Comparative Chloroplast Genome Analysis of Chinese Lacquer Tree (Toxicodendron vernicifluum, Anacardiaceae): East-West Divergence within Its Range in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling, DNA Extraction, and Sequencing
2.2. Chloroplast Genome Assembly and Annotation
2.3. Phylogenetic Analyses and Divergence Time Estimation Based on Chloroplast Genome Sequences
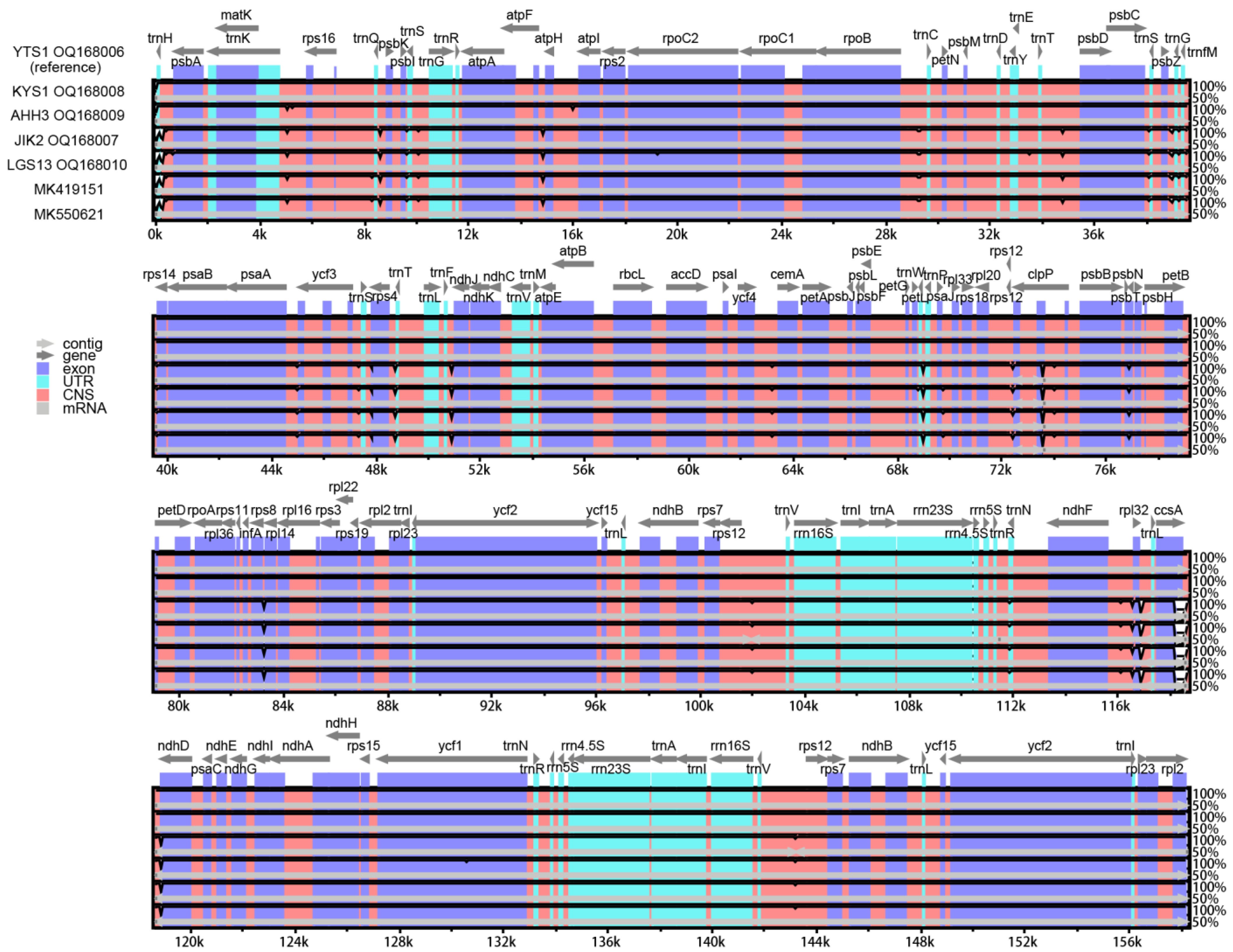
2.4. Comparative Chloroplast Genome Analyses
2.5. Repeat Sequence Identification
2.6. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Variation
3. Results and Discussion
3.1. Chloroplast Genome Assembly and Annotation
3.2. Phylogenetic Analyses Based on Chloroplast Genome Sequences
3.3. Divergence Time Estimation
3.4. Chloroplast Genome Comparison among the Accessions of T. vernicifluum, Toxicodendron, and the Tribe Rhoeae
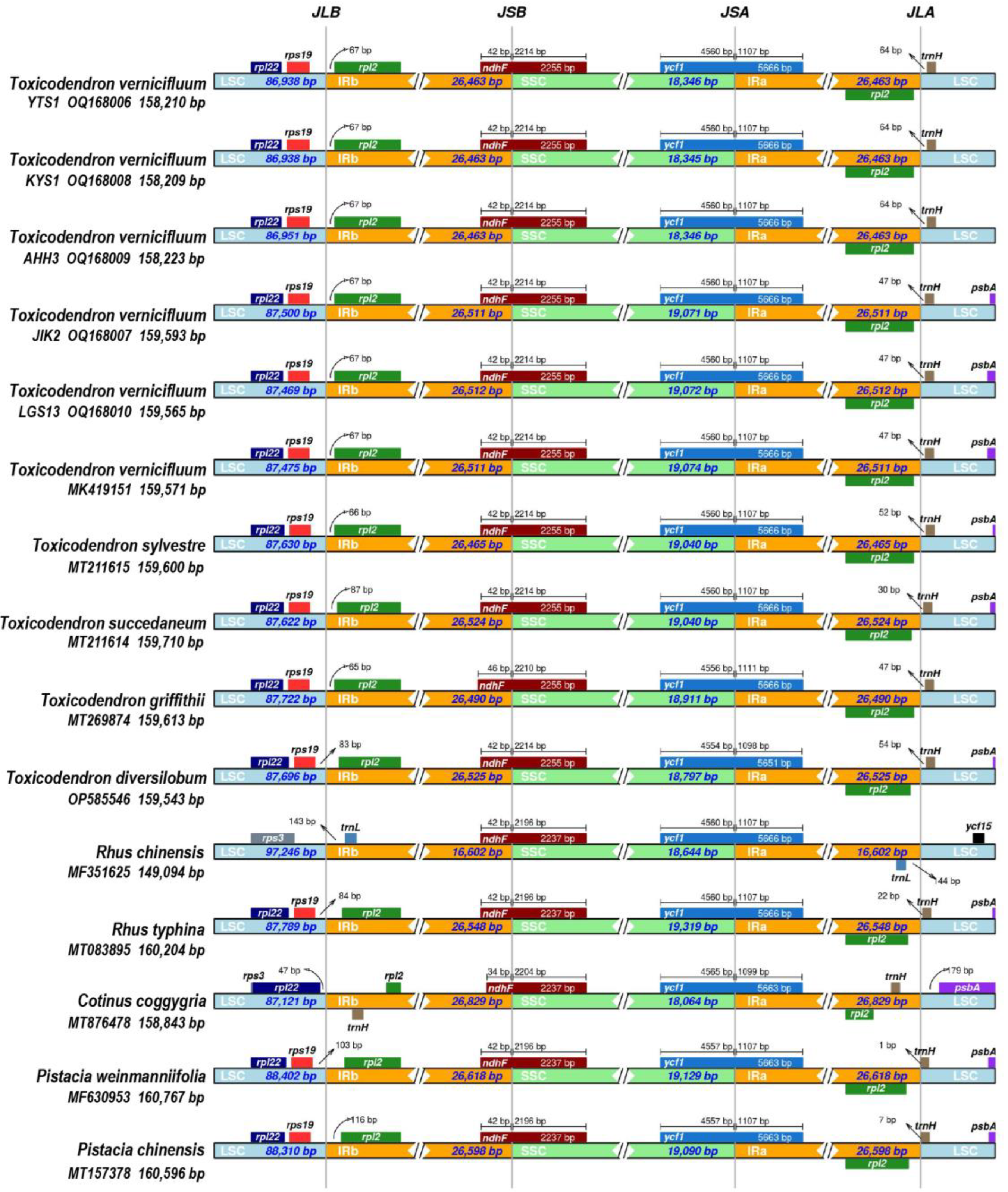
3.5. Expansion and Contraction of the IR Regions
3.6. Repeat Sequence Identification
3.7. Nuclear Ribosomal Internal Transcribed Spacer (ITS) Variation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suzuki, M.; Noshiro, S.; Tanaka, T. Origin of urushi (Toxicodendron vernicifluum) in the neolithic Jomon period of Japan. Bull. Nat. Mus. Jpn. Hist. 2014, 187, 49–70. [Google Scholar]
- Zhao, M.; Liu, C.; Zheng, G. Comparative studies of bark structure, lacquer yield and urushiol content of cultivated Toxicodendron vernicifluum varieties. New. Zeal. J. Bot. 2013, 51, 13. [Google Scholar] [CrossRef]
- Hong, D.H.; Han, S.B.; Lee, C.W.; Park, S.H.; Jeon, Y.J.; Kim, M.J.; Kwak, S.S.; Kim, H.M. Cytotoxicity of urushiols isolated from sap of Korean lacquer tree (Rhus vernicifera Stokes). Arch. Pharm. Res. 1999, 22, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.; Choi, J.H.; Yang, H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012, 50, 1940–1945. [Google Scholar] [CrossRef]
- Suzuki, M.; Yonekura, K.; Noshiro, S. Distribution and habitat of Toxicodendron vernicifluum (Stokes) F. A. Barkl. (Anacardiaceae) in China. Jpn. J. Histor. Bot. 2007, 15, 58–62. [Google Scholar]
- Noshiro, S.; Suzuki, M. Rhus verniciflua Stokes grew in Japan since the Early Jomon Period. Jpn. J. Histor. Bot. 2004, 12, 3–11. [Google Scholar]
