Biomass Models and Ecosystem Carbon Density: A Case Study of Two Coniferous Forest in Northern Hunan, China
Abstract
1. Introduction
2. Materials and Methods
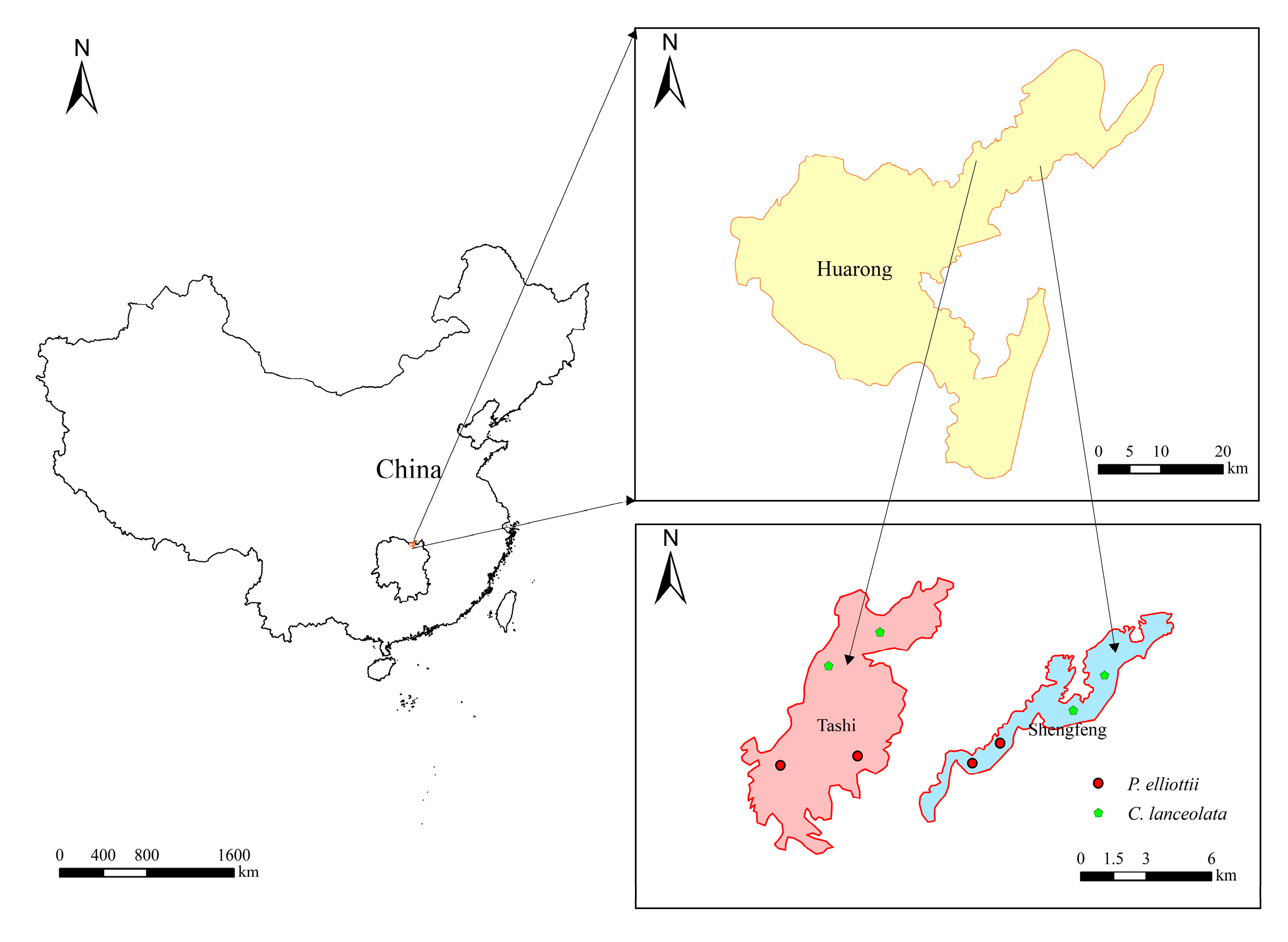
2.1. Study Area
2.2. Sample Plot Setting
2.3. Biomass Model Construction
2.4. Soil Sample Collection
2.5. Carbon Concentration Measurement
2.6. Forest Carbon Density Assessment
2.7. Statistical Analysis
3. Results
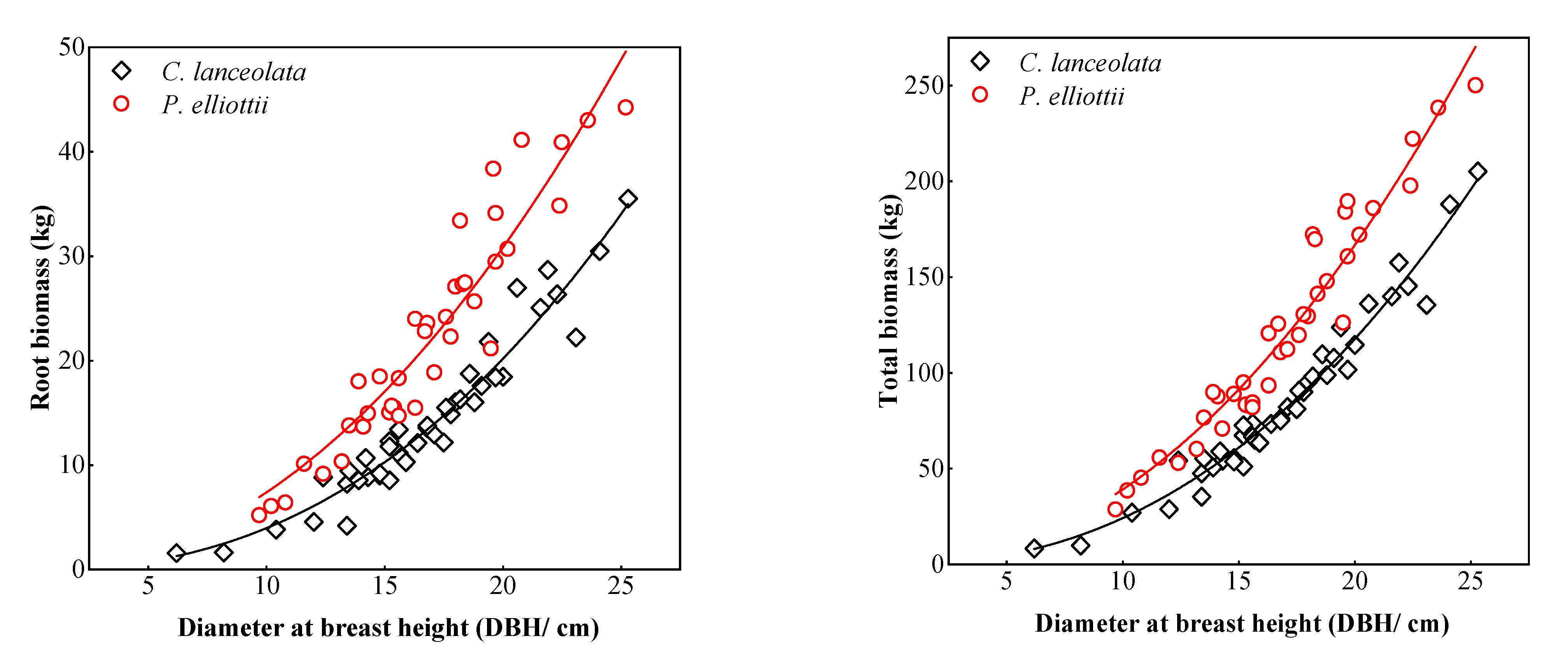
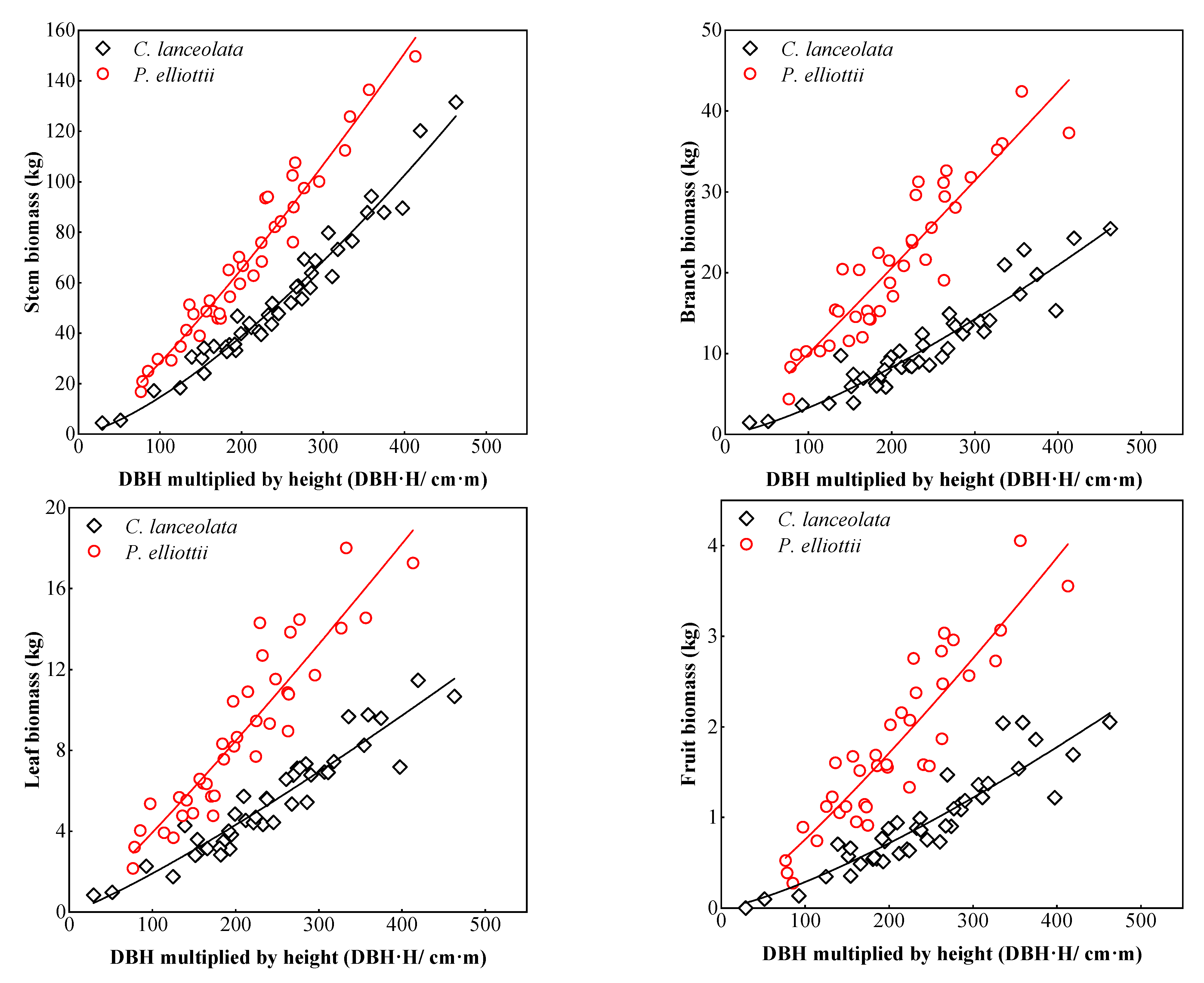
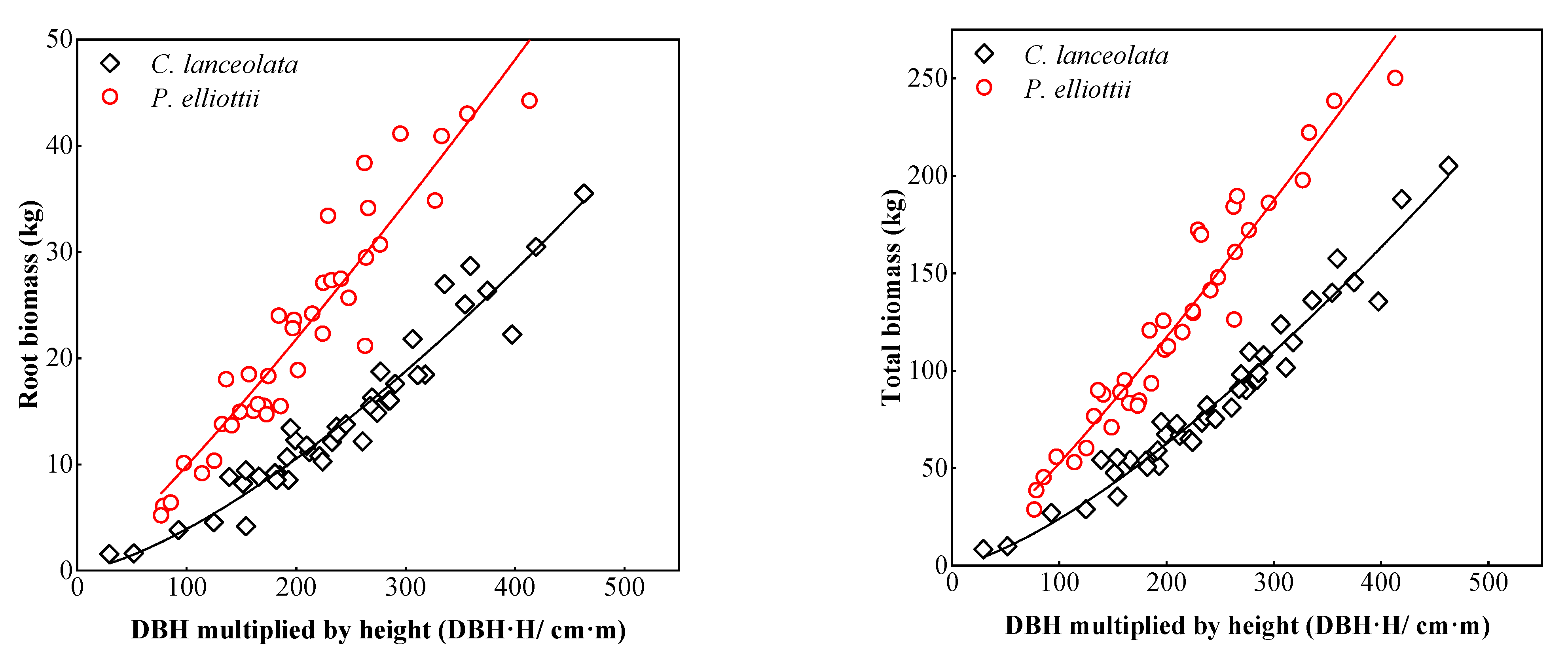
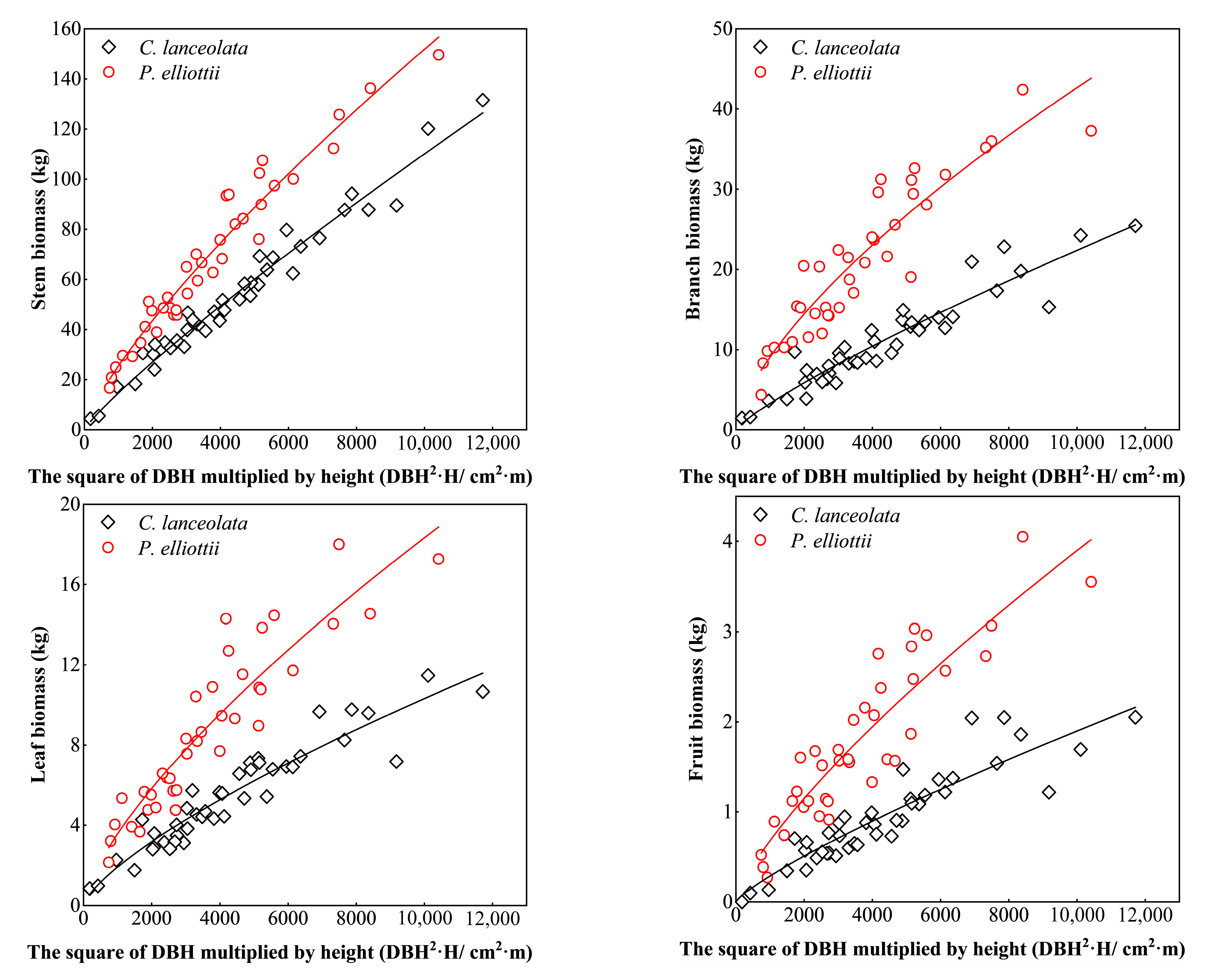
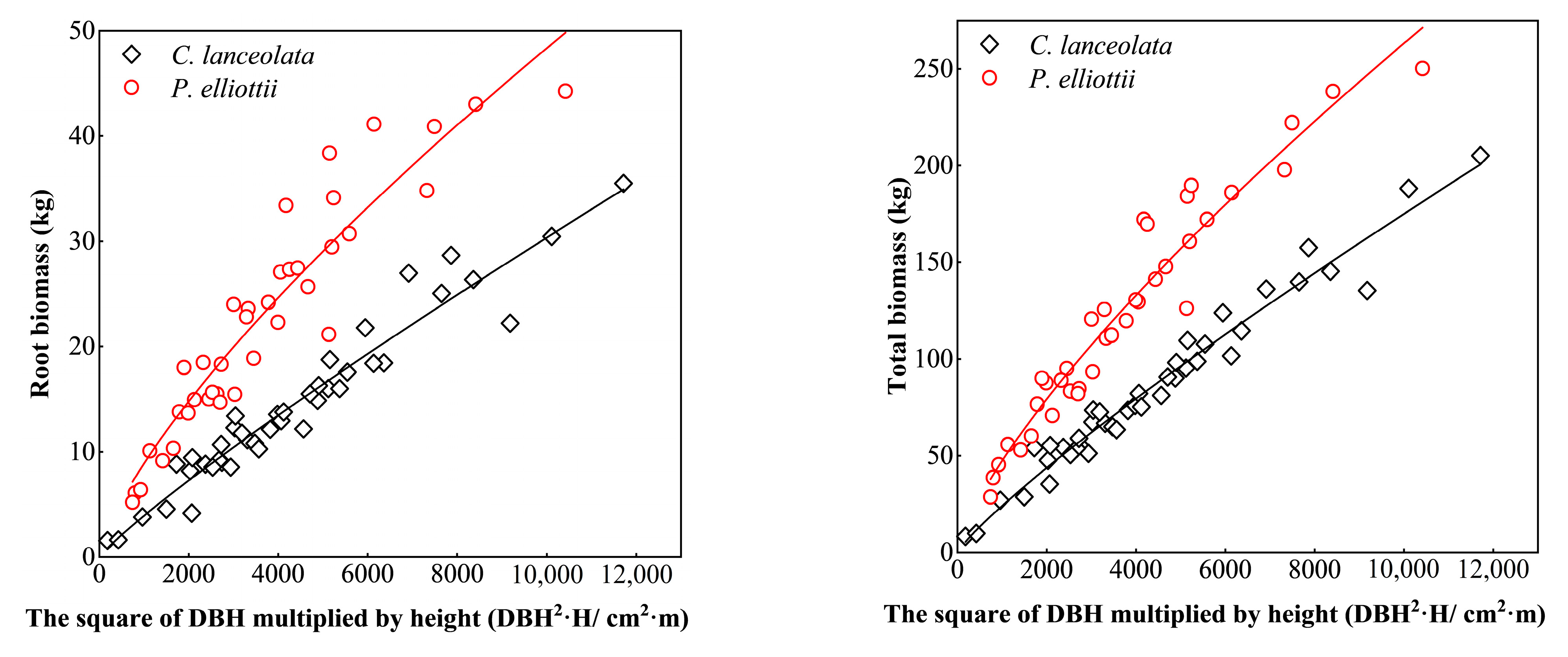
3.1. Biomass Models of Two Coniferous Forests
3.2. Forest Stand Biomass
3.2.1. Tree Layer Biomass
3.2.2. Understory Vegetation and Dead Cover Layer Biomass
3.3. Carbon Concentration of Forest Ecosystem Components
3.3.1. Carbon Concentration in the Tree Layer
3.3.2. Carbon Concentration of the Understory Vegetation and Dead Cover Layer
3.3.3. Soil Carbon Concentration
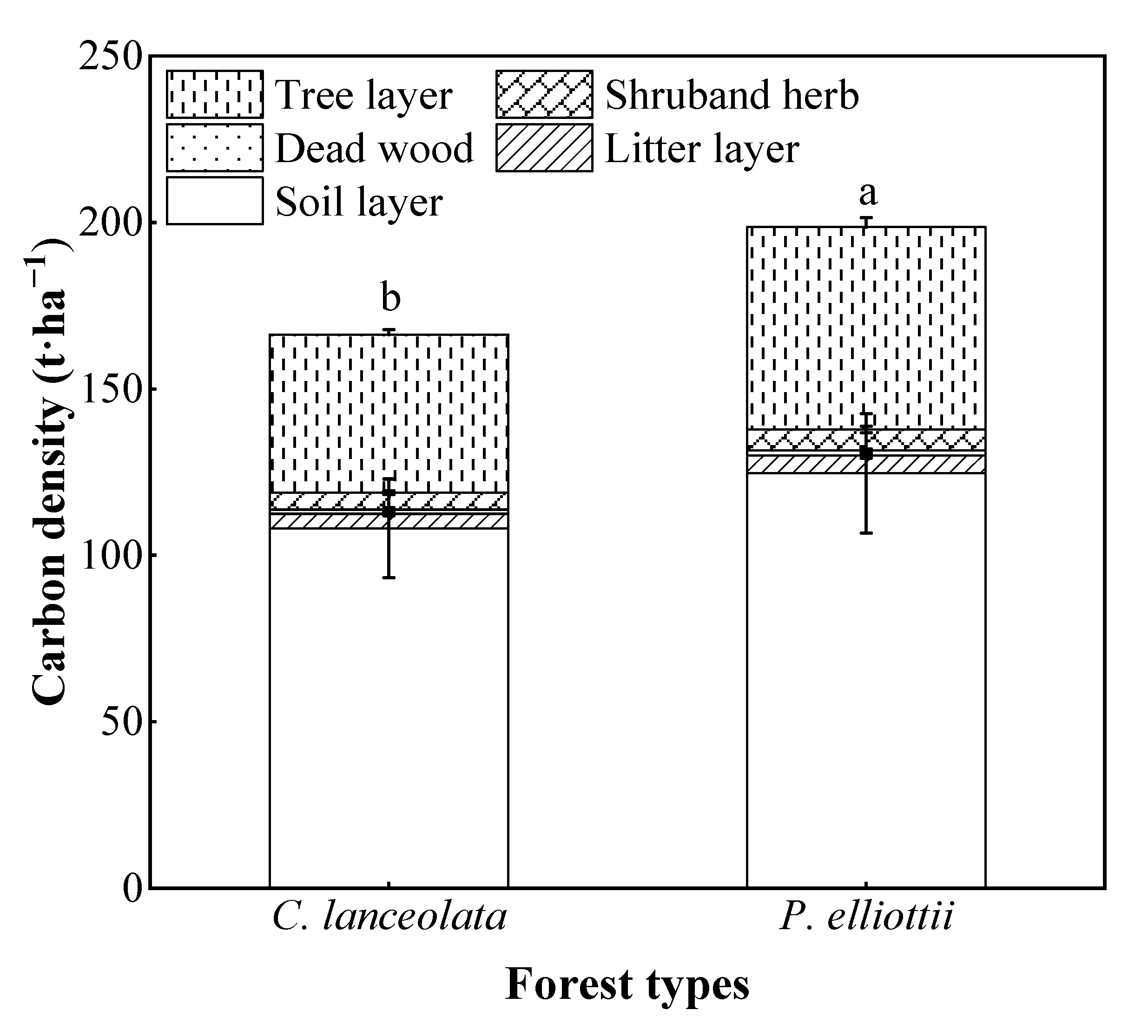
3.4. Carbon Density of Forest Ecosystem
3.4.1. Carbon Density in the Tree Layer
3.4.2. Carbon Density in the Understory Vegetation and Dead Cover Layer
3.4.3. Carbon Density in Soil
3.4.4. Total Carbon Density of Forest Ecosystem
4. Discussion
4.1. Single Tree Biomass Model
4.2. Biomass and Distribution in Plantations
4.3. Forest Carbon Concentration
4.4. Forest Carbon Density and Its Distribution
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robinson, R.A.; Crick, H.Q.P.; Learmonth, J.A.; Maclean, I.M.D.; Thomas, C.D.; Bairlein, F.; Forchhammer, M.C.; Francis, C.M.; Gill, J.A.; Godley, B.J.; et al. Travelling through a warming world: Climate change and migratory species. Endanger. Species Res. 2009, 7, 87–99. [Google Scholar] [CrossRef]
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. Lancet 2006, 367, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Pecl, G.T.; Araújo, M.B.; Bell, J.D.; Blanchard, J.; Bonebrake, T.C.; Chen, I.C.; Clark, T.D.; Colwell, R.K.; Danielsen, F.; Evengård, B.; et al. Biodiversity redistribution under climate change: Impacts on ecosystems and human well-being. Science 2017, 355, eaai9214. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Gren, I.M.; Zeleke, A.A. Policy design for forest carbon sequestration: A review of the literature. For. Policy Econ. 2016, 70, 128–136. [Google Scholar] [CrossRef]
