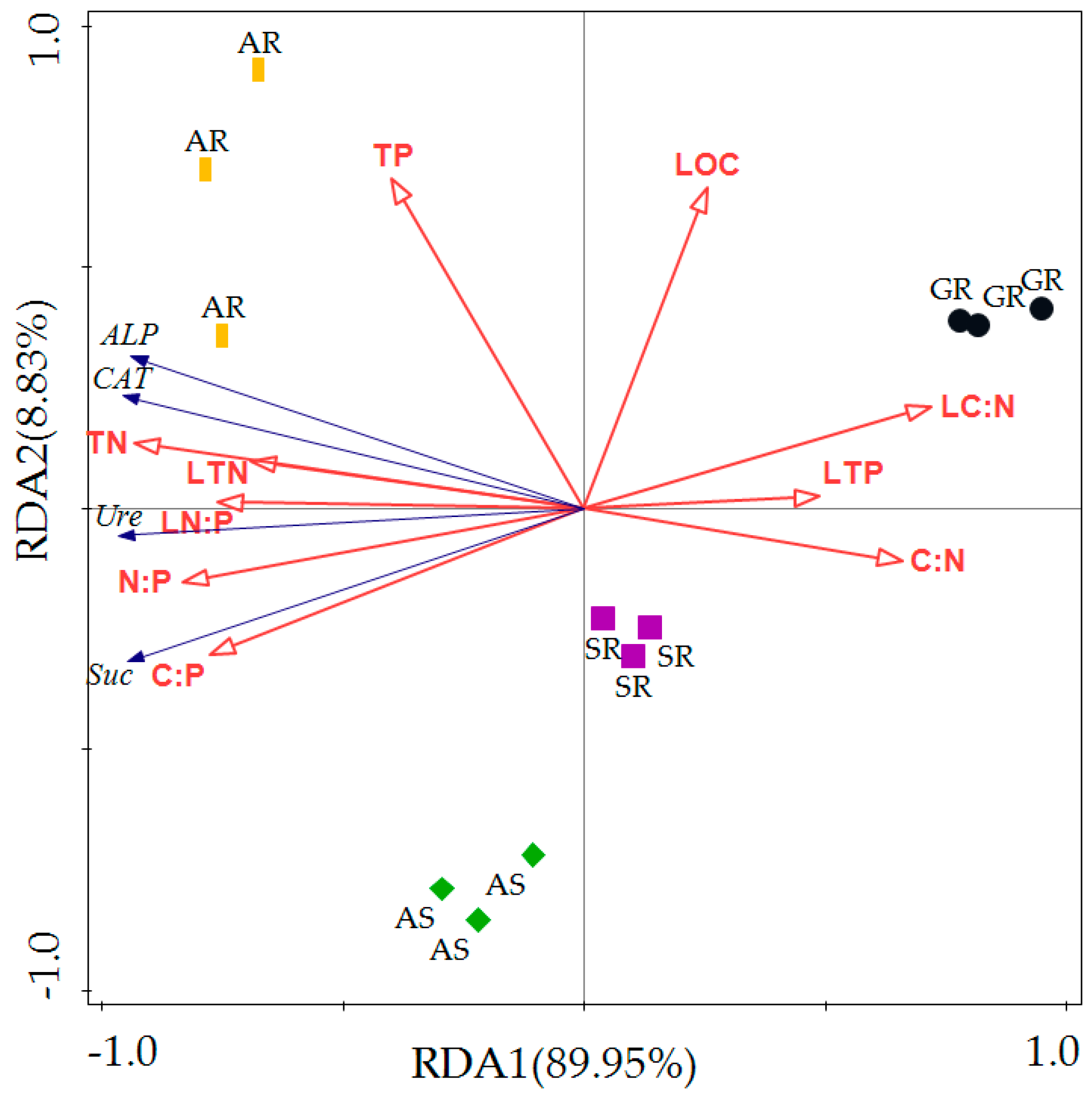
| Statistic | Axis 1 | Axis 2 | Axis 3 | Axis 4 |
|---|---|---|---|---|
| Eigenvalues | 0.8995 | 0.0883 | 0.0087 | 0.0001 |
| Explained Variation (Cumulative) | 89.95 | 98.78 | 99.64 | 99.65 |
| Pseudo-Canonical Correlation | 0.9986 | 0.996 | 0.9814 | 0.9814 |
| Explained Fitted Variation (Cumulative) | 90.26 | 99.13 | 99.99 | 100.00 |
| Total Canonical Eigenvalues | 0.981 | |||
| Total Eigenvalues | 1.000 | |||
| Name | Explains% | Pseudo-F | p |
|---|---|---|---|
| TN | 78.2 | 35.9 | 0.002 |
| N:P | 62.5 | 16.7 | 0.004 |
| C:P | 54.8 | 12.1 | 0.002 |
| LN:P | 51.8 | 10.7 | 0.006 |
| LC:N | 47.1 | 8.9 | 0.008 |
| LTN | 43 | 7.5 | 0.004 |
| C:N | 39.5 | 6.5 | 0.014 |
| LTP | 21.6 | 2.8 | 0.106 |
| TP | 18.5 | 2.3 | 0.146 |
| LOC | 9.8 | 1.1 | 0.322 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Dai, Q.; Zhu, L.; Ding, P.; Hu, Z.; Zhou, H. Influence of Vegetation Types on the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity in Karst Ecosystems. Forests 2023, 14, 771. https://doi.org/10.3390/f14040771
Zhao M, Dai Q, Zhu L, Ding P, Hu Z, Zhou H. Influence of Vegetation Types on the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity in Karst Ecosystems. Forests. 2023; 14(4):771. https://doi.org/10.3390/f14040771
Chicago/Turabian StyleZhao, Min, Quanhou Dai, Liekun Zhu, Pengwei Ding, Zeyin Hu, and Hong Zhou. 2023. "Influence of Vegetation Types on the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity in Karst Ecosystems" Forests 14, no. 4: 771. https://doi.org/10.3390/f14040771
APA StyleZhao, M., Dai, Q., Zhu, L., Ding, P., Hu, Z., & Zhou, H. (2023). Influence of Vegetation Types on the C, N, and P Stoichiometric Characteristics of Litter and Soil and Soil Enzyme Activity in Karst Ecosystems. Forests, 14(4), 771. https://doi.org/10.3390/f14040771







