The Importance of Initial Seedling Characteristics in Controlling Allocation to Growth and Reserves under Different Soil Moisture Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Initial Seedling Measurements
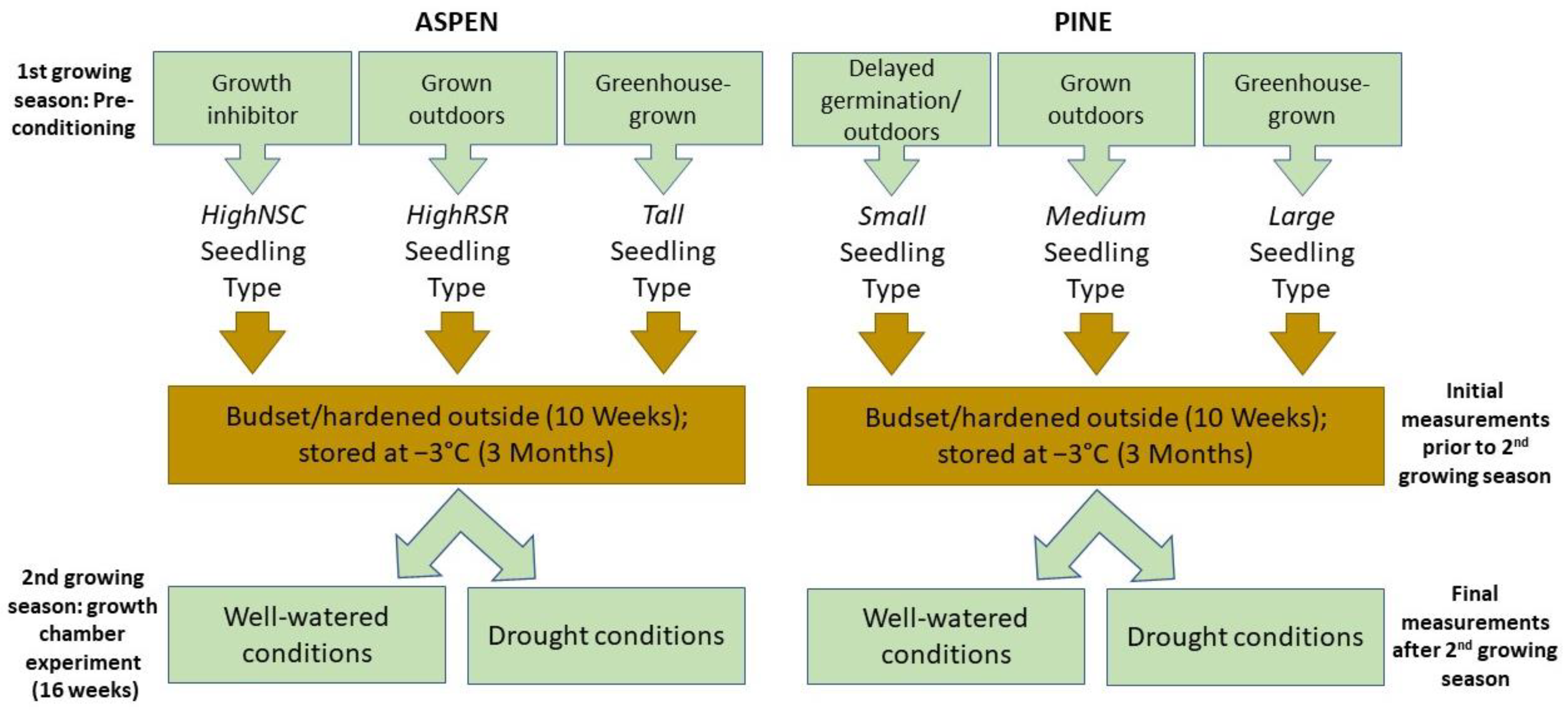
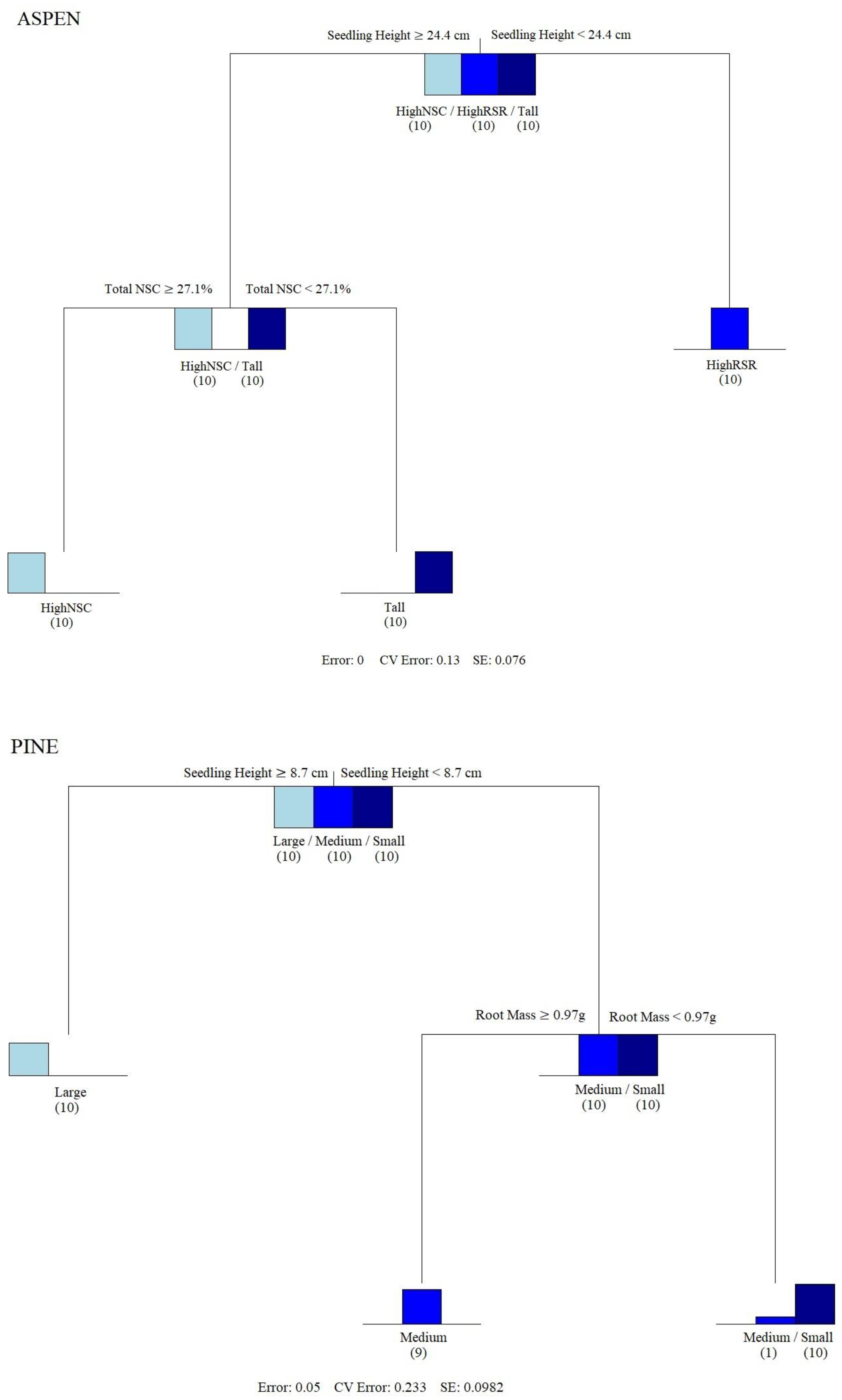
2.2. Characterizing and Separating Initial Seedling Conditions
2.3. Experimental Growing Conditions
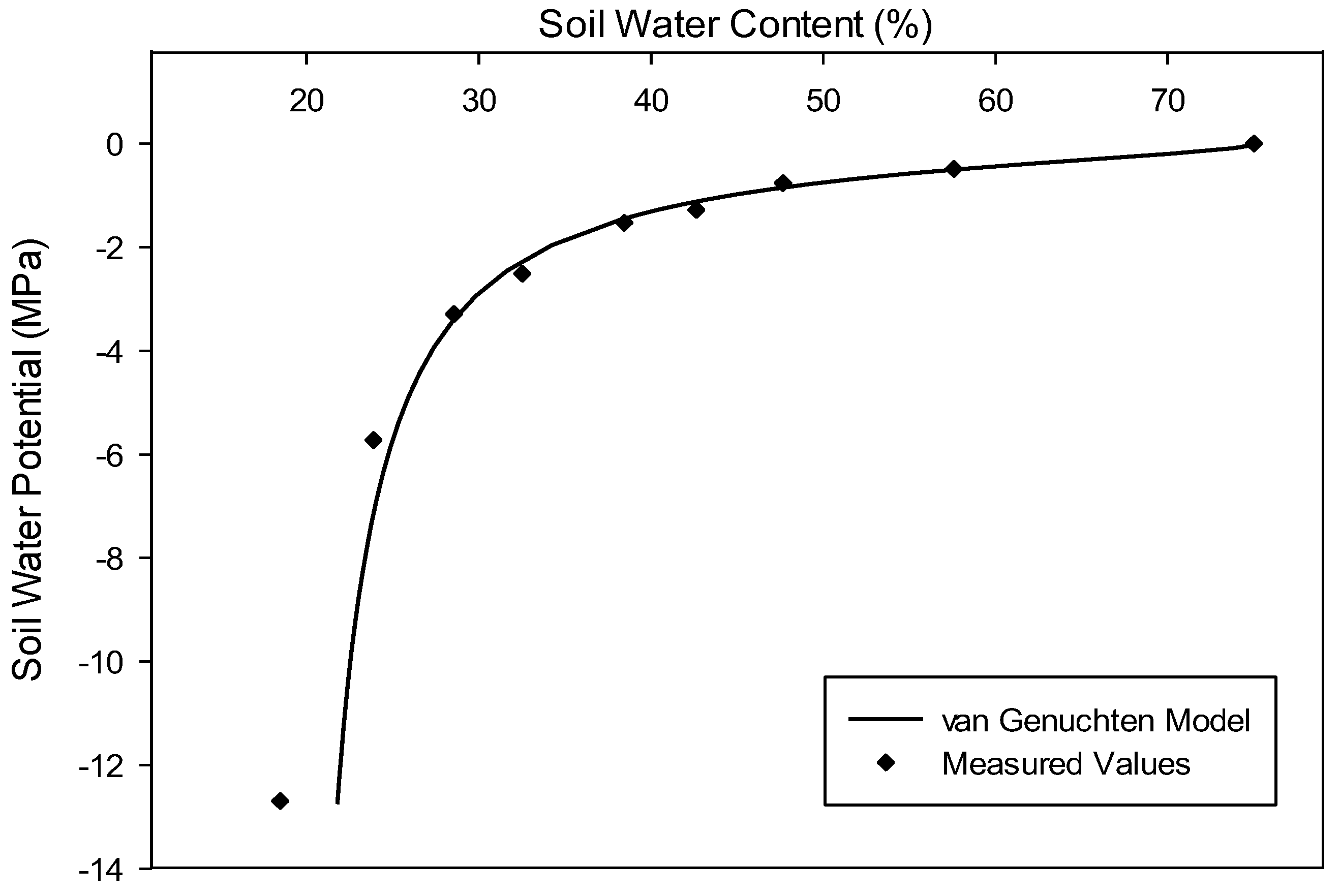
2.4. Drought Treatment
2.5. Post-Drought Seedling Measurements
2.6. Data Analysis
3. Results
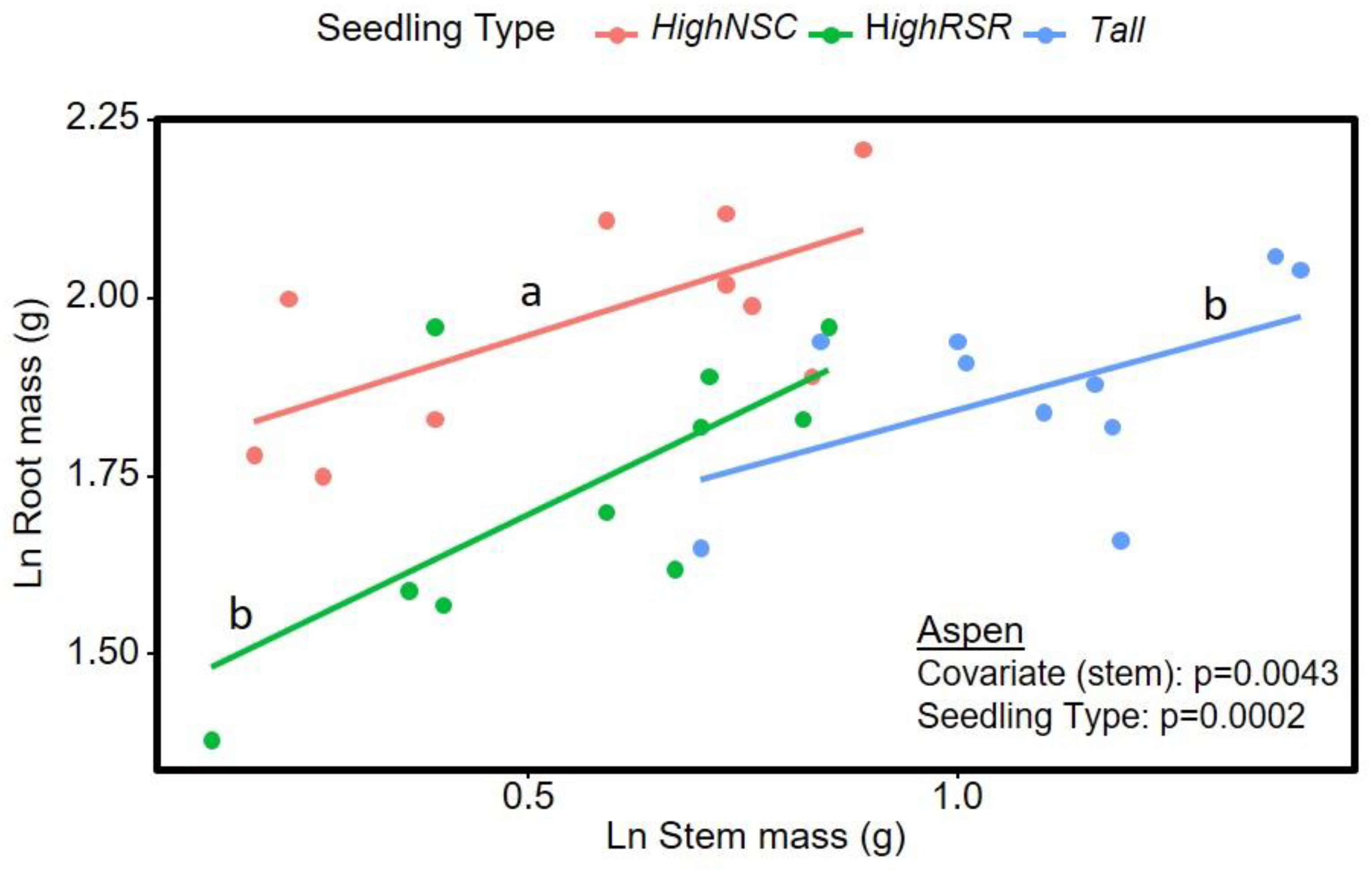
3.1. Aspen
3.2. Pine
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Measurement | Seedling Type | |||
|---|---|---|---|---|
| HighNSC | HighRSR | Tall | ||
| Height (cm) * | 32.35 (0.79) b | 15.90 (0.61) c | 51.78 (1.26) a | |
| Root Collar Diameter (mm) * | 3.91 (0.08) b | 3.03 (0.07) c | 4.76 (0.11) a | |
| Root Mass (g) | 3.04 (0.13) a | 1.64 (0.17) b | 2.72 (0.12) a | |
| Stem Mass (g) * | 1.05 (0.08) b | 0.35 (0.03) c | 1.89 (0.14) a | |
| Whole Seedling Mass (g) | 4.09 (0.16) a | 1.99 (0.19) b | 4.61 (0.19) a | |
| Root Stem Ratio (g g−1) * | 3.04 (0.25) b | 4.83 (0.31) a | 1.51 (0.13) c | |
| NSC concentration (%) | Root Sugar | 24.17 (0.92) a | 21.92 (0.60) a | 18.24 (0.74) b |
| Root Starch | 9.79 (0.46) a | 7.49 (0.87) b | 5.78 (0.77) b | |
| Root NSC | 33.96 (0.99) a | 29.41 (1.36) b | 24.01 (1.34) c | |
| Stem Sugar * | 16.52 (0.43) a | 15.27 (0.51) a | 12.72 (0.29) b | |
| Stem Starch | 0.21 (0.03) a | 0.22 (0.03) a | 0.26 (0.02) a | |
| Stem NSC * | 16.73 (0.43) a | 15.48 (0.49) a | 12.98 (0.29) b | |
| Whole Seedling NSC | 29.55 (0.76) a | 26.94 (1.19) a | 19.67 (1.08) b | |
| NSC mass (g) | Root NSC | 1.03 (0.05) a | 0.48 (0.06) b | 0.66 (0.06) c |
| Stem NSC * | 0.18 (0.01) b | 0.05 (0.006) c | 0.24 (0.02) a | |
| Whole Seedling | 1.20 (0.05) a | 0.53 (0.06) c | 0.90 (0.05) b | |
| Structural biomass (g) | Root | 2.01 (0.10) a | 1.16 (0.11) b | 2.06 (0.08) a |
| Stem * | 0.87 (0.06) b | 0.29 (0.03) c | 1.65 (0.12) a | |
| Whole Seedling | 2.88 (0.12) b | 1.45 (0.13) c | 3.71 (0.18) a | |
| Measurement | Seedling Type | |||
|---|---|---|---|---|
| Large | Medium | Small | ||
| Height (cm) * | 14.35 (0.62) a | 5.45 (0.28) b | 5.52 (0.34) b | |
| Root Collar Diameter (mm) | 2.73 (0.07) a | 2.49 (0.13) b | 1.98 (0.06) c | |
| Root Mass (g) * | 1.20 (0.10) a | 1.17 (0.06) a | 0.73 (0.05) b | |
| Stem Mass (g) * | 0.48 (0.03) a | 0.26 (0.02) b | 0.13 (0.01) c | |
| Needle Mass (g) | 1.56 (0.08) a | 0.86 (0.06) b | 0.51 (0.06) c | |
| Whole Seedling Mass (g) * | 3.24 (0.19) a | 2.29 (0.13) b | 1.36 (0.10) c | |
| Root:Shoot Ratio | 0.59 (0.04) c | 1.07 (0.03) b | 1.20 (0.08) a | |
| NSC concentration (%) | Root Sugar * | 4.21 (0.10) a | 4.57 (0.22) a | 4.43 (0.17) a |
| Root Starch | 6.12 (0.54) b | 7.57 (0.34) a | 7.56 (0.36) a | |
| Root NSC | 10.33 (0.57) b | 12.14 (0.46) a | 12.00 (0.39) a | |
| Stem Sugar | 13.87 (0.55) b | 15.77 (0.53) a | 16.47 (0.42) a | |
| Stem Starch | 0.38 (0.07) b | 1.15 (0.10) a | 1.13 (0.11) a | |
| Stem NSC | 14.26 (0.58) b | 16.92 (0.59) a | 17.60 (0.44) a | |
| Needle Sugar | 16.65 (0.31) b | 16.74 (0.30) b | 18.23 (0.38) a | |
| Needle Starch | 0.15 (0.01) a | 0.11 (0.01) b | 0.11 (0.01) a b | |
| Needle NSC | 16.63 (0.41) b | 16.63 (0.29) b | 18.16 (0.52) a | |
| Whole Seedling NSC | 14.03 (0.33) b | 14.47 (0.27) a | 14.85 (0.21) a | |
| NSC mass (g) | Root NSC | 0.123 (0.012) a | 0.141 (0.007) a | 0.088 (0.007) b |
| Stem NSC * | 0.070 (0.007) a | 0.043 (0.004) b | 0.023 (0.002) c | |
| Needle NSC * | 0.263 (0.017) a | 0.146 (0.011) b | 0.092 (0.011) c | |
| Whole Seedling NSC * | 0.457 (0.032) a | 0.330 (0.018) b | 0.203 (0.016) c | |
| Structural biomass (g) | Root * | 1.08 (0.09) a | 1.03 (0.06) a | 0.64 (0.04) b |
| Stem * | 0.41 (0.03) a | 0.22 (0.02) b | 0.11 (0.01) c | |
| Needle | 1.29 (0.06) a | 0.71 (0.05) b | 0.41 (0.05) c | |
| Whole Seedling * | 2.78 (0.16) a | 1.96 (0.11) b | 1.16 (0.08) c | |