- Brinck, K.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Dantas De Paula, M.; Pütz, S.; Sexton, J.O.; Song, D.; Huth, A. High resolution analysis of tropical forest fragmentation and its impact on the global carbon cycle. Nat. Commun. 2017, 8, 14855. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Murray, L.T.; Pearson, T.R.H. Global carbon dioxide removal rates from forest landscape restoration activities. Carbon Balance Manag. 2018, 13, 22. [Google Scholar] [CrossRef]
- Heinonen, T.; Pukkala, T.; Mehtätalo, L.; Asikainen, A.; Kangas, J.; Peltola, H. Scenario analyses for the effects of harvesting intensity on development of forest resources, timber supply, carbon balance and biodiversity of Finnish forestry. For. Policy Econ. 2017, 80, 80–98. [Google Scholar] [CrossRef]
- Gough, C.M.; Vogel, C.S.; Harrold, K.H.; George, K.; Curtis, P.S. The legacy of harvest and fire on ecosystem carbon storage in a north temperate forest. Glob. Chang. Biol. 2007, 13, 1935–1949. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Chen, A.; Gao, M.; Slette, I.J.; Piao, S. The impact of the 2009/2010 drought on vegetation growth and terrestrial carbon balance in Southwest China. Agric. For. Meteorol. 2019, 269, 239–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.; Qin, Q.; Chen, K. Value accounting of forest carbon sinks in China. J. Beijing For. Univ. 2013, 35, 124–131. [Google Scholar] [CrossRef]
- IGBP Terrestrial Carbon Working Group. The terrestrial carbon cycle: Implications for the Kyoto Protocol. Science 1998, 280, 1393–1394. [Google Scholar] [CrossRef]
- Ma, X.; Xiong, K.; Zhang, Y.; Lai, J.; Zhang, S.; Ji, C. Research progresses and prospects of carbon storage in forest ecosystems. J. Northwest For. Univ. 2019, 34, 62–72. [Google Scholar] [CrossRef]
- Sun, W.; Liu, X. Review on carbon storage estimation of forest ecosystem and applications in China. For. Ecosyst. 2020, 7, 4. [Google Scholar] [CrossRef]
- Fang, J.; Chen, A.; Peng, C.; Zhao, S.; Ci, L. Changes in forest biomass carbon storage in China between 1949 and 1998. Science 2001, 292, 2320–2322. [Google Scholar] [CrossRef]
- Schwendenmann, L.; Pendall, E. Effects of forest conversion into grassland on soil aggregate structure and carbon storage in Panama: Evidence from soil carbon fractionation and stable isotopes. Plant Soil 2006, 288, 217–232. [Google Scholar] [CrossRef]
- Luo, X.; Hou, E.; Zhang, L.; Wen, D. Soil carbon dynamics in different types of subtropical forests as determined by density fractionation and stable isotope analysis. For. Ecol. Manag. 2020, 475, 118401. [Google Scholar] [CrossRef]
