Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Material and Test Design
2.3. Measurement of Gas Exchange Parameters
2.4. Measurement of Chlorophyll Fluorescence Parameters and Chlorophyll Content
2.5. Measurement of O2−, H2O2 MDA Content, and Antioxidant Enzyme Activities
2.6. Statistical Analysis
3. Results
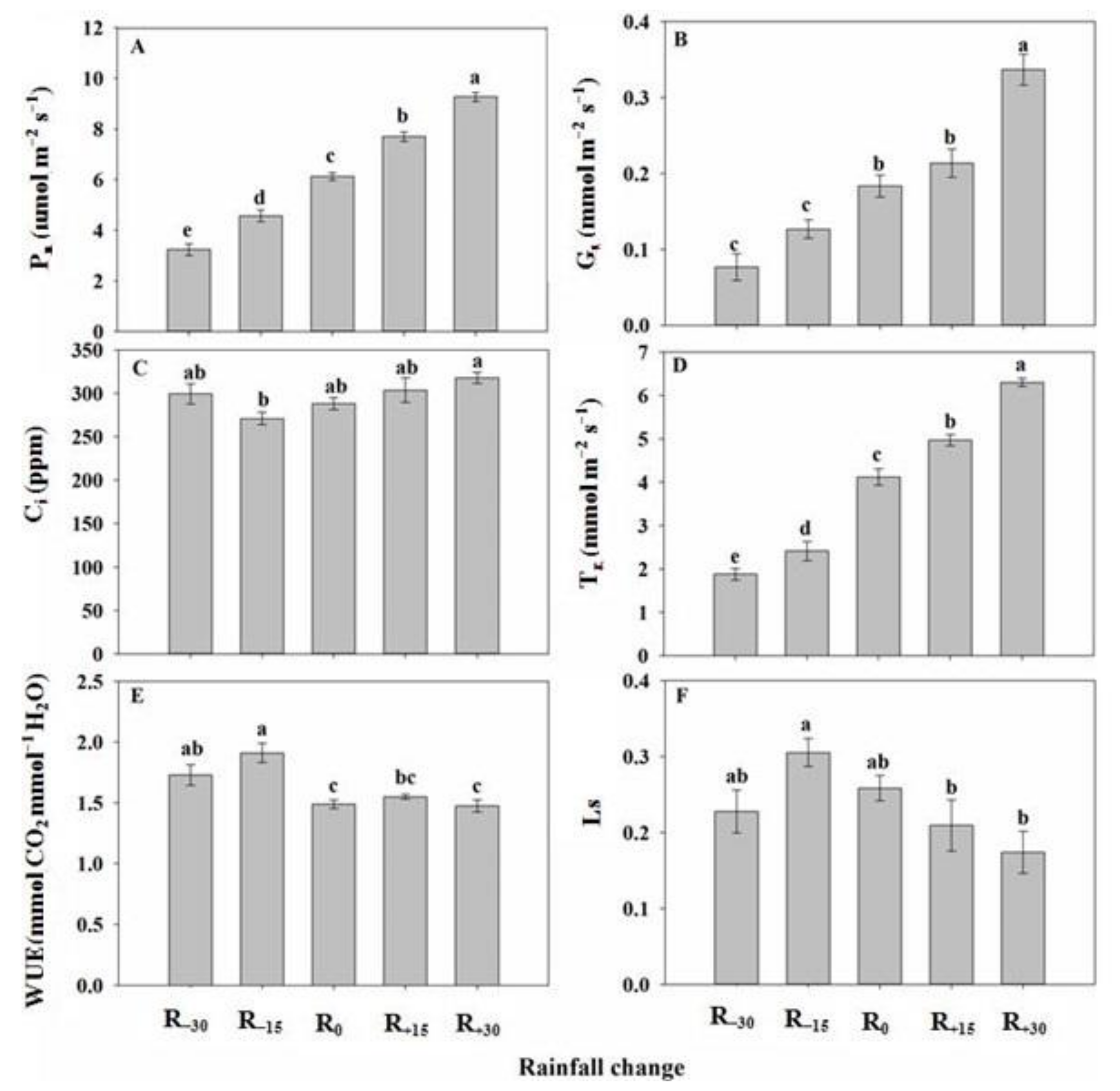
3.1. Effect of Rainfall Change on Photosynthetic Parameters
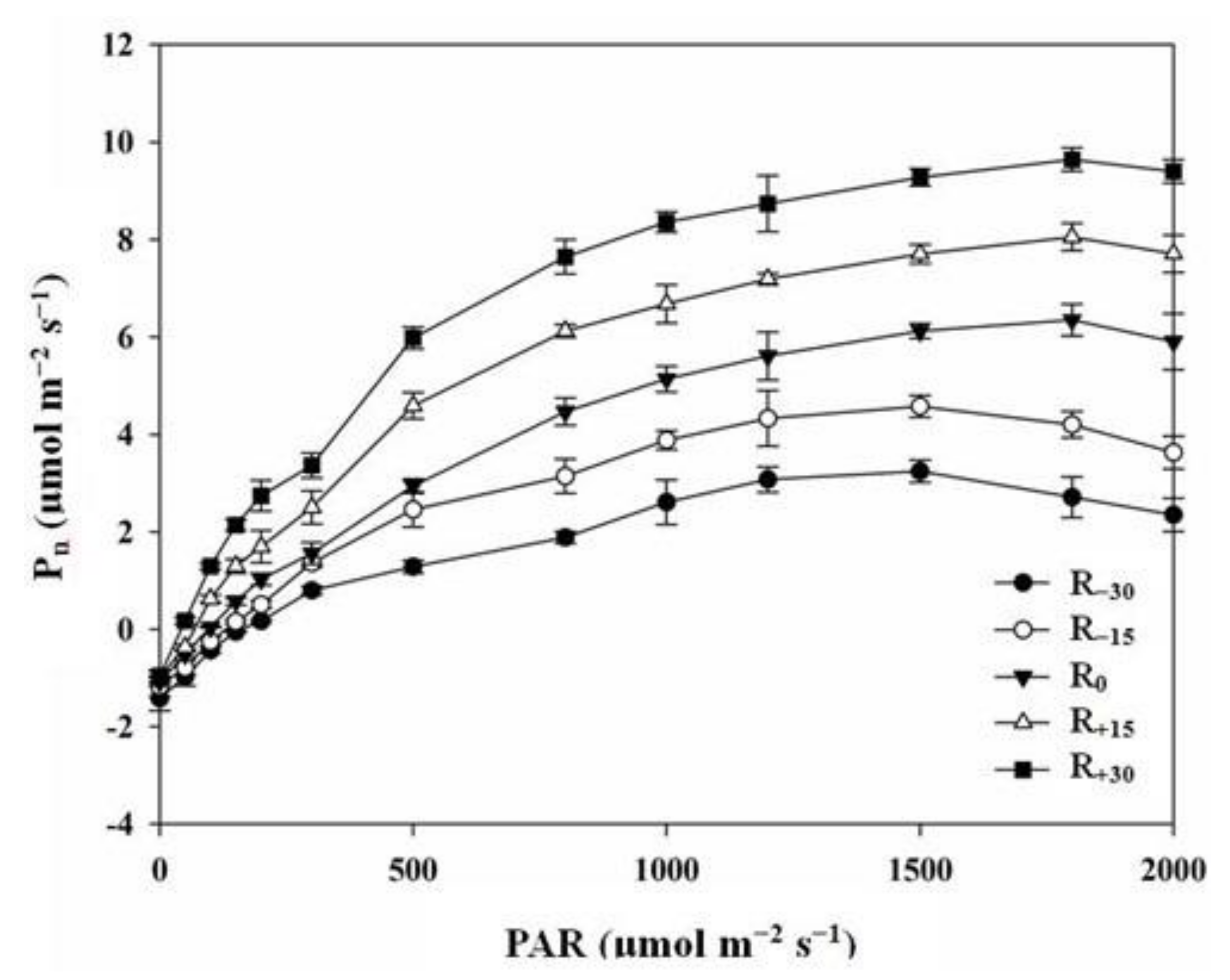
3.2. Effect of Rainfall Change on Light Response Parameters
3.3. Effect of Rainfall Change on Chlorophyll Content
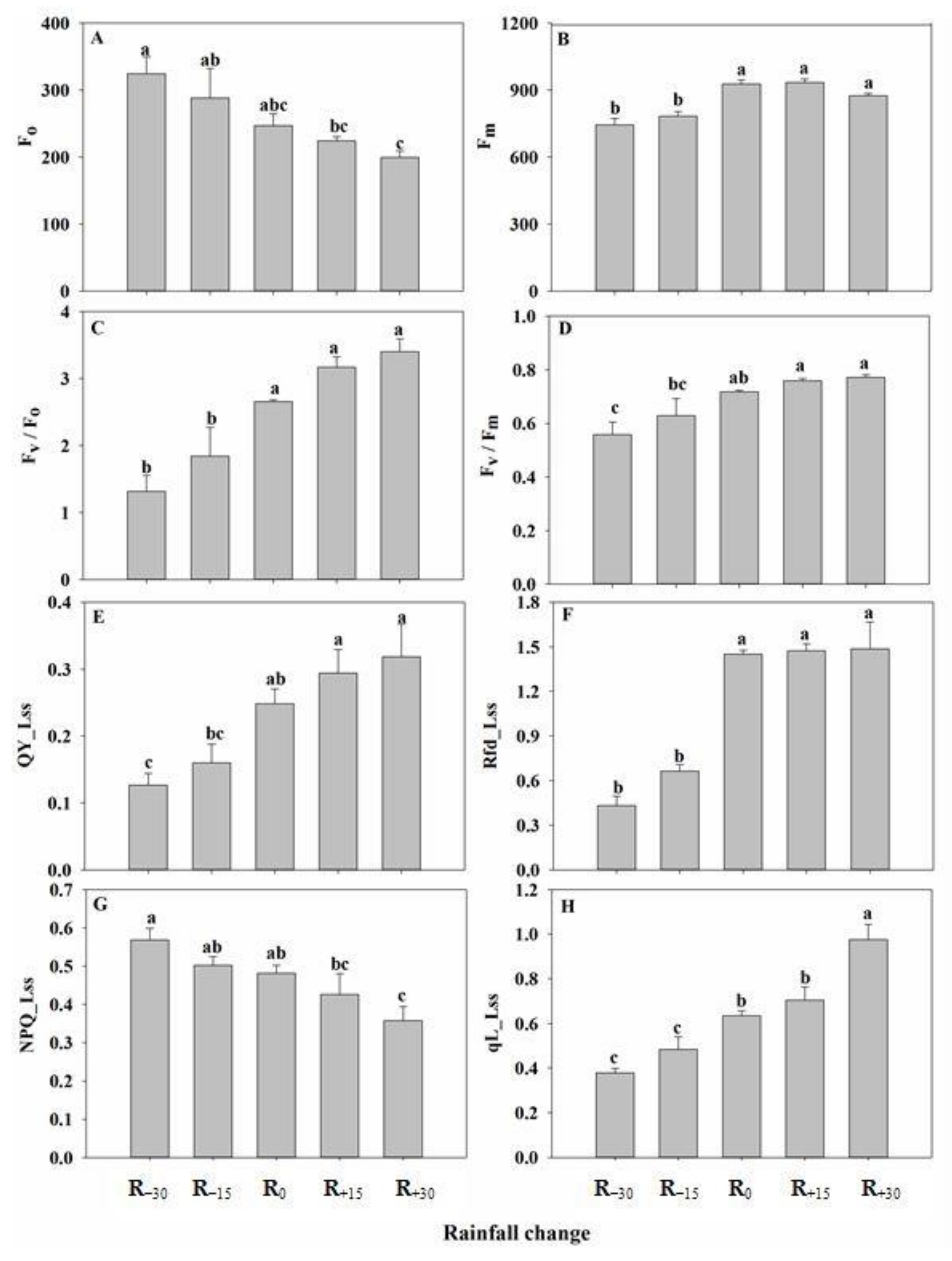
3.4. Effect of Rainfall Change on Chlorophyll Fluorescence Parameters
3.5. Correlation between Fluorescence and Light Response Parameters
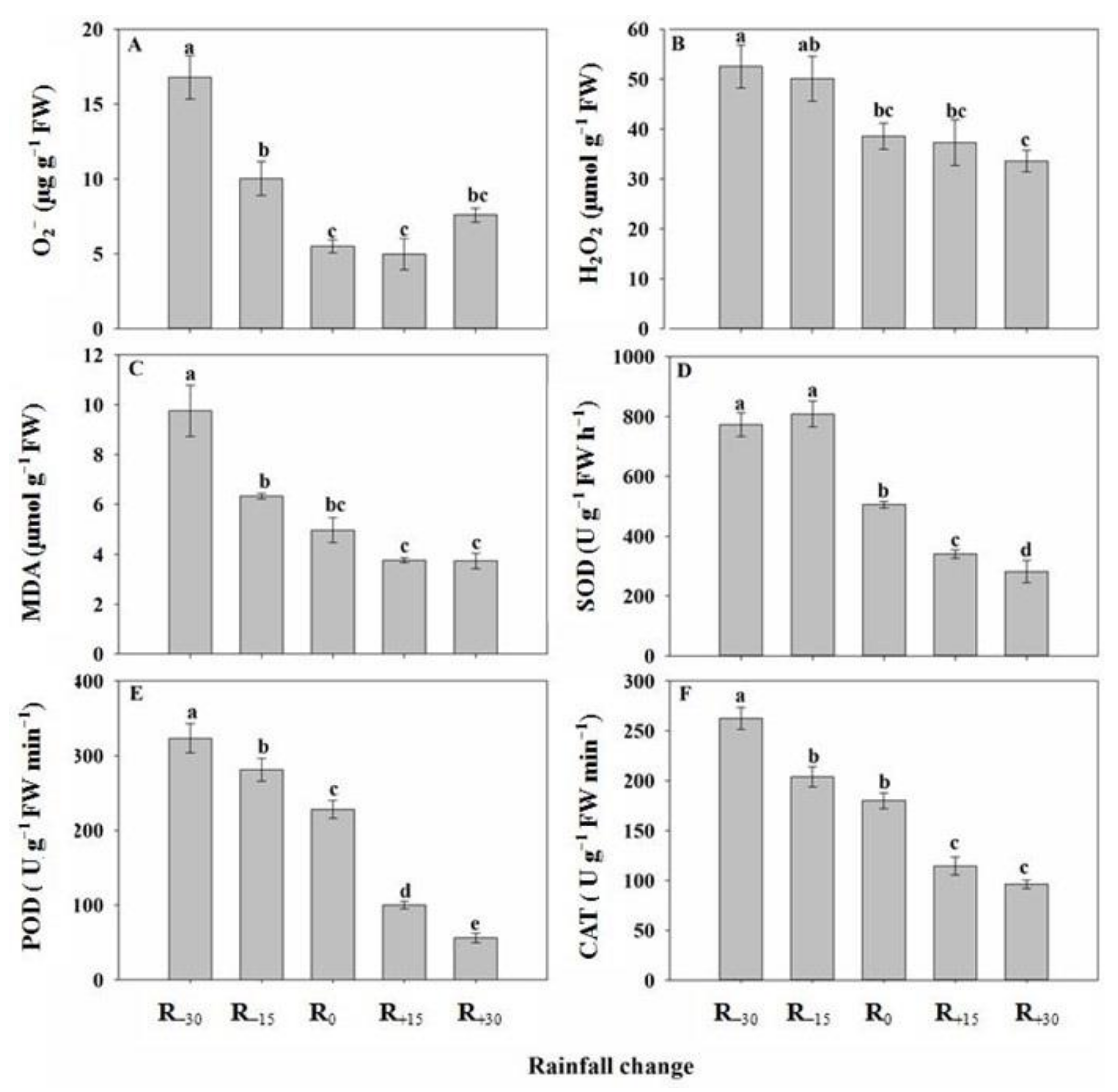
3.6. Effect of Rainfall Change on O2−, H2O2, and MDA Content
3.7. Effect of Rainfall Change on Antioxidant Enzyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallace, J.S. Increasing agricultural water use efficiency to meet future food production. Agric. Ecosyst. Environ. 2000, 82, 105–119. [Google Scholar] [CrossRef]
- Wang, H.J.; Chen, Y.N.; Chen, Z.S. Spatial distribution and temporal trends of mean precipitation and extremes in the arid region, northwest of China, during 1960–2010. Hydrol. Process. 2012, 27, 1807–1818. [Google Scholar] [CrossRef]
- Han, X.Q.; Xue, H.W.; Zhao, C.S.; Lu, D.R. The roles of convective and stratiform precipitation in the observed precipitation trends in Northwest China during 1961–2000. Atmos. Res. 2016, 169, 139–146. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhou, B.T.; Qin, D.H.; Wu, J.; Gao, R.; Song, L.C. Changes in mean and extreme temperature and precipitation over the arid region of northwestern China: Observation and projection. Adv. Atmos. Sci. 2017, 3, 9–25. [Google Scholar] [CrossRef]
- Dios, V.R.D.; Fischer, C.; Collinas, C. Climate change effects on Mediterranean forests and preventive measures. New For. 2007, 33, 29–40. [Google Scholar] [CrossRef]
- Weltzin, J.F.; Loik, M.E.; Schwinning, S.; Williams, D.G.; Zak, J.C. Assessing the response of terrestrial ecosystems to potential changes in precipitation. Bioscience 2003, 53, 941–952. [Google Scholar] [CrossRef]
- Patrick, L.D.; Ogle, K.; Bell, C.W.; Zak, J.; Tissue, D. Physiological responses of two contrasting desert plant species to precipitation variability are differentially regulated by soil moisture and nitrogen dynamics. Glob. Change Biol. 2009, 15, 1214–1229. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Christoffersen, B.O.; Barros, F.D.V.; Teodoro, G.S.; Bittencourt, P.; Brum, M.M., Jr.; Viani, R.A.G. Changing precipitation regimes and the water and carbon economies of trees. Theor. Exp. Plant Physiol. 2014, 26, 65–82. [Google Scholar] [CrossRef]