| Organ | Variable | Control | Drought | ||||
|---|---|---|---|---|---|---|---|
| HighNSC | HighRSR | Tall | HighNSC | HighRSR | Tall | ||
| Whole Seedling | BiomassTotal * | 10.6 (0.56) a | 10.3 (0.55) a | 11.9 (0.51) a | 6.3 (0.22) b | 4.61 (0.25) c | 6.81 (0.34) b |
| BiomassStructural * | 8.2 (0.38) ab | 8.0 (0.31) b | 9.4 (0.29) a | 4.9 (0.16) c | 3.72 (0.18) d | 5.56 (0.25) c | |
| NSCMass * | 2.4 (0.15) a | 2.3 (0.14) a | 2.5 (0.18) a | 1.4 (0.07) b | 0.89 (0.07) c | 1.26 (0.08) b | |
| NSCConcentration | 22.5 (0.9) a | 22.0 (0.5) ab | 20.7 (0.8) abc | 21.7 (0.9) ab | 19.0 (0.8) bc | 18.4 (0.6) c | |
| Leaves | BiomassTotal * | 1.57 (0.13) b | 2.7 (0.15) a | 2.28 (0.13) a | 0.66 (0.05) d | 0.91 (0.05) c | 0.57 (0.04) d |
| BiomassStructural * | 1.21 (0.09) b | 2.1 (0.12) a | 1.70 (0.09) a | 0.51 (0.03) d | 0.70 (0.03) c | 0.44 (0.02) d | |
| NSCMass * | 0.4 (0.05) b | 0.68 (0.04) a | 0.58 (0.05) a | 0.15 (0.02) cd | 0.21 (0.02) c | 0.13 (0.02) d | |
| NSCConcentration | 22.2 (1.1) a | 24.7 (0.6) a | 25.0 (1.1) a | 23.1 (1.5) a | 23.1 (1.1) a | 23.0 (1.1) a | |
| Stem | BiomassTotal * | 1.80 (0.15) b | 1.80 (0.13) b | 3.06 (0.20) a | 1.26 (0.09) c | 0.83 (0.04) d | 2.06 (0.10) b |
| BiomassStructural * | 1.51 (.12) b | 1.56 (0.11) b | 2.63 (0.17) a | 1.04 (0.07) c | 0.72 (0.03) d | 1.77 (0.09) b | |
| NSCMass * | 0.3 (0.02) b | 0.23 (0.02) b | 0.42 (0.03) a | 0.22 (0.02) b | 0.11 (0.01) c | 0.29 (0.02) b | |
| NSCConcentration | 15.9 (0.3) a | 13.1 (0.2) b | 13.9 (0.3) b | 17.3 (0.5) a | 13.1 (0.5) b | 14.0 (0.5) b | |
| Root | BiomassTotal * | 7.3 (0.35) a | 5.75 (0.34) b | 6.57 (0.28) ab | 4.35 (0.15) c | 2.87 (0.22) d | 4.19 (0.25) c |
| BiomassStructural * | 5.5 (0.32) a | 4.39 (0.25) a | 5.09 (0.22) a | 3.36 (0.13) b | 2.30 (0.16) c | 3.35 (0.20) b | |
| NSCMass * | 1.7 (0.10) a | 1.36 (0.10) ab | 1.48 (0.11) a | 0.99 (0.05) bc | 0.57 (0.06) d | 0.84 (0.06) c | |
| NSCConcentration | 24.3 (1.3) a | 23.4 (0.7) ab | 22.5 (1.2) ab | 22.8 (1.0) ab | 19.3 (1.1) b | 19.9 (0.6) b | |
| Organ | Source of Variation | Control | Drought | ||||
|---|---|---|---|---|---|---|---|
| Large | Medium | Small | Large | Medium | Small | ||
| Whole Seedling | BiomassTotal * | 11.2 (0.56) a | 6.59 (0.40) b | 4.92 (0.44) bc | 4.40 (0.22) c | 2.55 (0.25) d | 1.75 (0.18) e |
| BiomassStructural * | 9.78 (0.49) a | 5.76 (0.35) b | 4.30 (0.39) b | 4.07 (0.20) b | 2.37 (0.23) c | 1.63 (0.17) d | |
| MassNSC *† | 1.46 (0.08) a | 0.87 (0.10) b | 0.62 (0.06) b | 0.33 (0.02) c | 0.18 (0.02) d | 0.12 (0.01) e | |
| NSCConcentration† | 13.0 (0.3) a | 13.3 (0.9) a | 12.6 (0.5) a | 7.6 (0.3) b | 7.1 (0.5) b | 7.1 (0.3) b | |
| Needles | BiomassTotal * | 4.75 (0.25) a | 2.91 (0.20) b | 2.22 (0.25) bc | 1.91 (0.11) c | 0.93 (0.11) d | 0.72 (0.10) d |
| BiomassStructural * | 3.97 (0.19) a | 2.44 (0.17) b | 1.90 (0.21) b | 1.73 (0.10) b | 0.84 (0.10) c | 0.66 (0.09) c | |
| NSCMass * | 0.78 (0.07) a | 0.47 (0.06) b | 0.32 (0.05) b | 0.18 (0.02) c | 0.09 (0.01) d | 0.06 (0.01) d | |
| NSCConcentration * | 16.2 (0.6) a | 16.1 (1.4) a | 14.4 (0.8) a | 9.4 (0.5) b | 9.1 (0.6) b | 8.6 (0.3) b | |
| Stem | BiomassTotal * | 1.94 (0.19) a | 0.85 (0.07) b | 0.61 (0.07) c | 0.68 (0.06) b | 0.31 (0.04) c | 0.23 (0.03) c |
| BiomassStructural * | 1.75 (0.18) a | 0.76 (0.06) b | 0.55 (0.06) b | 0.61 (0.06) b | 0.28 (0.03) c | 0.21 (0.03) c | |
| NSCMass * | 0.19 (0.01) a | 0.09 (0.01) b | 0.07 (0.01) b | 0.06 (0.01) b | 0.03 (0.01) c | 0.02 (0.01) d | |
| NSCConcentration | 10.0 (0.4) a | 10.9 (0.8) a | 11.1 (0.6) a | 9.2 (0.4) a | 9.7 (0.6) a | 8.8 (0.6) a | |
| Roots | BiomassTotal * | 4.55 (0.23) a | 2.82 (0.20) b | 2.09 (0.15) bc | 1.81 (0.08) c | 1.31 (0.13) d | 0.81 (0.08) e |
| BiomassStructural * | 4.06 (0.22) a | 2.56 (0.18) b | 1.85 (0.13) bc | 1.72 (0.08) d | 1.24 (0.13) d | 0.76 (0.08) e | |
| NSCMass †* | 0.50 (0.03) a | 0.28 (0.03) b | 0.23 (0.02) b | 0.09 (0.01) c | 0.06 (0.01) cd | 0.04 (0.004) d | |