- Goetz, S.; Dubayah, R. Advances in remote sensing technology and implications for measuring and monitoring forest carbon stocks and change. Carbon Manag. 2011, 2, 231–244. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, H.; Ma, J.; Wang, K. Integrated remote sensing and model approach for impact assessment of future climate change on the carbon budget of global forest ecosystems. Glob. Planet. Chang. 2021, 203, 103542. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Wen, Y. Forest carbon sequestration potential in China under the background of carbon emission peak and carbon neutralization. J. Beijing For. Univ. 2022, 44, 38–47. [Google Scholar] [CrossRef]
- Qiu, Z.; Feng, Z.; Song, Y.; Li, M.; Zhang, P. Carbon sequestration potential of forest vegetation in China from 2003 to 2050: Predicting forest vegetation growth based on climate and the environment. J. Clean. Prod. 2020, 252, 119715. [Google Scholar] [CrossRef]
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- Mei, M.; Lei, Y. Analysis on development trend of China’s plantation in new era. World For. Res. 2019, 32, 73–77. [Google Scholar] [CrossRef]
- Liu, S.; Yang, Y.; Wang, H. Development strategy and management countermeasures of planted forests in China: Transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services. Acta Ecol. Sin. 2018, 38, 1–10. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, G.; Wang, Y.; Bai, S.; Sun, Z.; Berninger, F.; Bai, Y.; Penttinen, P. Thinning and species mixing in Chinese fir monocultures improve carbon sequestration in subtropical China. Eur. J. For. Res. 2019, 138, 433–443. [Google Scholar] [CrossRef]
- Bracho, R.; Starr, G.; Gholz, H.L.; Martin, T.A.; Cropper, W.P.; Loescher, H.W. Controls on carbon dynamics by ecosystem structure and climate for southeastern US slash pine plantations. Ecol. Monogr. 2012, 82, 101–128. [Google Scholar] [CrossRef]
- Zeng, W. Development of Volume-derived Biomass Models for Three Coniferous Forest Types in China. For. Res. 2021, 34, 49–57. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Berninger, F.; Duan, B. Net primary production of Chinese fir plantation ecosystems and its relationship to climate. Biogeosciences 2014, 11, 5595–5606. [Google Scholar] [CrossRef]
- Cai, H.; Di, X.; Chang, S.X.; Wang, C.; Shi, B.; Geng, P.; Jin, G. Carbon storage, net primary production, and net ecosystem production in four major temperate forest types in northeastern China. Can. J. For. Res. 2016, 46, 143–151. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, S.; Zhang, J. Assessing the effects of vegetation types on carbon storage fifteen years after reforestation on a Chinese fir site. For. Ecol. Manag. 2009, 258, 1437–1441. [Google Scholar] [CrossRef]