- He, J.; Wu, B.; Bao, F.; Li, J.Z.; Yao, B.; Gao, J.L.; Liu, M.H. Photosynthetic response mechanism of a desert plant species Nitraria tangutorum to rain addition. Sci. Silvae. Sin. 2015, 51, 27–35, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Song, X.L.; Wang, Y.H.; Lv, X.M. Responses of plant biomass, photosynthesis and lipid peroxidation to warming and precipitation change in two dominant species (Stipa grandis and Leymus chinensis) from north China grasslands. Ecol. Evol. 2016, 6, 1871–1882. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Limousin, J.M.; Misson, L.; Lavoir, A.V.; Martin, N.K.; Rambal, S. Do photosynthetic limitations of evergreen Quercus ilex leaves change with long-term increased drought severity? Plant Cell Environ. 2010, 33, 863–875. [Google Scholar] [CrossRef]
- Ye, Z.P.; Suggett, J.D.; Robakowski, P.; Kang, H.J. A mechanistic model for the photosynthesis-light response based on the photosynthetic electron transport of PSII in C3 and C4 species. New Phytol. 2013, 152, 1251–1262. [Google Scholar] [CrossRef]
- Xu, W.Z.; Deng, X.P.; Xu, B.C. Effects of water stress and fertilization on leaf gas exchange and photosynthetic light-response curves of Bothriochloa ischaemum L. Photosynthetica 2013, 51, 603–612. [Google Scholar] [CrossRef]
- Liang, G.T.; Liu, J.H.; Zhang, J.M. Effects of drought stress on photosynthetic and physiological parameters of tomato. J. Am. Soc. Hortic. Sci. 2020, 145, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicum Murr. seedlings. Photosynthetica 2016, 54, 524–531. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Khatri, K.; Patel, D.; Rathore, M.S. Photosynthetic gas exchange and chlorophyll a fluorescence in Salicornia brachiata (Roxb.) under osmotic stress. J. Plant Growth Regul. 2021, 41, 429–444. [Google Scholar] [CrossRef]
- Noctor, G.; Veljovic-Jovanovic, S.; Driscoll, S.; Novitskaya, L.; Foyer, C.H. Drought and oxidative load in the leaves of C3 plants: A predominant role for photorespiration? Ann. Bot. 2002, 89, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Peltzer, D.; Dreyer, E.; Polle, A. Differential temperature dependencies of antioxidative enzymes in two contrasting species: Fagus sylvatica and Coleus blumei. Plant Physiol. Biochem. 2002, 40, 141–150. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Q.; Zhou, R.L.; Liang, H.M.; Zhao, H.L.; Zhao, X.Y. Relationship of antioxidant enzyme and osmoregulation substance to photosynthesis in several desert plants. J. Desert Res. 2015, 35, 1557–1564, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Ma, S.M.; Zhang, M.L.; Sanderson, S.C. Phylogeography of the rare Gymnocarpos przewalskii (Caryophyllaceae): Indications of multiple glacial refugia in north-western China. Aust. J. Bot. 2012, 60, 20–31. [Google Scholar] [CrossRef]