| NSCConcentration † | 11.0 (0.6) a | 10.1 (0.9) a | 11.3 (0.4) a | 5.0 (0.3) b | 5.0 (0.3) b | 5.3 (0.4) b | |
References
- Adams, H.D.; Guardiola-Claramonte, M.; Barron-Gafford, G.A.; Villegas, J.C.; Breshears, D.D.; Zou, C.B.; Troch, P.A.; Huxman, T.E. Temperature Sensitivity of Drought-Induced Tree Mortality Portends Increased Regional Die-off under Global-Change-Type Drought. Proc. Natl. Acad. Sci. USA 2009, 106, 7063–7066. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A Global Overview of Drought and Heat-Induced Tree Mortality Reveals Emerging Climate Change Risks for Forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Trugman, A.T.; Badgley, G.; Anderson, C.M.; Bartuska, A.; Ciais, P.; Cullenward, D.; Field, C.B.; Freeman, J.; Goetz, S.J.; et al. Climate-Driven Risks to the Climate Mitigation Potential of Forests. Science 2020, 368, eaaz7005. [Google Scholar] [CrossRef]
- Beier, C.; Beierkuhnlein, C.; Wohlgemuth, T.; Penuelas, J.; Emmett, B.; Körner, C.; de Boeck, H.; Christensen, J.H.; Leuzinger, S.; Janssens, I.A.; et al. Precipitation Manipulation Experiments—Challenges and Recommendations for the Future. Ecol. Lett. 2012, 15, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.S.; Iverson, L.; Woodall, C.W.; Allen, C.D.; Bell, D.M.; Bragg, D.C.; D’Amato, A.W.; Davis, F.W.; Hersh, M.H.; Ibanez, I.; et al. The Impacts of Increasing Drought on Forest Dynamics, Structure, and Biodiversity in the United States. Glob. Chang. Biol. 2016, 22, 2329–2352. [Google Scholar] [CrossRef]
- Cobb, R.C.; Ruthrof, K.X.; Breshears, D.D.; Lloret, F.; Aakala, T.; Adams, H.D.; Anderegg, W.R.L.; Ewers, B.E.; Galiano, L.; Grünzweig, J.M.; et al. Ecosystem Dynamics and Management after Forest Die-off: A Global Synthesis with Conceptual State-and-Transition Models. Ecosphere 2017, 8, e02034. [Google Scholar] [CrossRef]
- Royer, P.D.; Cobb, N.S.; Clifford, M.J.; Huang, C.-Y.; Breshears, D.D.; Adams, H.D.; Villegas, J.C. Extreme Climatic Event-Triggered Overstorey Vegetation Loss Increases Understorey Solar Input Regionally: Primary and Secondary Ecological Implications. J. Ecol. 2011, 99, 714–723. [Google Scholar] [CrossRef]
- Hodgson, D.; McDonald, J.L.; Hosken, D.J. What Do You Mean, ‘Resilient’? Trends Ecol. Evol. 2015, 30, 503–506. [Google Scholar] [CrossRef]
- Grubb, P.J. Control of Forest Growth and Distribution on Wet Tropical Mountains: With Special Reference to Mineral Nutrition. Annu. Rev. Ecol. Syst. 1977, 8, 83–107. [Google Scholar] [CrossRef]
- Macdonald, S.E.; Landhäusser, S.M.; Skousen, J.; Franklin, J.; Frouz, J.; Hall, S.; Jacobs, D.F.; Quideau, S. Forest Restoration Following Surface Mining Disturbance: Challenges and Solutions. New For. 2015, 46, 703–732. [Google Scholar] [CrossRef]
- Hanson, P.J.; Todd, D.E.; Amthor, J.S. A Six-Year Study of Sapling and Large-Tree Growth and Mortality Responses to Natural and Induced Variability in Precipitation and Throughfall. Tree Physiol. 2001, 21, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Peñuelas, J.; Ogaya, R. Establishment of Co-Existing Mediterranean Tree Species under a Varying Soil Moisture Regime. J. Veg. Sci. 2004, 15, 237–244. [Google Scholar] [CrossRef]
- Poorter, L.; Hayashida-Oliver, Y. Effects of Seasonal Drought on Gap and Understory Seedlings in a Bolivian Moist Forest. J. Trop. Ecol. 2000, 16, 481–498. [Google Scholar] [CrossRef]
- Solarik, K.A.; Messier, C.; Ouimet, R.; Bergeron, Y.; Gravel, D. Local Adaptation of Trees at the Range Margins Impacts Range Shifts in the Face of Climate Change. Glob. Ecol. Biogeogr. 2018, 27, 1507–1519. [Google Scholar] [CrossRef]
- Kozlowski, T.T.; Pallardy, S.G. Acclimation and Adaptive Responses of Woody Plants to Environmental Stresses. Bot. Rev. 2002, 68, 270–334. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of Forest Trees to Single and Multiple Environmental Stresses from Seedlings to Mature Plants: Past Stress History, Stress Interactions, Tolerance and Acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Why Seedlings Grow: Influence of Plant Attributes. New For. 2018, 49, 1–34. [Google Scholar] [CrossRef]