- He, Y.; Qin, L.; Li, Z.; Liang, X.; Shao, M.; Tan, L. Carbon storage capacity of monoculture and mixed-species plantations in subtropical China. For. Ecol. Manag. 2013, 295, 193–198. [Google Scholar] [CrossRef]
- Qin, L.; He, Y.; Li, Z.; Shao, M.; Liang, X.; Tan, L. Allocation pattern of biomass and productivity for three plantations of Castanopsis hystrix, Pinus massoniana and their mixture in south subtropical area of Guangxi, China. Sci. Silvae Sin. 2011, 47, 17–21. [Google Scholar]
- Zheng, H.; Ouyang, Z.; Xu, W.; Wang, X.; Miao, H.; Li, X.; Tian, Y. Variation of carbon storage by different reforestation types in the hilly red soil region of southern China. For. Ecol. Manag. 2008, 255, 1113–1121. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H. Establishment of below-ground biomass equations for Chinese fir at tree and stand level. Sci. Silvae Sin. 2018, 54, 81–89. [Google Scholar] [CrossRef]
- Zhang, X.; Duan, A.; Zhang, J. Tree biomass estimation of Chinese fir (Cunninghamia lanceolata) based on Bayesian method. PLoS ONE 2013, 8, e79868. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.; Sun, Y.; Sajjad, S. Models for predicting the biomass of Cunninghamia lanceolata trees and stands in Southeastern China. PLoS ONE 2017, 12, e0169747. [Google Scholar] [CrossRef]
- Zhao, D.; Westfall, J.; Coulston, J.W.; Lynch, T.B.; Bullock, B.P.; Montes, C.R. Additive biomass equations for slash pine trees: Comparing three modeling approaches. Can. J. For. Res. 2019, 49, 27–40. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Duan, A.; Xiang, C. Selection of biomass estimation models for Chinese fir plantation. Chin. J. Appl. Ecol. 2010, 21, 3036–3046. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Chhin, S.; Zhang, J.; Duan, A. Disentangling the effects of stand and climatic variables on forest productivity of Chinese fir plantations in subtropical China using a random forest algorithm. Agric. For. Meteorol. 2021, 304, 108412. [Google Scholar] [CrossRef]
- Huang, Z.; He, Z.; Wan, X.; Hu, Z.; Fan, S.; Yang, Y. Harvest residue management effects on tree growth and ecosystem carbon in a Chinese fir plantation in subtropical China. Plant Soil 2013, 364, 303–314. [Google Scholar] [CrossRef]
- Cobb, W.R.; Will, R.E.; Daniels, R.F.; Jacobson, M.A. Aboveground biomass and nitrogen in four short-rotation woody crop species growing with different water and nutrient availabilities. For. Ecol. Manag. 2008, 255, 4032–4039. [Google Scholar] [CrossRef]