- Wang, L.; Huang, H.X.; Shan, L.S.; Gao, T.P.; Zhang, X.H. Population structure and spatial distribution pattern of Gymnocarpos przewalskii in Anxi extra-arid desert national nature reserve. J. Lanzhou Univ. (Nat. Sci.) 2015, 51, 690–698, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Huang, H.X.; Yang, Q.Q.; Cui, P.; Lu, G.; Han, G.J. Changes in morphological and physiological characteristics of Gymnocarpos przewalskii roots in response to water stress. Acta Prataculturae Sin. 2021, 30, 197–207, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Ma, S.; Zhang, M. Phylogeography and conservation genetics of the relic Gymnocarpos przewalskii (Caryophyllaceae) restricted to northwestern China. Conserv. Genet. 2012, 13, 1531–1541. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Xu, Z.P.; Wan, T.; Cai, P.; Yi, W.D. Study on potential distribution areas of Gymnocarpos przewalskii in China under future climatic conditions. J. Plant Resour. Environ. 2019, 28, 51–57, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Huang, H.X.; Lian, Z.H.; Wang, L.; Yang, Q.Q.; Wei, Z.Y.; Ma, Y.J.; Zhang, J.X. Response of osmotic regulation substances and antioxidant enzyme activity in leaves of Gymnocarpos przewalskii to drought. Arid Zone Res. 2020, 37, 227–235, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Wang, R.Z.; Gao, Q. Photosynthesis, transpiration and water use efficiency in two divergent Leymus chinensis populations from northeast China. Photosynthetica 2001, 39, 123–126. [Google Scholar] [CrossRef]
- Berry, J.A.; Downton, W.J.S. Environmental regulation of photosynthesis. In Photosynthesis; Academic Press: New York, NY, USA, 1982; ISBN 0-12-294302-3. [Google Scholar]
- Yin, C.Y.; Berninger, F.; Li, C.Y. Photosynthetic responses of Populus przewalski subjected to drought stress. Photosynthetica 2006, 44, 62–68. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts: Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef] [Green Version]
- Jiao, C.J.; Jiang, J.L.; Li, C.; Ke, L.M.; Cheng, W.; Li, F.M.; Li, Z.X.; Wang, C.Y. β-ODAP accumulation could be related to low levels of superoxide anion and hydrogen peroxide in Lathyrus sativus L. Food Chem. Toxicol. 2011, 49, 556–562. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the drought tolerance in rice (Oryza sativa L.) by exogenous application of salicylic acid. J. Agron. Crop Sci. 2009, 195, 237–246. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lin, H.W.; Chern, R.H.; Lo, H.F.; Li, L. Reduced susceptibility to water logging together with high-light stress is related to increases in superoxide dismutase and catalase activities in sweet potato. Plant Growth Regul. 1999, 27, 167–172. [Google Scholar] [CrossRef]