- Moler, E.R.V.; Nelson, A.S. Perspectives on Drought Preconditioning Treatments With a Case Study Using Western Larch. Front. Plant Sci. 2021, 12, 741027. [Google Scholar] [CrossRef] [PubMed]
- Sloan, J.L.; Burney, O.T.; Pinto, J.R. Drought-Conditioning of Quaking Aspen (Populus Tremuloides Michx.) Seedlings During Nursery Production Modifies Seedling Anatomy and Physiology. Front. Plant Sci. 2020, 11, 557894. [Google Scholar] [CrossRef] [PubMed]
- Villar-Salvador, P.; Ocaña, L.; Peñuelas, J.; Carrasco, I. Effect of Water Stress Conditioning on the Water Relations, Root Growth Capacity, and the Nitrogen and Non-Structural Carbohydrate Concentration of Pinus Halepensis Mill. (Aleppo Pine) Seedlings. Ann. For. Sci. 1999, 56, 459–465. [Google Scholar] [CrossRef]
- Dietze, M.C.; Sala, A.; Carbone, M.S.; Czimczik, C.I.; Mantooth, J.A.; Richardson, A.D.; Vargas, R. Nonstructural Carbon in Woody Plants. Annu. Rev. Plant Biol. 2014, 65, 667–687. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G. Carbon Reserves as Indicators for Carbon Limitation in Trees. In Progress in Botany: Vol. 76; Lüttge, U., Beyschlag, W., Eds.; Progress in Botany; Springer International Publishing: Cham, Switzerland, 2015; pp. 321–346. ISBN 978-3-319-08807-5. [Google Scholar]
- Piper, F.I.; Fajardo, A. Foliar Habit, Tolerance to Defoliation and Their Link to Carbon and Nitrogen Storage. J. Ecol. 2014, 102, 1101–1111. [Google Scholar] [CrossRef]
- Tomlinson, K.W.; van Langevelde, F.; Ward, D.; Bongers, F.; da Silva, D.A.; Prins, H.H.T.; de Bie, S.; Sterck, F.J. Deciduous and Evergreen Trees Differ in Juvenile Biomass Allometries Because of Differences in Allocation to Root Storage. Ann. Bot. 2013, 112, 575–587. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M.J.; Burslem, D.F.R.P.; Caduff, A.; Tay, J.; Hector, A. Contrasting Nonstructural Carbohydrate Dynamics of Tropical Tree Seedlings under Water Deficit and Variability. New Phytol. 2015, 205, 1083–1094. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought Survival of Tropical Tree Seedlings Enhanced by Non-Structural Carbohydrate Levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Dumroese, K.; Landis, T.D.; Pinto, J.; Haase, D.L.; Wilkinson, K.W.; Davis, A.S. Meeting Forest Restoration Challenges: Using the Target Plant Concept. Reforesta 2016, 1, 37–52. [Google Scholar] [CrossRef]
- Landis, T.D. The Target Plant Concept-a History and Brief Overview. In National Proceedings: Forest and Conservation Nursery Associations—2010; Proc. RMRS-P-65; Riley, L.E., Haase, D.L., Pinto, J.R., Eds.; USDA Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2011; Volume 65, pp. 61–66. [Google Scholar]
- Rose, R.; Campbell, S.J.; Landis, T.D. Target Seedling Symposium: Proceedings, Combined Meeting of the Western Forest Nursery Associations; Gen. Tech. Rep. RM-GTR-200; U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station: Fort Collins, CO, USA, 1990; Volume 200. [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass Allocation to Leaves, Stems and Roots: Meta-Analyses of Interspecific Variation and Environmental Control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Bloom, A.J.; Chapin, F.S.; Mooney, H.A. Resource Limitation in Plants-An Economic Analogy. Annu. Rev. Ecol. Syst. 1985, 16, 363–392. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Lieffers, V.J. Photosynthesis and Carbon Allocation of Six Boreal Tree Species Grown in Understory and Open Conditions. Tree Physiol. 2001, 21, 243–250. [Google Scholar] [CrossRef]
- Niinemets, Ü. Photosynthesis and Resource Distribution through Plant Canopies. Plant Cell Environ. 2007, 30, 1052–1071. [Google Scholar] [CrossRef]
- Buckley, T.N.; John, G.P.; Scoffoni, C.; Sack, L. The Sites of Evaporation within Leaves. Plant Physiol 2017, 173, 1763–1782. [Google Scholar] [CrossRef]