- Wang, L.; Li, Y.; Noshiro, S.; Suzuki, M.; Arai, T.; Kobayashi, K.; Xie, L.; Zhang, M.Y.; He, N.; Fang, Y.M.; et al. Stepped geomorphology shaped the phylogeographic structure of a widespread tree species (Toxicodendron vernicifluum, Anacardiaceae) in East Asia. Front. Plant Sci. 2022, 13, 920054. [Google Scholar] [CrossRef]
- Moore, M.J.; Soltis, P.S.; Bell, C.D.; Burleigh, J.G.; Soltis, D.E. Phylogenetic analysis of 83 plastid genes further resolves the early diversification of eudicots. Proc. Natl. Acad. Sci. USA 2010, 107, 4623–4628. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; He, P.; Li, P.; Lee, J.; Soltis, D.E. Chloroplast genome analyses and genomic resource development for epilithic sister genera Oresitrophe and Mukdenia (Saxifragaceae), using genome skimming data. BMC Genom. 2018, 19, 235. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [PubMed]
- Birky, C.W. Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc. Natl. Acad. Sci. USA 1995, 92, 11331–11338. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, C.; Cheng, T.; Lin, K.; Zhou, S. Sequencing angiosperm plastid genomes made easy: A complete set of universal primers and a case study on the phylogeny of Saxifragales. Genome Biol. Evol. 2013, 5, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Sajjadian, S.; Eichler, E.E. Limitations of next-generation genome sequence assembly. Nat. Methods 2011, 8, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, F.; Li, Z.; Xu, Q.; Chen, Y.; Yu, Z.; Chen, N.S. Development of a high-resolution molecular marker for tracking Phaeocystis globosa genetic diversity through comparative analysis of chloroplast genomes. Harmful Algae 2020, 99, 101911. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Lin, C.; Lee, S.Y.; Mao, L.; Meng, K. Comparative analysis of complete Ilex (Aquifoliaceae) chloroplast genomes: Insights into evolutionary dynamics and phylogenetic relationships. BMC Genom. 2022, 23, 203. [Google Scholar] [CrossRef]
- Huang, D.; Hefer, C.; Kolosova, N.; Douglas, C.; Cronk, Q. Whole plastome sequencing reveals deep plastid divergence and cytonuclear discordance between closely related balsam poplars, Populus balsamifera and P. trichocarpa (Salicaceae). New Phytol. 2014, 204, 693–703. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, X.; Kang, H.; Liu, C.; Mao, L.; Fang, Y. Extensive sharing of chloroplast haplotypes among East Asian Cerris oaks: The imprints of shared ancestral polymorphism and introgression. Ecol. Evol. 2022, 12, e9142. [Google Scholar] [CrossRef]