- Subin, M.P.; Francis, S. Phytotoxic effects of cadmium on seed germination, early seedling growth and antioxidant enzyme activities in Cucurbita maxima Duchesne. Int. Res. J. Biol. Sci. 2013, 2, 40–47. [Google Scholar]
- Pereira, G.J.G.; Molina, S.M.G.; Lea, P.J.; Azevedo, R.A. Activity of antioxidant enzymes in response to cadmium in Crotalaria juncea. Plant Soil 2022, 239, 123–132. [Google Scholar] [CrossRef]
- Ekmekci, Y.; Terzioglu, S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic. Biochem. Physiol. 2005, 83, 69–81. [Google Scholar] [CrossRef]
- Meehl, G.A.; Arblaster, J.M.; Tebaldi, C. Understanding future patterns of increased precipitation intensity in climate model simulations. Geophys. Res. Lett. 2005, 32, 109–127. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.T.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystem to temperature and precipitation change: A meta-analysis of experimental manipulation. Global Change Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef] [Green Version]
- Chong, P.F.; Ji, J.L.; Li, Y.; Shan, L.S.; Su, S.P. Photosynthetic physiology responses to elevated CO2 concentration and changing precipitation in desert plant Reaumuria soongorica. J. Desert Res. 2017, 37, 714–723, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Loik, M.E. Sensitivity of water relations and photosynthesis to summer precipitation pulses for Artemisia tridentata and Purshia tridentate. Plant Ecol. 2007, 191, 95–108. [Google Scholar] [CrossRef]
- Ghotbi-Ravandi, A.A.; Shahbazi, M.; Shariati, M.; Mulo, P. Effects of mild and severe drought stress on photosynthetic efficiency in tolerant and susceptible barley (Hordeum vulgare L.) genotypes. J. Agron. Crop Sci. 2014, 200, 403–415. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhang, G.C.; Gu, S.Y.; Xia, J.B.; Zhao, J.K. Critical responses of photosynthetic efficiency of goldspur apple tree to soil water variation in semiarid loess hilly area. Photosynthetica 2010, 48, 589–595. [Google Scholar] [CrossRef]
- Pilon, C.; Snider, J.L.; Sobolev, V.; Chastain, D.R.; Sorensen, R.B.; Meeks, C.D.; Massa, A.N.; Walk, T.; Singh, B.; Earl, H.J. Assessing stomatal and non-stomatal limitations to carbon assimilation under progressive drought in peanut (Arachis hypogaea L.). J. Plant Physiol. 2018, 231, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Gao, H.; Li, Y.H.; Kong, D.S.; Guo, Y.Y.; Yan, F.; Wang, Y.W.; Lu, K.; Tian, J.W.; Lu, Y.L. Effect of water stress on photosynthesis, chlorophyll fluorescence parameters and water use efficiency of common reed in the Hexi Corridor. Russ. J. Plant Physiol. 2019, 66, 556–563. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, W.Z.; Wen, Z.J. Photosynthetic response of two shrubs to rainfall pulses in desert regions of northwestern China. Photosynthetica 2012, 50, 109–119. [Google Scholar] [CrossRef]