- Claeys, H.; Inzé, D. The Agony of Choice: How Plants Balance Growth and Survival under Water-Limiting Conditions. Plant Physiol. 2013, 162, 1768–1779. [Google Scholar] [CrossRef]
- Valladares, F.; Gianoli, E.; Gómez, J.M. Ecological Limits to Plant Phenotypic Plasticity. New Phytol. 2007, 176, 749–763. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.T.; Merlin, M.; Wiley, E.; Landhäusser, S.M. Splitting the Difference: Heterogeneous Soil Moisture Availability Affects Aboveground and Belowground Reserve and Mass Allocation in Trembling Aspen. Front. Plant Sci. 2021, 12, 654159. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Cochard, H.; Mencuccini, M.; Sterck, F.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; Nolè, A.; Poyatos, R.; et al. Hydraulic Adjustment of Scots Pine across Europe. New Phytol. 2009, 184, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Rood, S.B.; Patiño, S.; Coombs, K.; Tyree, M.T. Branch Sacrifice: Cavitation-Associated Drought Adaptation of Riparian Cottonwoods. Trees 2000, 14, 248–257. [Google Scholar] [CrossRef]
- Sterck, F.J.; Martínez-Vilalta, J.; Mencuccini, M.; Cochard, H.; Gerrits, P.; Zweifel, R.; Herrero, A.; Korhonen, J.F.J.; Llorens, P.; Nikinmaa, E.; et al. Understanding Trait Interactions and Their Impacts on Growth in Scots Pine Branches across Europe. Funct. Ecol. 2012, 26, 541–549. [Google Scholar] [CrossRef]
- Canham, C.D.; Kobe, R.K.; Latty, E.F.; Chazdon, R.L. Interspecific and Intraspecific Variation in Tree Seedling Survival: Effects of Allocation to Roots versus Carbohydrate Reserves. Oecologia 1999, 121, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Galiano, L.; Martínez-Vilalta, J.; Eugenio, M.; Granzow-de la Cerda, Í.; Lloret, F. Seedling Emergence and Growth of Quercus Spp. Following Severe Drought Effects on a Pinus Sylvestris Canopy. J. Veg. Sci. 2013, 24, 580–588. [Google Scholar] [CrossRef]
- Suarez, M.L.; Kitzberger, T. Recruitment Patterns Following a Severe Drought: Long-Term Compositional Shifts in Patagonian Forests. Can. J. For. Res. 2008, 38, 3002–3010. [Google Scholar] [CrossRef]
- Chapin, F.S.; Schulze, E.; Mooney, H.A. The Ecology and Economics of Storage in Plants. Annu. Rev. Ecol. Syst. 1990, 21, 423–447. [Google Scholar] [CrossRef]
- Hartmann, H.; Adams, H.D.; Hammond, W.M.; Hoch, G.; Landhäusser, S.M.; Wiley, E.; Zaehle, S. Identifying Differences in Carbohydrate Dynamics of Seedlings and Mature Trees to Improve Carbon Allocation in Models for Trees and Forests. Environ. Exp. Bot. 2018, 152, 7–18. [Google Scholar] [CrossRef]
- Maguire, A.J.; Kobe, R.K. Drought and Shade Deplete Nonstructural Carbohydrate Reserves in Seedlings of Five Temperate Tree Species. Ecol. Evol. 2015, 5, 5711–5721. [Google Scholar] [CrossRef] [PubMed]
- Landhäusser, S.M.; Pinno, B.D.; Lieffers, V.J.; Chow, P.S. Partitioning of Carbon Allocation to Reserves or Growth Determines Future Performance of Aspen Seedlings. For. Ecol. Manag. 2012, 275, 43–51. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Rodriguez-Alvarez, J.; Marenholtz, E.H.; Lieffers, V.J. Effect of Stock Type Characteristics and Time of Planting on Field Performance of Aspen (Populus Tremuloides Michx.) Seedlings on Boreal Reclamation Sites. New For. 2012, 43, 679–693. [Google Scholar] [CrossRef]
- Signori-Müller, C.; Oliveira, R.S.; de Barros, F.V.; Tavares, J.V.; Gilpin, M.; Diniz, F.C.; Zevallos, M.J.M.; Yupayccana, C.A.S.; Acosta, M.; Bacca, J.; et al. Non-Structural Carbohydrates Mediate Seasonal Water Stress across Amazon Forests. Nat. Commun. 2021, 12, 2310. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Root Carbon Reserve Dynamics in Aspen Seedlings: Does Simulated Drought Induce Reserve Limitation? Tree Physiol. 2011, 31, 250–257. [Google Scholar] [CrossRef]
- Galvez, D.A.; Landhäusser, S.M.; Tyree, M.T. Low Root Reserve Accumulation during Drought May Lead to Winter Mortality in Poplar Seedlings. New Phytol. 2013, 198, 139–148. [Google Scholar] [CrossRef]