- Fahey, P.S.; Fowler, R.M.; Udovicic, F.; Cantrill, D.J.; Bayly, M.J. Use of plastid genome sequences in phylogeographic studies of tree species can be misleading without comprehensive sampling of co-occurring, related species. Tree Genet. Genomes 2021, 17, 43. [Google Scholar] [CrossRef]
- Min, T.; Barfod, A. Anacardiaceae. In Flora of China; Wu, Z., Raven, P.H., Hong, D., Eds.; Science Press: Beijing, China; Missouri Botanical Garden Press: St. Louis, MA, USA, 2008; Volume 11, pp. 335–357. [Google Scholar]
- Wang, L.; He, N.; Li, Y.; Fang, Y.; Zhang, F. The complete chloroplast genome sequence of Toxicodendron succedaneum (Anacardiaceae). Mitochondrial DNA Part B 2020, 5, 1956–1957. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Y.; Wu, T. The complete chloroplast genome of Toxicodendron griffithii. Mitochondrial DNA Part B 2020, 5, 2211–2212. [Google Scholar] [CrossRef]
- Weisberg, A.J.; Kim, G.; Westwood, J.H.; Jelesko, J.G. Sequencing and de novo assembly of the Toxicodendron radicans (poison ivy) transcriptome. Genes 2017, 8, 317. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, N.; Zhao, S.; Li, J.; Pei, X.; Yu, J.; Guo, D. The complete plastid genome of Cotinus coggygria and phylogenetic analysis of the Anacardiaceae. Genet. Mol. Biol. 2021, 44, 3. [Google Scholar] [CrossRef] [PubMed]
- Rossini, B.C.; de Moraes, M.L.T.; Marino, C.L. Complete chloroplast genome of Myracrodruon urundeuva and its phylogenetics relationships in Anacardiaceae family. Physiol. Mol. Biol. Plants 2021, 27, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Zarei, A.; Ebrahimi, A.; Mathur, S.; Lawson, S. The first complete chloroplast genome sequence and phylogenetic analysis of pistachio (Pistacia vera). Diversity 2022, 14, 577. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Ren, Z. Complete chloroplast genome of Pistacia chinensis Bunge (Anacardiaceae: Rhoideae), an important economical and ornamental plant. Mitochondrial DNA Part B 2020, 5, 1931–1932. [Google Scholar] [CrossRef]
- Xu, Y.; Wen, J.; Su, X.; Ren, Z. Variation among the complete chloroplast genomes of the sumac species Rhus chinensis: Reannotation and comparative analysis. Genes 2022, 13, 1936. [Google Scholar] [CrossRef]