- Yin, C.Y.; Wang, X.; Duan, B.L.; Luo, J.X.; Li, C.Y. Early growth, dry matter allocation and water use efficiency of two sympatric Populus species as affected by water stress. Environ. Exp. Bot. 2005, 53, 315–322. [Google Scholar] [CrossRef]
- Gao, S.; Su, P.X.; Yan, Q.D.; Ding, S.S. Canopy and leaf gas exchange of Haloxylon ammodendron under different soil moisture regimes. Sci. China Life Sci. 2010, 53, 718–728. [Google Scholar] [CrossRef]
- Guo, W.H.; Li, B.; Zhang, X.S.; Wang, R.Q. Effects of water stress on water use efficiency and water balance components of Hippophae rhamnoides and Caragana intermedia in the soil-plant-atmosphere continuum. Agrofor. Syst. 2010, 80, 423–435. [Google Scholar] [CrossRef]
- Cai, F.; Zhang, Y.; Mi, N.; Ming, H.Q.; Zhang, S.J.; Zhang, H.; Zhao, X.L. Maize (Zea mays L.) physiological responses to drought and rewatering, and the associations with water stress degree. Agric. Water Manag. 2020, 241, 106379. [Google Scholar] [CrossRef]
- Ye, Z.P. A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Phtosynthetica 2007, 45, 637–640. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Z.Q.; Zhang, L.T.; Sun, M.M.; Lin, T.B. Photosynthetic responses of wheat (Triticum aestivum L.) to combined effects of drought and exogenous methyl jasmonate. Photosynthetica 2014, 52, 377–385. [Google Scholar] [CrossRef]
- Iryna, I.T.; Michael, M.B. Effect of delayed fruit harvest on photosynthesis, transpiration and nutrient remobilization of apple leaves. New Phytol. 2004, 164, 441–450. [Google Scholar] [CrossRef]
- Wang, R.R.; Xia, J.B.; Yang, J.H.; Zhao, Y.Y.; Liu, J.T.; Sun, J.K. Comparison of light response models of photosynthesis in leaves of Periploca sepium under drought stress in sand habitat formed from seashells. Chin. J. Plant Ecol. 2013, 37, 111–121, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Datt, B. A new reflectance index for remote sensing of chlorophyll content in higher plants: Tests using eucalyptus leaves. J. Plant Physiol. 1999, 154, 30–36. [Google Scholar] [CrossRef]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Fang, Z.; Bouwkamp, J.; Solomos, T. Chlorophyllase activities and chlorophyll degradation during leaf senescence in non-yellowing mutant and wild type of Phaseolus vulgaris L. J. Exp. Bot. 1998, 49, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Jajoo, A.; Oukarroum, A.; Brestic, M.; Zivcak, M.; Samborska, I.A.; Cetner, M.D.; Lukasik, I.; Goltsev, V.; Ladle, R.J. Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol. Plant. 2016, 38, 102–112. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.K.; Andersen, S.B.; Ottosen, C.O.; Rosenquist, E. Wheat cultivars selected for high Fv/Fm under heat stress maintain high photosynthesis, total chlorophyll, stomatal conductance, transpiration and dry matter. Physiol. Plant. 2015, 153, 284–298. [Google Scholar] [CrossRef] [PubMed]
- Rahbarian, R.; Khavari-Nejad, R.; Ganjeali, A.; Bagheri, A.; Najafi, F. Drought stress effects on photosynthesis, chlorophyll fluorescence and water relations in tolerant and susceptible chickpea (Cicer arietinum L.) genotypes. Acta. Biol. Cracov. Ser. Bot. 2011, 53, 47–56. [Google Scholar] [CrossRef]
- Krall, J.P.; Edward, G.E. Relationship between photosystem II activity and CO2 fixation in leaves. Physiol. Plant. 1992, 86, 180–187. [Google Scholar] [CrossRef]
- Martínez, C.R.; Sánchez, R.J.; Pérez, P. Changes in chlorophyll fluorescence during the course of photoperiod and in response to drought in Casuarina equisetifolia Forst. and Forst. Photosynthetica 2002, 40, 363–368. [Google Scholar] [CrossRef]
- Pellegrini, E.; Francini, A.; Lorenzini, G.; Nali, C. PSII photochemistry and carboxylation efficiency in Liriodendron tulipifera under ozone exposure. Environ. Exp. Bot. 2011, 70, 217–226. [Google Scholar] [CrossRef]
- Piñol, R.; Simón, E. Effect of 24-Epibrassinolide on chlorophyll fluorescence and photosynthetic CO2 assimilation in Vicia faba plants treated with the photosynthesis-inhibiting herbicide terbutryn. J. Plant Growth Regul. 2009, 28, 97–105. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Energy dissipation in C3 plants under drought. Funct. Plant Biol. 2002, 29, 1209–1215. [Google Scholar] [CrossRef]
- Stefanov, D.; Terashima, I. Non-photochemical loss in PSII in high-and low-light-grown leaves of Vicia faba quantified by several fluorescence parameters including LNP, F0/F’m, a novel parameter. Physiol. Plant. 2008, 133, 327–338. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Carvalho, M. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Jiang, J.L.; Su, M.; Chen, Y.R.; Gao, N.; Jiao, C.J.; Sun, Z.X.; Li, F.M.; Wang, C.Y. Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biologia 2013, 68, 231–240. [Google Scholar] [CrossRef]