- Rennenberg, H.; Loreto, F.; Polle, A.; Brilli, F.; Fares, S.; Beniwal, R.S.; Gessler, A. Physiological Responses of Forest Trees to Heat and Drought. Plant Biol. 2006, 8, 556–571. [Google Scholar] [CrossRef]
- Kobe, R.K.; Iyer, M.; Walters, M.B. Optimal Partitioning Theory Revisited: Nonstructural Carbohydrates Dominate Root Mass Responses to Nitrogen. Ecology 2010, 91, 166–179. [Google Scholar] [CrossRef]
- Barbaroux, C.; Breda, N. Contrasting Distribution and Seasonal Dynamics of Carbohydrate Reserves in Stem Wood of Adult Ring-Porous Sessile Oak and Diffuse-Porous Beech Trees. Tree Physiol. 2002, 22, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Hoch, G.; Richter, A.; Körner, C. Non-Structural Carbon Compounds in Temperate Forest Trees. Plant Cell Environ. 2003, 26, 1067–1081. [Google Scholar] [CrossRef]
- Uscola, M.; Villar-Salvador, P.; Gross, P.; Maillard, P. Fast Growth Involves High Dependence on Stored Resources in Seedlings of Mediterranean Evergreen Trees. Ann. Bot. 2015, 115, 1001–1013. [Google Scholar] [CrossRef]
- Kelly, J.W.G.; Landhäusser, S.M.; Chow, P.S. The Impact of Light Quality and Quantity on Root-to-Shoot Ratio and Root Carbon Reserves in Aspen Seedling Stock. New For. 2015, 46, 527–545. [Google Scholar] [CrossRef]
- Le, K.D.; Schreiber, S.G.; Landhäusser, S.M.; Schoonmaker, A.L. Manipulating Aspen (Populus Tremuloides) Seedling Size Characteristics to Improve Initial Establishment and Growth on Competitive Sites. Scand. J. For. Res. 2020, 35, 29–45. [Google Scholar] [CrossRef]
- Chow, P.S.; Landhäusser, S.M. A Method for Routine Measurements of Total Sugar and Starch Content in Woody Plant Tissues. Tree Physiol. 2004, 24, 1129–1136. [Google Scholar] [CrossRef]
- Landhäusser, S.M.; Chow, P.S.; Dickman, L.T.; Furze, M.E.; Kuhlman, I.; Schmid, S.; Wiesenbauer, J.; Wild, B.; Gleixner, G.; Hartmann, H.; et al. Standardized Protocols and Procedures Can Precisely and Accurately Quantify Non-Structural Carbohydrates. Tree Physiol. 2018, 38, 1764–1778. [Google Scholar] [CrossRef]
- De’ath, G. Multivariate Regression Trees: A New Technique for Modeling Species–Environment Relationships. Ecology 2002, 83, 1105–1117. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- van Genuchten, M.T. A Closed-Form Equation for Predicting the Hydraulic Conductivity of Unsaturated Soils. Soil Sci. Soc. Am. J. 1980, 44, 892–898. [Google Scholar] [CrossRef]
- Cai, J.; Tyree, M.T. The Impact of Vessel Size on Vulnerability Curves: Data and Models for within-Species Variability in Saplings of Aspen, Populus Tremuloides Michx. Plant Cell Env. 2010, 33, 1059–1069. [Google Scholar] [CrossRef]
- Schoonmaker, A.L.; Hacke, U.G.; Landhäusser, S.M.; Lieffers, V.J.; Tyree, M.T. Hydraulic Acclimation to Shading in Boreal Conifers of Varying Shade Tolerance. Plant Cell Env. 2010, 33, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.; King, C.M.; Landhäusser, S.M. Identifying the Relevant Carbohydrate Storage Pools Available for Remobilization in Aspen Roots. Tree Physiol. 2019, 39, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Wyka, T.P.; Karolewski, P.; Żytkowiak, R.; Chmielarz, P.; Oleksyn, J. Whole-Plant Allocation to Storage and Defense in Juveniles of Related Evergreen and Deciduous Shrub Species. Tree Physiol. 2016, 36, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Schott, K.M.; Pinno, B.D.; Landhäusser, S.M. Premature Shoot Growth Termination Allows Nutrient Loading of Seedlings with an Indeterminate Growth Strategy. New For. 2013, 44, 635–647. [Google Scholar] [CrossRef]
- Jordan, M.-O.; Habib, R. Mobilizable Carbon Reserves in Young Peach Trees as Evidenced by Trunk Girdling Experiments. J. Exp. Bot. 1996, 47, 79–87. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Lethaus, G.; Winkler, A.; Wieser, G. Stem Girdling Indicates Prioritized Carbon Allocation to the Root System at the Expense of Radial Stem Growth in Norway Spruce under Drought Conditions. Environ. Exp. Bot. 2017, 138, 109–118. [Google Scholar] [CrossRef]