- Barrett, C.F. Plastid genomes of the North American Rhus integrifolia-ovata complex and phylogenomic implications of inverted repeat structural evolution in Rhus L. PeerJ 2020, 8, e9315. [Google Scholar] [CrossRef]
- Zong, D.; Qiao, Z.; Zhou, J.; Li, P.; Gan, P.; Ren, M.; He, C. Chloroplast genome sequence of triploid Toxicodendron vernicifluum and comparative analyses with other lacquer chloroplast genomes. BMC Genom. 2023, 24, 56. [Google Scholar] [CrossRef]
- Doyle, J.J. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Chen, Y.; Chen, Y.; Shi, C.; Huang, Z.; Zhang, Y.; Li, S.; Chen, Q. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2018, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; de Pamphilis, W.C.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef]
- Wick, R.R.; Schultz, M.B.; Zobel, J.; Holt, K.E. Bandage: Interactive visualization of de novo genome assemblies. Bioinformatics 2015, 31, 3350–3352. [Google Scholar] [CrossRef]
- Liu, C.; Shi, L.; Zhu, Y.; Chen, H.; Zhang, J.; Lin, X.; Guan, X. CpGAVAS, an integrated web server for the annotation, visualization, analysis, and GenBank submission of completely sequenced chloroplast genome sequences. BMC Genom. 2012, 13, 715. [Google Scholar] [CrossRef]
- Liu, S.; Ni, Y.; Li, J.; Zhang, X.; Yang, H.; Chen, H.; Liu, C. CPGView: A package for visualizing detailed chloroplast genome structures. Mol. Ecol. Resour. 2023, 23, 694–704. [Google Scholar] [CrossRef]
- Nie, Z.L.; Sun, H.; Meng, Y.; Wen, J. Phylogenetic analysis of Toxicodendron (Anacardiaceae) and its biogeographic implications on the evolution of north temperate and tropical intercontinental disjunctions. J. Syst Evol. 2009, 47, 416–430. [Google Scholar] [CrossRef]
- Angiosperm Phylogeny Group; Chase, M.W.; Christenhusz, M.J.; Fay, M.F.; Byng, J.W.; Judd, W.S.; Stevens, P.F. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetic studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; Haeseler, A.V.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Drummond, A.J. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, M.; Meng, Y.; Wen, J.; Ge, X.J.; Nie, Z.L. The importance of the North Atlantic land bridges and eastern Asia in the post-Boreotropical biogeography of the Northern Hemisphere as revealed from the poison ivy genus (Toxicodendron, Anacardiaceae). Mol. Phylogenet. Evol. 2019, 139, 106561. [Google Scholar] [CrossRef]