- Guo, S.; Zhao, H.; Zhou, G.; Long, W.; Gan, G.; Wu, S.; Meng, M.; Chen, J. Biomass and Its Distribution Pattern of Four Tree Species Plantation in South Subtropical China. For. Res. 2022, 35, 182–189. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, L.; Li, F. Developing two additive biomass equations for three coniferous plantation species in Northeast China. Forests 2016, 7, 136. [Google Scholar] [CrossRef]
- Labrecque, M.; Teodorescu, T.I. Field performance and biomass production of 12 willow and poplar clones in short-rotation coppice in southern Quebec (Canada). Biomass Bioenergy 2005, 29, 1–9. [Google Scholar] [CrossRef]
- Yu, R.; Xiang, W.; Ning, C.; Luo, Z. Carbon storage and sequestration in four urban forest ecosystems in Changsha, Hunan. Acta Ecol. Sin 2016, 36, 3499–3509. [Google Scholar]
- Bunnell, F.L.; Houde, I. Down wood and biodiversity—Implications to forest practices. Environ. Rev. 2010, 18, 397–421. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Zou, D.; Lei, J.; Wu, X.; Wang, J.; Yan, W.; Liang, X. Biomass, carbon storage and nutrient content of coarse woody debris in secondary-growth forests in southern Hunan. Acta Ecol. Sin. 2022, 42, 3441–3448. [Google Scholar] [CrossRef]
- Krishna, M.P.; Mohan, M. Litter decomposition in forest ecosystems: A review. Energy Ecol. Environ. 2017, 2, 236–249. [Google Scholar] [CrossRef]
- Santonja, M.; Fernandez, C.; Proffit, M.; Gers, C.; Gauquelin, T.; Reiter, I.M.; Cramer, W.; Baldy, V. Plant litter mixture partly mitigates the negative effects of extended drought on soil biota and litter decomposition in a Mediterranean oak forest. J. Ecol. 2017, 105, 801–815. [Google Scholar] [CrossRef]
- Oliveira, R.A.C.; Marques, R.; Marques, M.C.M. Plant diversity and local environmental conditions indirectly affect litter decomposition in a tropical forest. Appl. Soil Ecol. 2019, 134, 45–53. [Google Scholar] [CrossRef]
- Houghton, R.A.; Skole, D.L.; Nobre, C.A.; Hackler, J.L.; Lawrence, K.T.; Chomentowski, W.H. Annual fluxes of carbon from deforestation and regrowth in the Brazilian Amazon. Nature 2000, 403, 301–304. [Google Scholar] [CrossRef]
- Liu, X.; Xu, X.; Hong, A. Study on forest carbon storage and carbon density in Hefei City. J. Soil Water Conserv. 2011, 25, 192–196. [Google Scholar] [CrossRef]
- Chen, L.; Guan, X.; Li, H.; Wang, Q.; Zhang, W.; Yang, Q.; Wang, S. Spatiotemporal patterns of carbon storage in forest ecosystems in Hunan Province, China. For. Ecol. Manag. 2019, 432, 656–666. [Google Scholar] [CrossRef]
- Ma, Z.; Hartmann, H.; Wang, H.; Li, Q.; Wang, Y.; Li, S. Carbon dynamics and stability between native Masson pine and exotic slash pine plantations in subtropical China. Eur. J. For. Res. 2014, 133, 307–321. [Google Scholar] [CrossRef]