- Akyol, T.Y.; Ylmaz, O.; Uzilday, B.; Ozgur, R.; Turkan, I. Plant response to salinity: An analysis of ROS formation, signaling, and antioxidant defense. Turk. J. Bot. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.Y.; Luo, X.L.; Wei, M.G.; Huang, T.W.; Khan, A.; Zhu, Y.M. Physiological and proteomic analysis on long-term drought resistance of cassava (Manihot esculenta Crantz). Sci. Rep. 2018, 8, 17982. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.Q.; Bai, J.D.; Liu, Y.J.; Meng, Y.P.; Yang, Z.; Liu, T. Drought resistance of ten ground cover seedling species during roof greening. PLoS ONE 2020, 15, e0220598. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, H.M.; Zeng, W.J.; Li, J.; Wang, Y.; Wang, S.Q. Physiological responses of five plants in northwest China arid area under drought stress. Chin. J. Appl. Ecol. 2017, 8, 1455–1463, (In Chinese with English Summary). [Google Scholar] [CrossRef]
- Uzilday, B.; Turkan, I.; Sekmen, A.H.; Ozgur, R.; Karakaya, H.C. Comparison of ROS formation and antioxidant enzymes in Cleome gynandra (C4) and Cleome spinosa (C3) under drought stress. Plant Sci. 2020, 182, 59–70. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Duan, X.L.; Xu, S.; Wang, R.; Ouyang, Z.Z.; Shen, W.B. Linking hydrogen-mediated boron toxicity tolerance with improvement of root elongation, water status and reactive oxygen species balance: A case study for rice. Ann. Bot. 2016, 118, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Bragg, W.K.; Knapp, A.K.; Briggs, J.M. Comparative water relations of seedling and adult Quercus species during gallery forest expansion in tallgrass prairie. For. Ecol. Manag. 1993, 56, 29–41. [Google Scholar] [CrossRef]
- Cui, M.; Smith, W.K. Photosynthesis, water relations and mortality in Abies lasiocarpa seedlings during natural establishment. Tree Physiol. 1991, 8, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.L.; Kilminster, K.L.; Sánchez Alarcón, M.; Bennett, K.; Strydom, S.; McNamara, S.; Lavery, P.S.; McMahon, K.M. Population-specific resilience of Halophila ovalis seagrass habitat to unseasonal rainfall, an extreme climate event in estuaries. J. Ecol. 2021, 109, 3260–3279. [Google Scholar] [CrossRef]
- Trenberth, K.E. Changes in precipitation with climate change. Clim. Res. 2011, 47, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Guehl, J.M.; Picon, C.; Aussenac, G.; Gross, P. Interactive effects of elevated CO2 and soil drought on growth and transpiration efficiency and its determinants in two European forest tree species. Tree Physiol. 1994, 14, 707–724. [Google Scholar] [CrossRef]

| Month | Rainfall (mm) | Irrigation Volume (mL) | ||||
|---|---|---|---|---|---|---|
| R−30 | R−15 | R0 | R+15 | R+30 | ||
| May | 11.7 | 1366.4 | 1659.2 | 1952.0 | 2244.7 | 2537.5 |
| June | 14.5 | 1686.4 | 2047.7 | 2409.1 | 2770.4 | 3131.8 |
| July | 23.8 | 2776.1 | 3370.9 | 3965.8 | 4560.7 | 5155.5 |
| August | 28.7 | 3347.5 | 4064.8 | 4782.1 | 5499.4 | 6216.7 |