- Manchester, S.R. Fruits and seeds of the middle Eocene Nut Beds flora, Clarno Formation, Oregon. Palaeontogr. Am. 1994, 58, 1–205. [Google Scholar]
- MacGinitie, H.D. Fossil Plants of the Florissant Beds; Carnegie Institute: Washington, DC, USA, 1953. [Google Scholar]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarisation in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E.; Sánchez-Gracia, A. DnaSP 6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef] [PubMed]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, 273–279. [Google Scholar] [CrossRef]
- Darling, A.E.; Mau, B.; Perna, N.T. progressiveMauve: Multiple genome alignment with gene gain, loss and rearrangement. PLoS ONE 2010, 5, e11147. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, S.; Choudhuri, J.V.; Ohlebusch, E.; Schleiermacher, C.; Stoye, J.; Giegerich, R. REPuter: The manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res. 2001, 29, 4633–4642. [Google Scholar] [CrossRef]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef]
- Beier, S.; Thiel, T.; Münch, T.; Scholz, U.; Mascher, M. MISA-web: A web server for microsatellite prediction. Bioinformatics 2017, 33, 2583–2585. [Google Scholar] [CrossRef]
- Thiel, T.; Michalek, W.; Varshney, R.; Graner, A. Exploiting EST databases for the development and characterization of gene-derived SSR-markers in barley (Hordeum vulgare L.). Theor. Appl. Genet. 2003, 106, 411–422. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Wang, L.; He, N.; Li, Y.; Fang, Y.M.; Zhang, F.L. Complete chloroplast genome sequence of Chinese lacquer tree (Toxicodendron vernicifluum, Anacardiaceae) and its phylogenetic significance. BioMed Res. Int. 2020, 2020, 9014873. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Zong, D.; Zhou, A.; He, X.F.; He, C.Z. The complete chloroplast genome of the Toxicodendron vernicifluum cv. Dahongpao, an elite natural triploid lacquer tree. Mitochondrial DNA B 2019, 4, 1227–1228. [Google Scholar] [CrossRef]
- He, N.; Wang, L.; Li, Y.; Fang, Y.; Zhang, F. The complete chloroplast genome sequence of Toxicodendron sylvestre (Anacardiaceae). Mitochondrial DNA Part B 2020, 5, 2008–2009. [Google Scholar] [CrossRef]