- Liu, Z.; Ning, C.; Yan, W.; Ni, X.; Chen, Y.; Ning, X. Three kinds of pine forest biomass and carbon storage research in karst landform. J. Cent. South Univ. For. Technol. 2017, 37, 105–111. [Google Scholar] [CrossRef]
- Zhong, C.; Huang, Y.; Zhang, Q.; Wen, H. The stoichiometric characteristics and carbon, nitrogen stores of four main coastal shelterbelt forests in Pingtan. J. Southwest For. Univ. 2016, 36, 96–102. [Google Scholar]
- Lin, W.; Li, J.; Zhou, P.; Wei, L. Spatial distribution of carbon storage of three forests ecosystem in Guangzhou, China. For. Environ. Sci. 2014, 30, 1–7. [Google Scholar]
- Wei, X.; Li, Q.; Liu, Y.; Liu, S.; Guo, X.; Zhang, L.; Niu, D. Restoring ecosystem carbon sequestration through afforestation: A sub-tropic restoration case study. For. Ecol. Manag. 2013, 300, 60–67. [Google Scholar] [CrossRef]
- Shan, J.; Morris, L.A.; Hendrick, R.L. The effects of management on soil and plant carbon sequestration in slash pine plantations. J. Appl. Ecol. 2001, 38, 932–941. [Google Scholar] [CrossRef]

| Parameter | Forest Type | |
|---|---|---|
| C. lanceolata | P. elliottii | |
| Age | 19 | 19 |
| Stand density | 1363 (43) | 1088 (25) |
| Average DBH/cm | 15.6 (2.8) | 16.3 (2.7) |
| Soil bulk density/g·cm−3 | 1.34 (0.11) | 1.36 (0.12) |
| Soil pH | 4.60 (0.20) | 4.60 (0.26) |
| Soil organic carbon/g·kg−1 | 10.4 (5.3) | 11.8 (6.0) |
| Total nitrogen/g·kg−1 | 1.24 (0.47) | 1.31 (0.53) |
| Total phosphorus/g·kg−1 | 0.32 (0.14) | 0.33 (0.13) |
| Total kalium/g·kg−1 | 5.14 (1.94) | 5.72 (2.51) |
| Sand (>0.02 mm):Silt (0.002–0.02 mm):Clay (<0.002 mm)/(%:%:%) | 40.5:38.1:21.4 | 44.6:35.9:19.5 |
| Forest management measure | In 2019, the thinning intensity was 20%–30% | In 2018, the thinning intensity was 20%–30% |
| Main understory plants | Dicranopteris dichotoma Camellia sinensis Symplocos sumuntia Lygodium japonicum | Loropetalum chinense Symplocos sumuntia Dicranopteris dichotoma Macleaya cordata |
| Equation | Forest Type | Components | Model Parameters | R2 | P | |
|---|---|---|---|---|---|---|
| a | b | |||||
| Equation (1) | C. lanceolata | Stem | 0.07087 | 2.31836 | 0.971 | 0.000 |
| Branch | 0.02027 | 2.21076 | 0.890 | 0.003 | ||
| Leaf | 0.02184 | 1.94271 | 0.885 | 0.003 | ||
| Fruit | 0.00196 | 2.16921 | 0.832 | 0.017 | ||
| Root | 0.01788 | 2.34608 | 0.938 | 0.001 | ||
| Total | 0.12624 | 2.28239 | 0.965 | 0.000 | ||
| P. elliottii | Stem | 0.13913 | 2.17639 | 0.951 | 0.000 | |
| Branch | 0.09688 | 1.89479 | 0.861 | 0.005 | ||
| Leaf | 0.03015 | 1.99425 | 0.850 | 0.011 | ||
| Fruit | 0.00405 | 2.13861 | 0.836 | 0.016 | ||