- Tuttle, C.L.; South, D.B.; Golden, M.S.; Meldahl, R.S. Relationship between Initial Seedling Height and Survival and Growth of Loblolly Pine Seedlings Planted during a Droughty Year. South. J. Appl. For. 1987, 11, 139–143. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in Size and Nitrogen Concentration Enhances Seedling Survival in Mediterranean Plantations. Insights from an Ecophysiological Conceptual Model of Plant Survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Navarro, R.M.; Retamosa, M.J.; Lopez, J.; del Campo, A.; Ceaceros, C.; Salmoral, L. Nursery Practices and Field Performance for the Endangered Mediterranean Species Abies Pinsapo Boiss. Ecol. Eng. 2006, 27, 93–99. [Google Scholar] [CrossRef]
- Myers, J.A.; Kitajima, K. Carbohydrate Storage Enhances Seedling Shade and Stress Tolerance in a Neotropical Forest. J. Ecol. 2007, 95, 383–395. [Google Scholar] [CrossRef]
- Wiley, E.; Hoch, G.; Landhäusser, S.M. Dying Piece by Piece: Carbohydrate Dynamics in Aspen (Populus Tremuloides) Seedlings under Severe Carbon Stress. J. Exp. Bot. 2017, 68, 5221–5232. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, C.L.; South, D.B.; Golden, M.S.; Meldahl, R.S. Initial Pinustaeda Seedling Height Relationships with Early Survival and Growth. Can. J. For. Res. 1988, 18, 867–871. [Google Scholar] [CrossRef]
- Tan, W.; Hogan, G.D. Physiological and Morphological Responses to Nitrogen Limitation in Jack Pine Seedlings: Potential Implications for Drought Tolerance. New For. 1997, 14, 19–31. [Google Scholar] [CrossRef]
- Trubat, R.; Cortina, J.; Vilagrosa, A. Nutrient Deprivation Improves Field Performance of Woody Seedlings in a Degraded Semi-Arid Shrubland. Ecol. Eng. 2011, 37, 1164–1173. [Google Scholar] [CrossRef]
- Furze, M.E.; Huggett, B.A.; Aubrecht, D.M.; Stolz, C.D.; Carbone, M.S.; Richardson, A.D. Whole-Tree Nonstructural Carbohydrate Storage and Seasonal Dynamics in Five Temperate Species. New Phytol. 2019, 221, 1466–1477. [Google Scholar] [CrossRef]
- Schoonmaker, A.L.; Hillabrand, R.M.; Lieffers, V.J.; Chow, P.S.; Landhäusser, S.M. Seasonal Dynamics of Non-Structural Carbon Pools and Their Relationship to Growth in Two Boreal Conifer Tree Species. Tree Physiol. 2021, 41, 1563–1582. [Google Scholar] [CrossRef]
- Pinto, J.R.; Marshall, J.D.; Dumroese, R.K.; Davis, A.S.; Cobos, D.R. Photosynthetic Response, Carbon Isotopic Composition, Survival, and Growth of Three Stock Types under Water Stress Enhanced by Vegetative Competition. Can. J. For. Res. 2012, 42, 333–344. [Google Scholar] [CrossRef]
- Hillabrand, R.M.; Gordon, H.; Hynes, B.; Constabel, C.P.; Landhäusser, S.M. Populus Root Salicinoid Phenolic Glycosides Are Not Mobilized to Support Metabolism and Regrowth under Carbon Limited Conditions. Tree Physiol. 2023, tpad020. [Google Scholar] [CrossRef] [PubMed]
- Najar, A.; Landhäusser, S.M.; Whitehill, J.G.A.; Bonello, P.; Erbilgin, N. Reserves Accumulated in Non-Photosynthetic Organs during the Previous Growing Season Drive Plant Defenses and Growth in Aspen in the Subsequent Growing Season. J. Chem. Ecol. 2014, 40, 21–30. [Google Scholar] [CrossRef]
- Wiley, E.; Rogers, B.J.; Hodgkinson, R.; Landhäusser, S.M. Nonstructural Carbohydrate Dynamics of Lodgepole Pine Dying from Mountain Pine Beetle Attack. New Phytol. 2016, 209, 550–562. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest Trees and Stands under Severe Drought: A Review of Ecophysiological Responses, Adaptation Processes and Long-Term Consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Hartmann, H.; Ziegler, W.; Trumbore, S. Lethal Drought Leads to Reduction in Nonstructural Carbohydrates in Norway Spruce Tree Roots but Not in the Canopy. Funct. Ecol. 2013, 27, 413–427. [Google Scholar] [CrossRef]