- Wicke, S.; Schneeweiss, G.M.; dePamphilis, C.W.; Müller, K.F.; Quandt, D. The evolution of the plastid chromosome in land plants: Gene content, gene order, gene function. Plant Mol. Biol. 2011, 76, 273–297. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, N.; Wolf, E.M.; Rigault, P.; Zhou, W.; Kiefer, M.; Zhao, Y.; Koch, M.A. Ginkgo biloba’s footprint of dynamic Pleistocene history dates back only 390,000 years ago. BMC Genom. 2018, 9, 299. [Google Scholar] [CrossRef]
- Renoult, J.P.; Kjellberg, F.; Grout, C.; Santoni, S.; Khadari, B. Cyto-nuclear discordance in the phylogeny of Ficus section Galoglychia and host shifts in plant-pollinator associations. BMC Evol. Biol. 2009, 9, 248. [Google Scholar] [CrossRef]
- Luo, D.; Xu, B.; Li, Z.M.; Sun, H. Biogeographical divides delineated by the three-step landforms of China and the East China Sea: Insights from the phylogeography of Kerria japonica. J. Biogeogr. 2021, 48, 372–385. [Google Scholar] [CrossRef]
- Wang, W.; Chen, S.; Zhang, X. Whole-genome comparison reveals heterogeneous divergence and mutation hotspots in chloroplast genome of Eucommia ulmoides Oliver. Int. J. Mol. Sci. 2018, 19, 1037. [Google Scholar] [CrossRef]
- Park, I.; Yang, S.; Kim, W. Sequencing and comparative analysis of the chloroplast genome of Angelica polymorpha and the development of a novel indel marker for species identification. Molecules 2019, 24, 1038. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, J.; Yang, Y. Phylogenomic analysis and dynamic evolution of chloroplast genomes in Salicaceae. Front. Plant Sci. 2017, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kong, H.; Zhou, J. Complete chloroplast genome of Cercis chuniana (Fabaceae) with structural and genetic comparison to six species in Caesalpinioideae. Int. J. Mol. Sci. 2018, 19, 1286. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Guo, W.; Gupta, S. Evolutionary dynamics of the plastid inverted repeat: The effects of expansion, contraction, and loss on substitution rates. New Phytol. 2016, 209, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Weng, M.L.; Blazier, J.C.; Govindu, M.; Jansen, R.K. Reconstruction of the ancestral plastid genome in Geraniaceae reveals a correlation between genome rearrangements, repeats, and nucleotide substitution rates. Mol. Biol. Evol. 2014, 31, 645–659. [Google Scholar] [CrossRef] [PubMed]
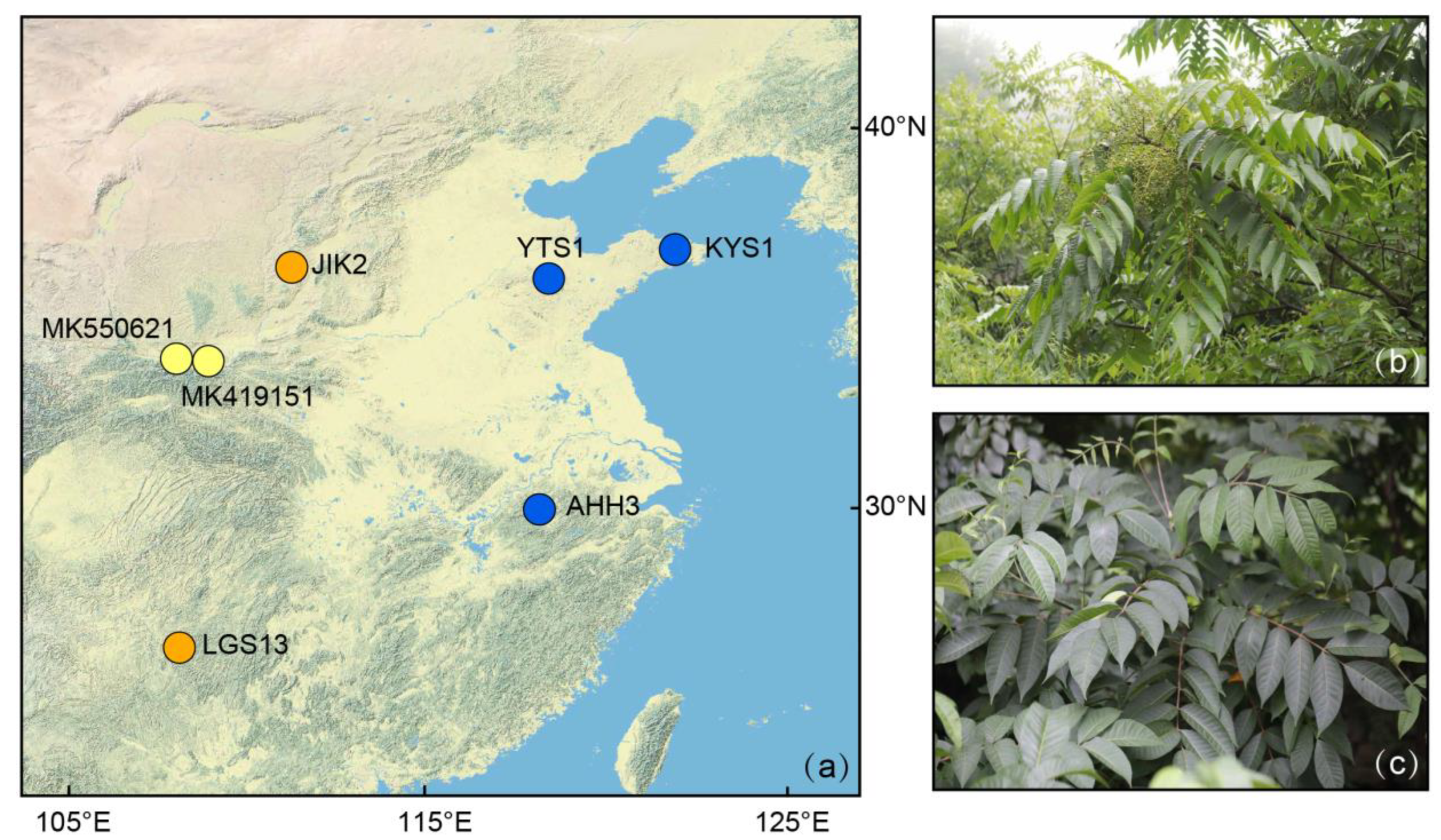

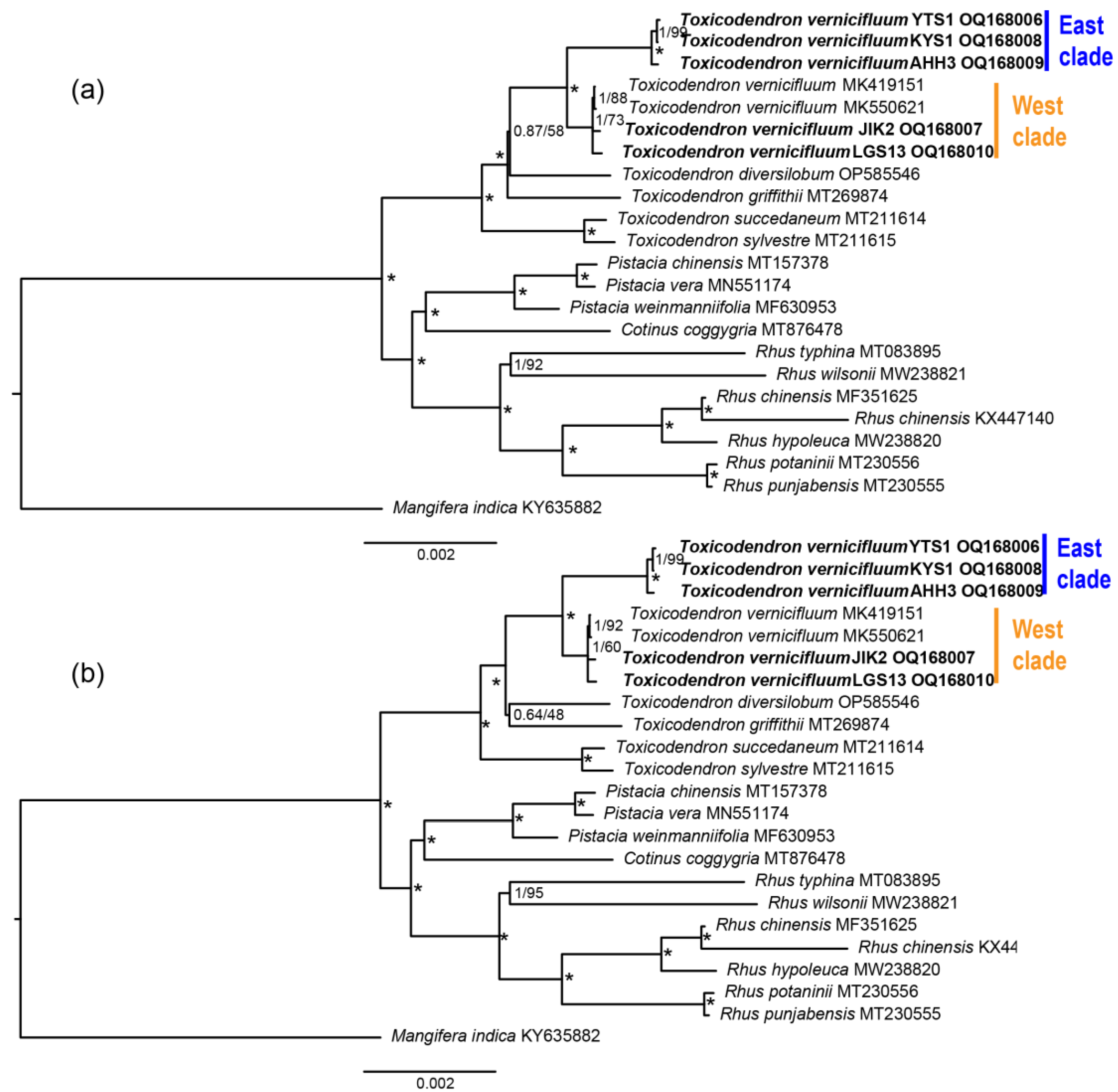