| Root | 0.06423 | 2.06071 | 0.888 | 0.003 | ||
| Total | 0.31744 | 2.09088 | 0.937 | 0.001 | ||
| Equation (2) | C. lanceolata | Stem | 0.02272 | 1.40461 | 0.964 | 0.000 |
| Branch | 0.00696 | 1.33681 | 0.885 | 0.003 | ||
| Leaf | 0.00863 | 1.17303 | 0.895 | 0.002 | ||
| Fruit | 0.00068 | 1.31255 | 0.836 | 0.014 | ||
| Root | 0.00551 | 1.42608 | 0.935 | 0.001 | ||
| Total | 0.04121 | 1.38277 | 0.961 | 0.000 | ||
| P. elliottii | Stem | 0.10970 | 1.20620 | 0.951 | 0.000 | |
| Branch | 0.08195 | 1.04288 | 0.852 | 0.007 | ||
| Leaf | 0.02439 | 1.10425 | 0.849 | 0.010 | ||
| Fruit | 0.00339 | 1.17512 | 0.825 | 0.024 | ||
| Root | 0.05119 | 1.14247 | 0.891 | 0.002 | ||
| Total | 0.25459 | 1.15740 | 0.935 | 0.001 | ||
| Equation (3) | C. lanceolata | Stem | 0.03485 | 0.87491 | 0.972 | 0.000 |
| Branch | 0.01039 | 0.83336 | 0.892 | 0.002 | ||
| Leaf | 0.01222 | 0.73167 | 0.896 | 0.002 | ||
| Fruit | 0.00101 | 0.81802 | 0.840 | 0.010 | ||
| Root | 0.00859 | 0.88712 | 0.941 | 0.001 | ||
| Total | 0.06277 | 0.86132 | 0.968 | 0.000 | ||
| P. elliottii | Stem | 0.11908 | 0.77640 | 0.951 | 0.000 | |
| Branch | 0.08676 | 0.67294 | 0.856 | 0.007 | ||
| Leaf | 0.02624 | 0.71102 | 0.850 | 0.008 | ||
| Fruit | 0.00360 | 0.75877 | 0.829 | 0.021 | ||
| Root | 0.05537 | 0.73529 | 0.890 | 0.003 | ||
| Stem | 0.27471 | 0.74532 | 0.937 | 0.001 | ||
| Forest Type | Stem | Branch | Leaf | Fruit | Root | Total |
|---|---|---|---|---|---|---|
| C. lanceolata | 58.8(1.9) b | 12.4(0.4) b | 6.4(0.2) b | 1.1(0.1) b | 16.0(0.5) b | 94.7(3.0) b |
| P. elliottii | 68.2(3.4) a | 21.4(1.0) a | 8.8(0.4) a | 1.8(0.1) a | 22.7(1.1) a | 122.9(5.9) a |
| Layer | C. lanceolata | P. elliottii | |
|---|---|---|---|
| Shrub layer biomass | 7.8 (3.5) a | 9.9 (3.4) a | |
| Herb layer biomass | 2.8 (1.7) a | 3.5 (1.8) a | |
| Dead wood biomass | 2.4 (1.3) a | 2.9 (0.9) a | |
| Litter layer biomass | Undecomposed | 3.8 (0.9) b | 6.6 (1.4) a |
| Semi-decomposed | 3.1 (0.7) a | 3.5 (0.8) a | |
| Decomposed | 2.5 (0.7) a | 2.5 (0.7) a | |
| Sum | 9.4 (2.2) b | 12.6 (2.6) a | |
| Total biomass | 22.4 | 28.9 |
| Forest Type | Stem | Branch | Leaf | Fruit | Root |
|---|---|---|---|---|---|
| C. lanceolata | 50.6 (3.2) a | 48.2 (3.6) a | 51.4 (3.4) a | 46.9 (2.9) a | 49.6 (3.6) a |
| P. elliottii | 51.6 (3.2) a | 47.6 (3.8) a | 45.0 (2.7) b | 45.1 (2.9) b | 46.7(4.1) b |
| Forest Type | Shrub Layer | Herb Layer | Dead Wood | Litter Layer | ||
|---|---|---|---|---|---|---|
| Undecomposed | Semi-Decomposed | Decomposed | ||||
| C. lanceolata | 49.8 (3.5) a | 43.3 (2.5) a | 49.2 (2.8) a | 49.9 (2.9) a | 45.5 (1.9) a | 44.1 (2.2) a |