| Treatment | AQY | Rd/ (µmol m−2 s−1) | Pnmax/ (µmol m−2 s−1) | Lsp/ (µmol m−2 s−1) | Lcp/ (µmol m−2 s−1) |
|---|---|---|---|---|---|
| R−30 | 0.006 ± 0.001 c | 1.16 ± 0.24 a | 3.01 ± 0.47 d | 1408.96 ± 45.11 b | 195.88 ± 9.86 a |
| R−15 | 0.009 ± 0.002 bc | 1.12 ± 0.11 a | 4.39 ± 0.38 cd | 1423.50 ± 27.93 b | 134.28 ± 2.31 b |
| R0 | 0.010 ± 0.003 bc | 0.96 ± 0.25 a | 6.17 ± 0.45 bc | 1719.98 ± 46.44 a | 99.94 ± 4.64 c |
| R+15 | 0.016 ± 0.004 ab | 0.99 ± 0.23 a | 7.86 ± 0.53 ab | 1860.54 ± 30.13 a | 65.03 ± 3.00 d |
| R+30 | 0.022 ± 0.003 a | 0.84 ± 0.36 a | 9.50 ± 0.46 a | 1938.27 ± 56.28 a | 40.25 ± 5.11 e |
| Treatment | Chla (mg g−1 FW) | Chlb (mg g−1 FW) | Chl (mg g−1 FW) | Chla/b |
|---|---|---|---|---|
| R−30 | 0.74 ± 0.12 e | 1.55 ± 0.22 d | 2.29 ± 0.03 e | 0.48 ± 0.01 c |
| R−15 | 0.91 ± 0.08 d | 1.69 ± 0.15 c | 2.60 ± 0.98 d | 0.55 ± 0.92 c |
| R0 | 1.25 ± 0.06 c | 1.76 ± 0.07 bc | 3.01 ± 0.37 c | 0.71 ± 0.06 b |
| R+15 | 1.55 ± 0.98 b | 1.86 ± 0.87 b | 3.41 ± 0.87 b | 0.83 ± 0.07 a |
| R+30 | 1.67 ± 0.04 a | 2.00 ± 0.17 a | 3.67 ± 0.67 a | 0.84 ± 0.05 a |
| Parameters | Fv/Fo | Fv/Fm | QY_Lss | NPQ_Lss | qL_Lss | AQY | Rd | Pnmax | Lsp | Lcp |
|---|---|---|---|---|---|---|---|---|---|---|
| Fv/Fo | 1 | |||||||||
| Fv/Fm | 0.973 ** | 1 | ||||||||
| QY_Lss | 0.826 ** | 0.763 ** | 1 | |||||||
| NPQ_Lss | −0.752 ** | −0.662 ** | −0.698 ** | 1 | ||||||
| qL_Lss | 0.835 ** | 0.766 ** | 0.891 ** | −0.770 ** | 1 | |||||
| AQY | 0.635 * | 0.548 * | 0.676 ** | −0.558 * | −0.792 ** | 1 | ||||
| Rd | −0.234 | −0.128 | −0.133 | 0.062 | −0.177 | −0.358 | 1 | |||
| Pnmax | 0.815 ** | 0.716 ** | 0.828 ** | −0.797 ** | 0.892 ** | 0.812 ** | −0.229 | 1 | ||
| Lsp | 0.932 ** | 0.878 ** | 0.905 ** | −0.684 ** | 0.863 ** | 0.684 ** | −0.140 | 0.817 ** | 1 | |
| Lcp | −0.909 ** | −0.848 ** | −0.836 ** | 0.813 ** | −0.872 ** | −0.761 ** | 0.319 | −0.888 ** | −0.854 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Cui, P.; Lu, G.; Wang, X.; Jiang, L.; Luo, Y. Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes. Forests 2023, 14, 789. https://doi.org/10.3390/f14040789
Huang H, Cui P, Lu G, Wang X, Jiang L, Luo Y. Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes. Forests. 2023; 14(4):789. https://doi.org/10.3390/f14040789
Chicago/Turabian StyleHuang, Haixia, Peng Cui, Gang Lu, Xuhu Wang, Le Jiang, and Yongzhong Luo. 2023. "Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes" Forests 14, no. 4: 789. https://doi.org/10.3390/f14040789
APA StyleHuang, H., Cui, P., Lu, G., Wang, X., Jiang, L., & Luo, Y. (2023). Photosynthetic and Antioxidant Responses of Gymnocarpos przewalskii to Simulated Rainfall Changes. Forests, 14(4), 789. https://doi.org/10.3390/f14040789