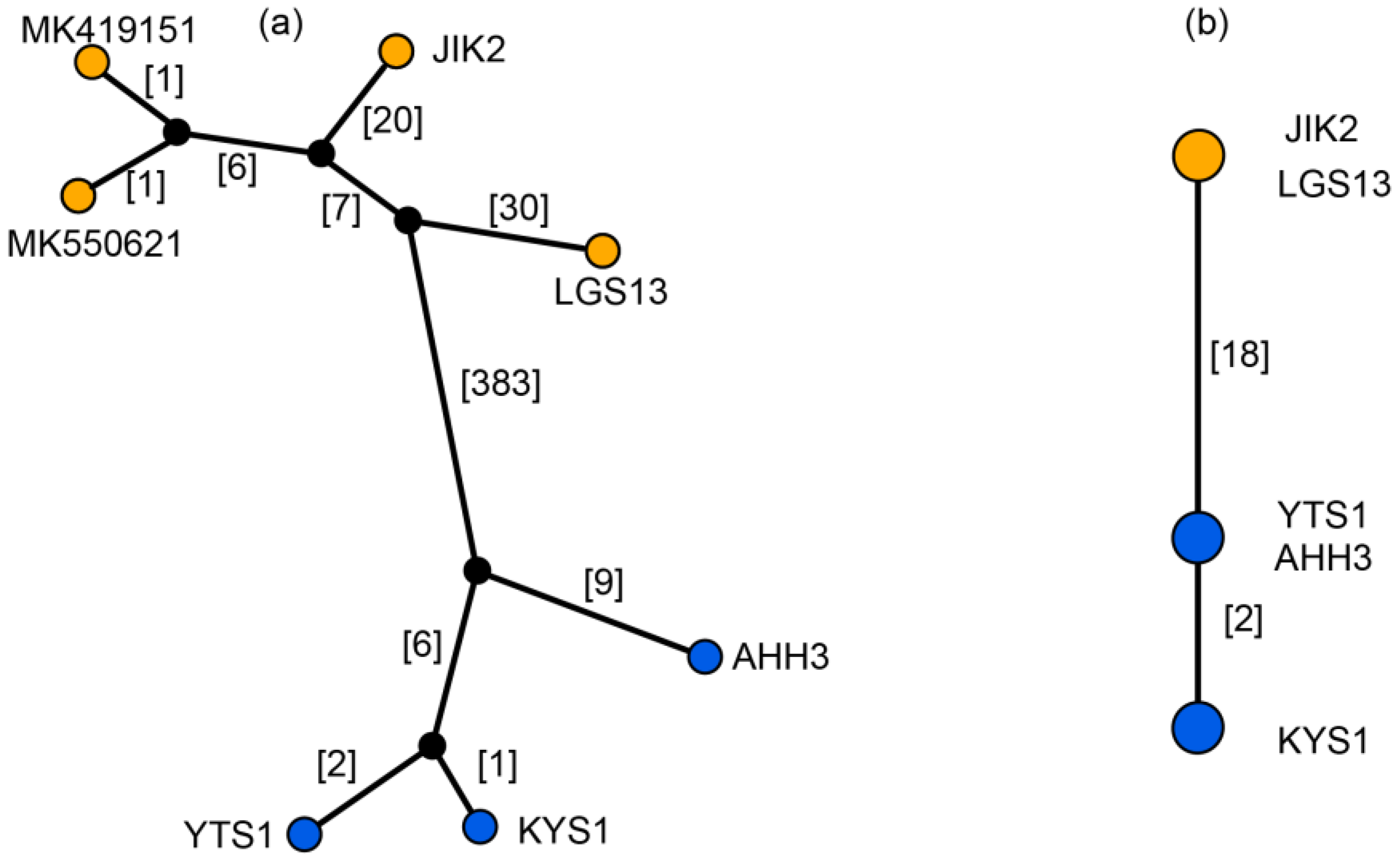
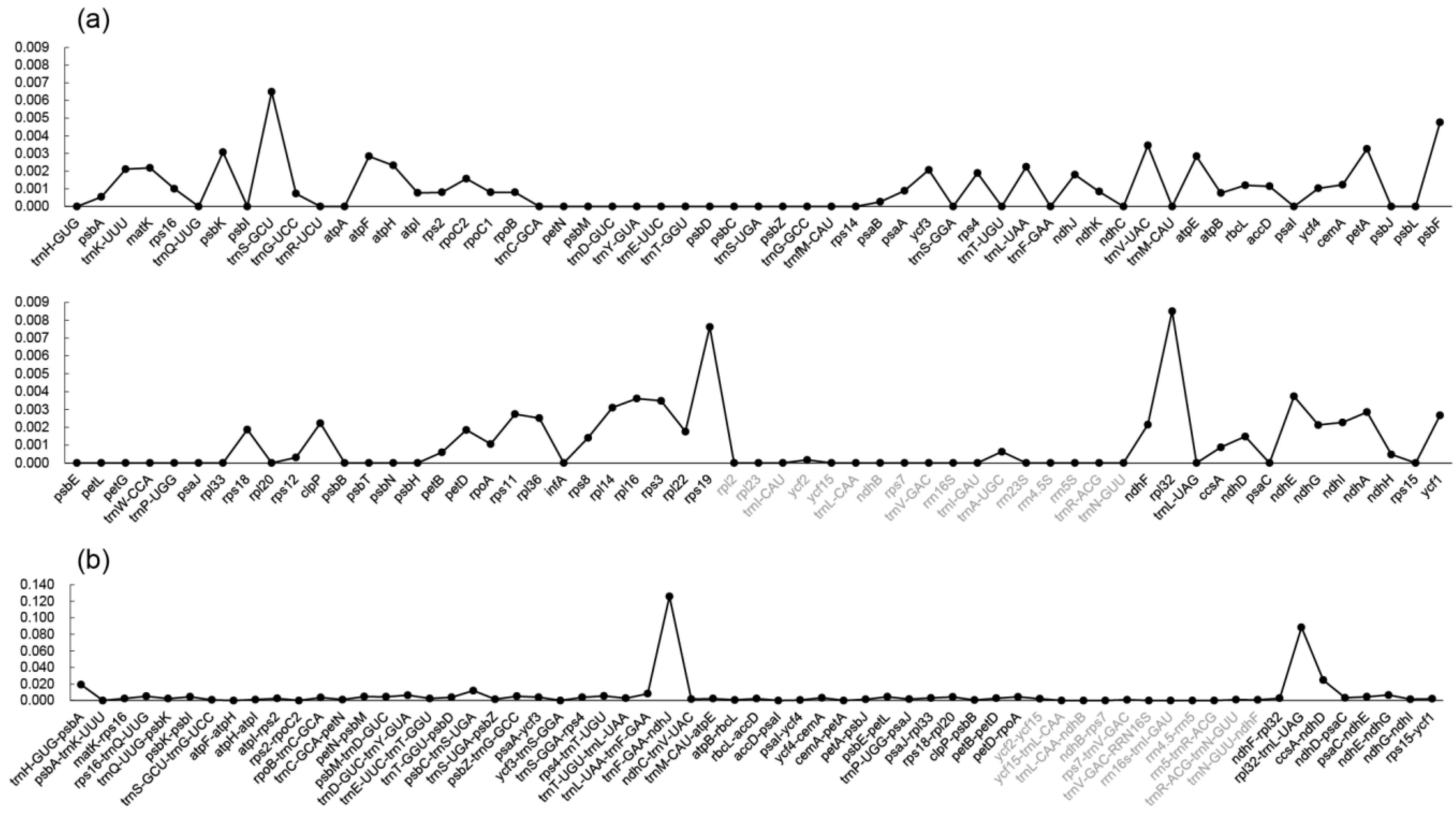
| Species | Sources (Voucher Number) | GenBank acc. # | Length (bp) | GC (%) | Gene Number | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plastome | IRa/IRb | SSC | LSC | Full | PCG | tRNA | rRNA | ||||
| T. vernicifluum | Qingzhou, Shandong, China (YTS1) | OQ168006 * | 158,210 | 26,464 | 18,345 | 86,937 | 38.0 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Yantai, Shandong, China (KYS1) | OQ168008 * | 158,209 | 26,464 | 18,344 | 86,937 | 38.0 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Huangshan, Anhui, China (AHH3) | OQ168009 * | 158,223 | 26,464 | 18,345 | 86,950 | 38.0 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Jiaokou, Shanxi, China (JIK2) | OQ168007 * | 159,593 | 26,512 | 19,070 | 87,499 | 37.9 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Leishan, Guizhou, China (LGS13) | OQ168010 * | 159,565 | 26,513 | 19,071 | 87,468 | 38.0 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Xi’an, Shaanxi, China | MK419151 | 159,571 | 26,512 | 19,073 | 87,474 | 38.0 | 132 | 87 | 37 | 8 |
| T. vernicifluum | Yangling, Shaanxi, China | MK550621 | 159,571 | 26,512 | 19,073 | 87,474 | 38.0 | 132 | 87 | 37 | 8 |
| T. sylvestre | Lin’an, Zhejiang, China | MT211615 | 159,600 | 26,466 | 19,039 | 87,629 | 37.9 | 132 | 87 | 37 | 8 |
| T. succedaneum | Chishui, Guizhou, China | MT211614 | 159,710 | 26,525 | 19,039 | 87,621 | 37.9 | 132 | 87 | 37 | 8 |
| T. griffithii | Chenggan, Yunnan, China | MT269874 | 159,613 | 26,491 | 18,910 | 87,721 | 37.9 | 132 | 87 | 37 | 8 |
| T. diversilobum | Washington Park, CA, USA | OP585546 | 159,543 | 26,526 | 18,796 | 87,695 | 38.0 | 132 | 87 | 37 | 8 |
| R. chinensis | Shandong or Henan, China | MF351625 | 149,094 | 16,603 | 18,643 | 97,245 | 37.9 | 125 | 81 | 36 | 8 |
| R. chinensis | Yanggu, Gangwon, South Korea | KX447140 | 149,011 | 16,742 | 18,646 | 96,881 | 37.8 | 125 | 81 | 36 | 8 |
| R. hypoleuca | Unknown | MW238820 | 159,472 | 26,561 | 18,780 | 87,570 | 37.9 | 131 | 86 | 37 | 8 |
| R. potaninii | Danfeng, Shaanxi, China | MT230556 | 159,620 | 26,476 | 18,947 | 87,721 | 37.9 | 132 | 87 | 37 | 8 |
| R. punjabensis | Enshi, Hubei, China | MT230555 | 159,617 | 26,477 | 18,970 | 87,693 | 37.9 | 132 | 87 | 37 | 8 |