| P. elliottii | 49.9 (4.0) a | 43.6 (2.9) a | 50.5 (3.3) a | 44.2 (2.5) b | 42.4 (1.9) b | 40.4 (2.0) b |
| Forest Type | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | |
|---|---|---|---|---|---|
| C. lanceolata | SOC (g·kg−1) | 17.5 (1.4) b A | 12.5 (1.9) b B | 7.2 (1.3) a C | 4.3 (1.0) a D |
| BD (g·cm−3) | 1.21 (0.07) A | 1.30 (0.06) B | 1.40 (0.05) C | 1.46 (0.05) C | |
| P. elliottii | SOC (g·kg−1) | 19.4 (2.3) a A | 15.2 (1.7) a B | 7.7 (1.6) a C | 5.1 (0.9) a D |
| BD (g·cm−3) | 1.21 (0.06) A | 1.33 (0.06) B | 1.44 (0.06) C | 1.48 (0.04) C |
| Forest Type | Stem | Branch | Leaf | Fruit | Root | Total |
|---|---|---|---|---|---|---|
| C. lanceolata | 29.8 (0.9) b | 6.0 (0.2) b | 3.3 (0.1) b | 0.5 (0.1) b | 7.9 (0.3) b | 47.5 (1.5) b |
| P. elliottii | 35.2 (1.7) a | 10.2 (0.5) a | 4.0 (0.2) a | 0.8 (0.1) a | 10.6 (0.5) a | 60.8 (2.9) a |
| Layer | C. lanceolata | P. elliottii | |
|---|---|---|---|
| Shrub layer biomass | 3.9 (0.6) | 4.9 (1.0) | |
| Herb layer biomass | 1.2 (0.2) | 1.5 (0.6) | |
| Dead wood biomass | 1.2 (0.6) | 1.5 (0.5) | |
| Litter layer biomass | Undecomposed | 1.9 (0.3) b | 2.9 (0.4) a |
| Semi-decomposed | 1.4 (0.1) | 1.5 (0.2) | |
| Decomposed | 1.1 (0.2) | 1.0 (0.2) | |
| Sum | 4.4 (0.6) | 5.4 (0.8) | |
| Total biomass | 10.7 | 13.3 | |
| Forest Type | 0–20 cm | 20–40 cm | 40–60 cm | 60–80 cm | Total |
|---|---|---|---|---|---|
| C. lanceolata | 42.5 (4.9) b A | 32.6 (5.8) b B | 20.3 (3.8) a C | 12.7 (2.9) b D | 108.1 (14.8) b |
| P. elliottii | 46.8 (6.4) a A | 40.4 (5.1) a B | 22.3 (5.1) a C | 15.1 (3.0) a D | 124.6 (17.9) a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Chen, J.; He, J.; Li, J.; Li, J.; Kang, W. Biomass Models and Ecosystem Carbon Density: A Case Study of Two Coniferous Forest in Northern Hunan, China. Forests 2023, 14, 814. https://doi.org/10.3390/f14040814
Luo H, Chen J, He J, Li J, Li J, Kang W. Biomass Models and Ecosystem Carbon Density: A Case Study of Two Coniferous Forest in Northern Hunan, China. Forests. 2023; 14(4):814. https://doi.org/10.3390/f14040814
Chicago/Turabian StyleLuo, Hang, Jiao Chen, Jienan He, Jianjun Li, Jianan Li, and Wenxing Kang. 2023. "Biomass Models and Ecosystem Carbon Density: A Case Study of Two Coniferous Forest in Northern Hunan, China" Forests 14, no. 4: 814. https://doi.org/10.3390/f14040814
APA StyleLuo, H., Chen, J., He, J., Li, J., Li, J., & Kang, W. (2023). Biomass Models and Ecosystem Carbon Density: A Case Study of Two Coniferous Forest in Northern Hunan, China. Forests, 14(4), 814. https://doi.org/10.3390/f14040814