- Sala, A.; Piper, F.; Hoch, G. Physiological Mechanisms of Drought-Induced Tree Mortality Are Far from Being Resolved: Letters. New Phytol. 2010, 186, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sevanto, S.; Mcdowell, N.G.; Dickman, L.T.; Pangle, R.; Pockman, W.T. How Do Trees Die? A Test of the Hydraulic Failure and Carbon Starvation Hypotheses. Plant Cell Env. 2014, 37, 153–161. [Google Scholar] [CrossRef]
- Brodribb, T.J.; Holbrook, N.M.; Hill, R.S.; Brodribb, T.J.; Holbrook, N.M.; Hill, R.S. Seedling Growth in Conifers and Angiosperms: Impacts of Contrasting Xylem Structure. Aust. J. Bot. 2005, 53, 749–755. [Google Scholar] [CrossRef]
- Carnicer, J.; Barbeta, A.; Sperlich, D.; Coll, M.; Penuelas, J. Contrasting Trait Syndromes in Angiosperms and Conifers Are Associated with Different Responses of Tree Growth to Temperature on a Large Scale. Front. Plant Sci. 2013, 4, 409. [Google Scholar] [CrossRef]
- Givnish, T.J. Adaptive Significance of Evergreen vs. Deciduous Leaves: Solving the Triple Paradox. Silva Fenn. 2002, 36, 703–743. [Google Scholar] [CrossRef]

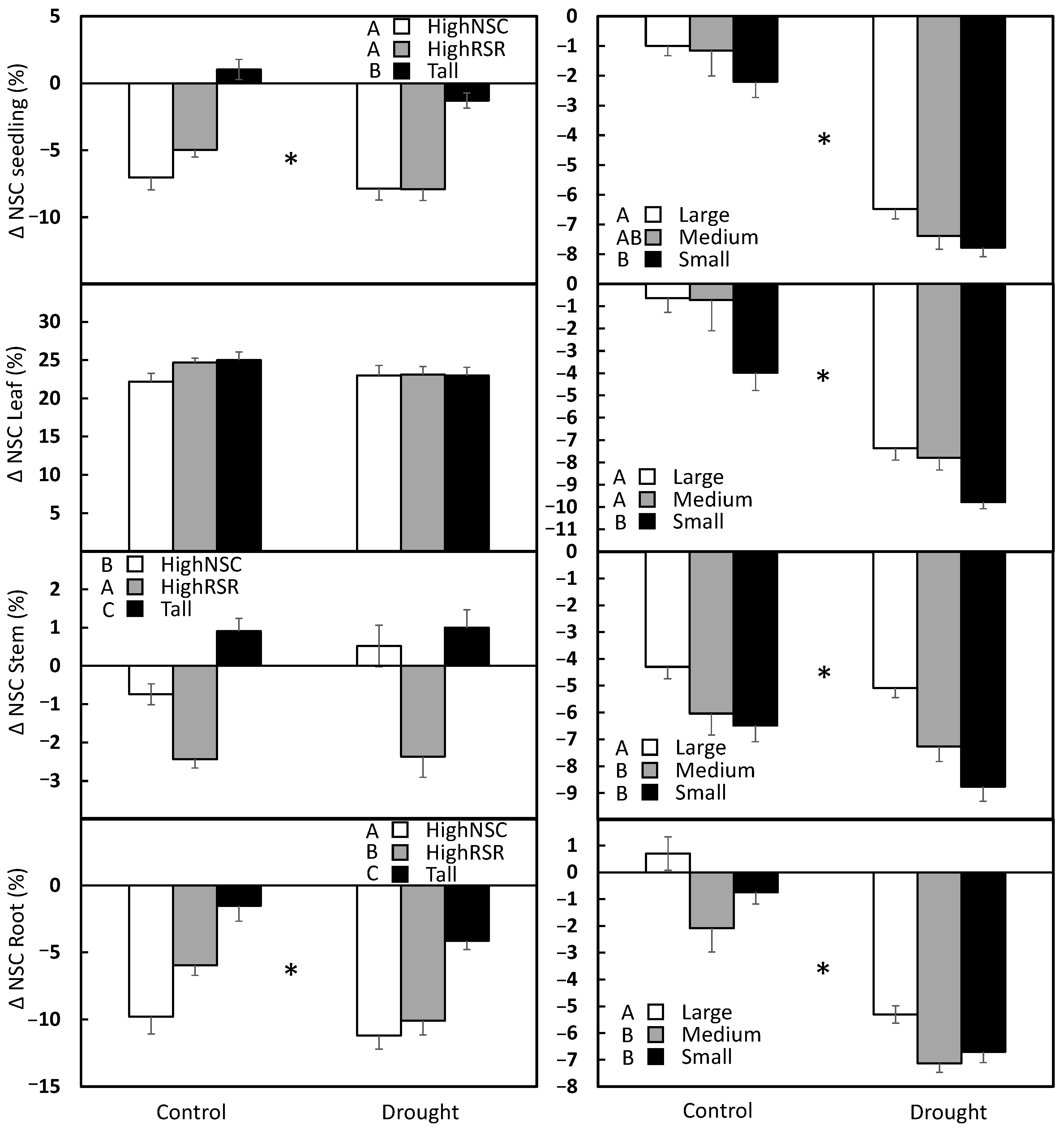
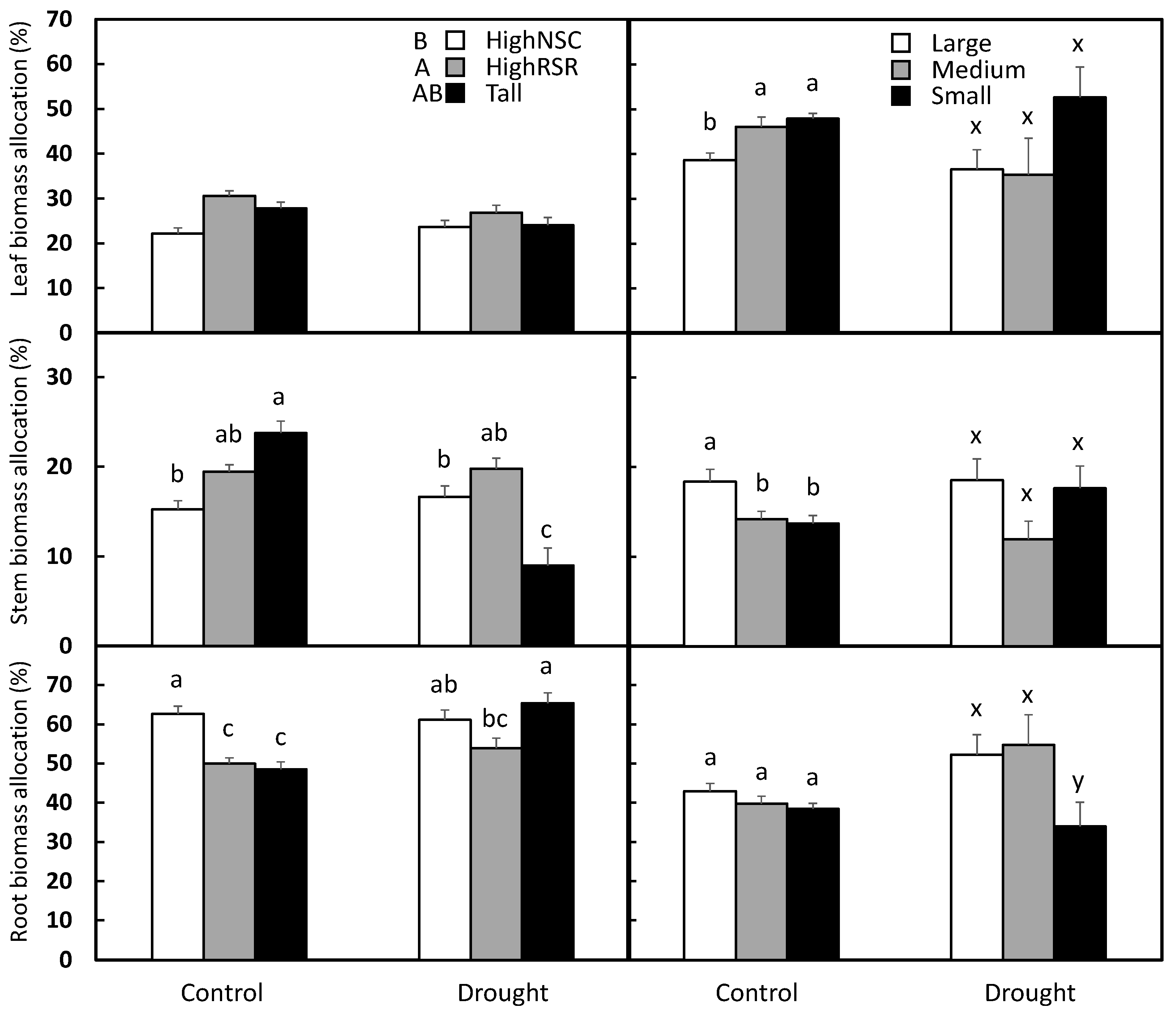

| Species | Organ | Response Variable | Seedling Type | Drought | Seedling Type × Drought | Error |
|---|---|---|---|---|---|---|
| DF | 2 | 1 | 2 | 54 or 53 | ||
| Aspen | Seedling | ∆Biomass * | 0.0379 | <0.0001 | 0.21 | |
| ∆NSCconcentration | <0.0001 | 0.0018 | 0.36 | |||
| Leaves | ∆ Biomass * | <0.0001 | <0.0001 | 0.0007 | ||
| ∆NSCconcentration | 0.34 | 0.28 | 0.37 | |||
| Stem | ∆ Biomass * | 0.0006 | <0.0001 | <0.0001 | ||
| ∆NSCconcentration * | <0.0001 | 0.17 | 0.27 | |||
| Roots | ∆Biomass * | 0.61 | <0.0001 | 0.92 | ||
| ∆NSCconcentration | <0.0001 | 0.0017 | 0.40 | |||
| Pine | Seedling | ∆Biomass ** | <0.0001 | <0.0001 | 0.0174 | |
| ∆NSCconcentration † | 0.0423 | <0.0001 | 0.72 | |||
| Needles | ∆Biomass ** | <0.0001 | <0.0001 | 0.0271 | ||
| ∆NSCconcentration * | <0.0001 | <0.0001 | 0.20 | |||
| Stem | ∆Biomass * | <0.0001 | <0.0001 | 0.0027 | ||
| ∆NSCconcentration | <0.0001 | 0.0032 | 0.41 | |||
| Roots | ∆Biomass ** | <0.0001 | <0.0001 | 0.0135 | ||
| ∆NSCconcentration † | 0.0001 | <0.0001 | 0.59 |
| Species | Organ | Seedling Type | Drought | Seedling Type × Drought | Error |
|---|---|---|---|---|---|
| DF | 2 | 1 | 2 | ||
| Aspen | Leaves * | 0.0008 | 0.102 | 0.122 | 53 |
| Stem ** | 0.0116 | 0.0001 | <0.0001 | 52 | |
| Roots * | 0.0002 | 0.0007 | 0.0003 | 53 |
| Species | Organ | Seedling Type (for Control) | n | Seedling Type (for Drought) | n | Main Drought Effect | n |
|---|---|---|---|---|---|---|---|
| DF | 2 | 2 | 1 | ||||
| Pine | Needles | 0.0016 | 10 | 0.12 | 9,6,8 | 0.56 | 30,23 |
| Stem | 0.0075 | 10 | 0.20 | 8,5,8 | 0.46 | 30,21 | |
| Roots | 0.19 | 10 | 0.0633 | 10,6,7 | 0.0940 | 30,23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landhäusser, S.M.; Wiley, E.T.; Solarik, K.A.; Kulbaba, S.P.; Goeppel, A.E. The Importance of Initial Seedling Characteristics in Controlling Allocation to Growth and Reserves under Different Soil Moisture Conditions. Forests 2023, 14, 796. https://doi.org/10.3390/f14040796
Landhäusser SM, Wiley ET, Solarik KA, Kulbaba SP, Goeppel AE. The Importance of Initial Seedling Characteristics in Controlling Allocation to Growth and Reserves under Different Soil Moisture Conditions. Forests. 2023; 14(4):796. https://doi.org/10.3390/f14040796
Chicago/Turabian StyleLandhäusser, Simon M., Erin T. Wiley, Kevin A. Solarik, Shaun P. Kulbaba, and Alexander E. Goeppel. 2023. "The Importance of Initial Seedling Characteristics in Controlling Allocation to Growth and Reserves under Different Soil Moisture Conditions" Forests 14, no. 4: 796. https://doi.org/10.3390/f14040796
APA StyleLandhäusser, S. M., Wiley, E. T., Solarik, K. A., Kulbaba, S. P., & Goeppel, A. E. (2023). The Importance of Initial Seedling Characteristics in Controlling Allocation to Growth and Reserves under Different Soil Moisture Conditions. Forests, 14(4), 796. https://doi.org/10.3390/f14040796