| R. typhina | Mts. Lushan, Shandong, China | MT083895 | 160,204 | 26,549 | 19,318 | 87,788 | 37.8 | 132 | 87 | 37 | 8 |
| R. wilsonii | Unknown | MW238821 | 159,814 | 26,528 | 18,842 | 87,916 | 37.8 | 132 | 87 | 37 | 8 |
| C. coggygria | Mts. Jinyun, Chongqing, China | MT876478 | 158,843 | 26,830 | 18,063 | 87,120 | 38.0 | 131 | 85 | 38 | 8 |
| P. weinmanniifolia | Unknown | MF630953 | 160,767 | 26,619 | 19,128 | 88,401 | 37.8 | 132 | 87 | 37 | 8 |
| P. chinensis | Bazhong, Sichuan, China | MT157378 | 160,596 | 26,599 | 19,089 | 88,309 | 37.9 | 132 | 87 | 37 | 8 |
| P. vera | Longnan, Gansu, China | MN551174 | 160,654 | 26,597 | 19,085 | 88,375 | 37.9 | 132 | 87 | 37 | 8 |
| Index | Eastern Group | Western Group | All Samples |
|---|---|---|---|
| Number of samples | 3 | 4 | 7 |
| Aligned length (bp) | 158,244 | 159,608 | 159,932 |
| Total numbers of SNPs and indels | 18/20 | 68/17 | 466/141 |
| Numbers of SNPs and indels in LSC | 11/17 | 50/13 | 331/109 |
| Numbers of SNPs and indels in SSC | 7/3 | 16/2 | 111/20 |
| Numbers of SNPs and indels in IRs | 0/0 | 2/2 | 24/12 |
| Numbers of SNPs and indels in PCGs | 14/0 | 19/0 | 150/39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Li, Y.; He, N.; Peng, Y.; Fang, Y.; Zhang, X.; Zhang, F. Comparative Chloroplast Genome Analysis of Chinese Lacquer Tree (Toxicodendron vernicifluum, Anacardiaceae): East-West Divergence within Its Range in China. Forests 2023, 14, 818. https://doi.org/10.3390/f14040818
Wang L, Li Y, He N, Peng Y, Fang Y, Zhang X, Zhang F. Comparative Chloroplast Genome Analysis of Chinese Lacquer Tree (Toxicodendron vernicifluum, Anacardiaceae): East-West Divergence within Its Range in China. Forests. 2023; 14(4):818. https://doi.org/10.3390/f14040818
Chicago/Turabian StyleWang, Lu, Yao Li, Na He, Ye Peng, Yanming Fang, Xingwang Zhang, and Feilong Zhang. 2023. "Comparative Chloroplast Genome Analysis of Chinese Lacquer Tree (Toxicodendron vernicifluum, Anacardiaceae): East-West Divergence within Its Range in China" Forests 14, no. 4: 818. https://doi.org/10.3390/f14040818
APA StyleWang, L., Li, Y., He, N., Peng, Y., Fang, Y., Zhang, X., & Zhang, F. (2023). Comparative Chloroplast Genome Analysis of Chinese Lacquer Tree (Toxicodendron vernicifluum, Anacardiaceae): East-West Divergence within Its Range in China. Forests, 14(4), 818. https://doi.org/10.3390/f14040818





