Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Soil Sampling and Chemical Properties Analysis
2.4. Determination of Soil Microbial Respiration
2.5. Soil DNA Extraction and Quantitative Real-Time PCR
2.6. Soil Enzyme Activity Measurement and Their Stoichiometry Analysis
2.7. Statistical Analysis
3. Results
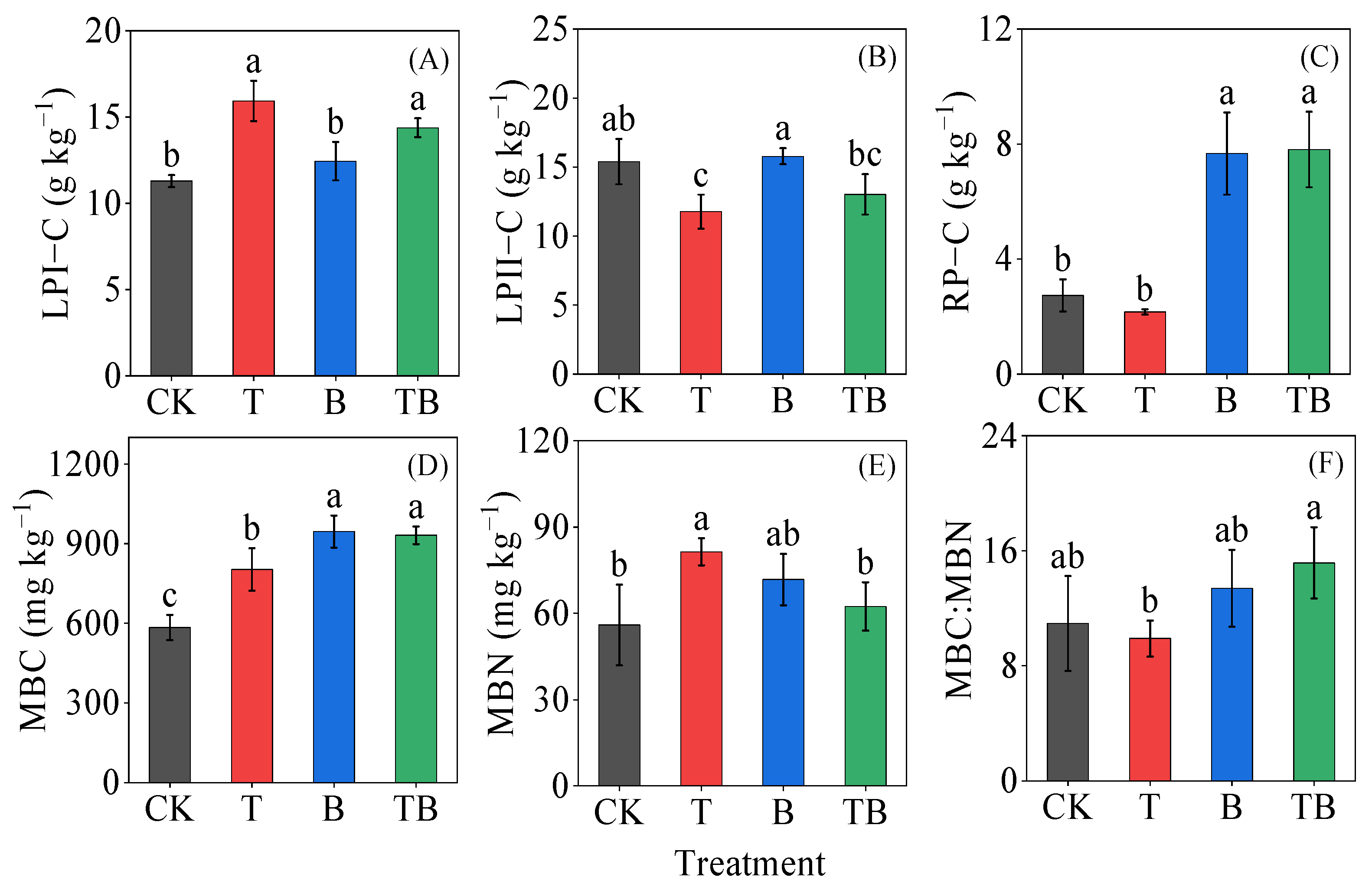
3.1. Soil Chemical Properties
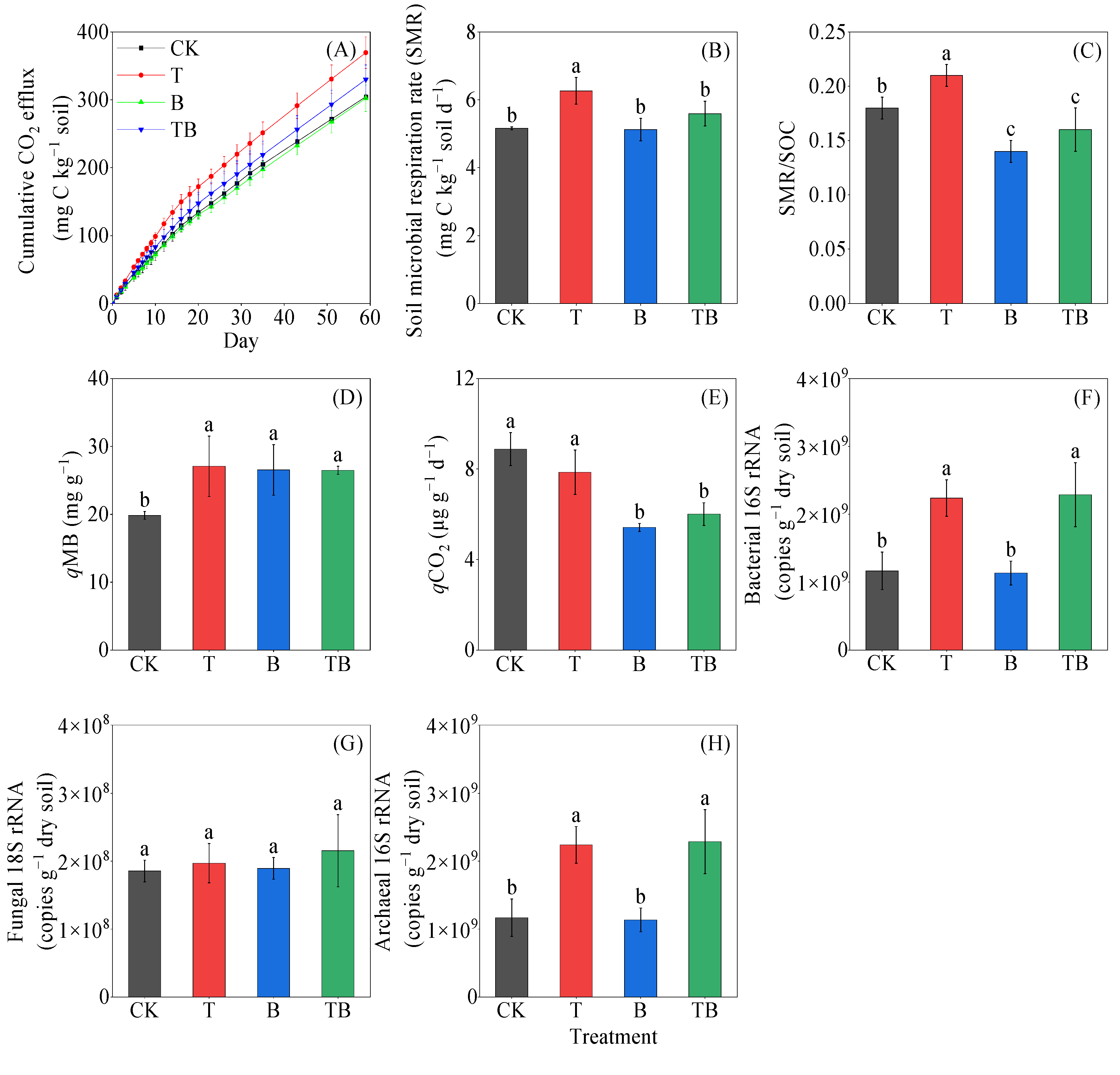
3.2. Soil Microbial Respiration, Microbial Metabolic Quotient and Microbial Biomass
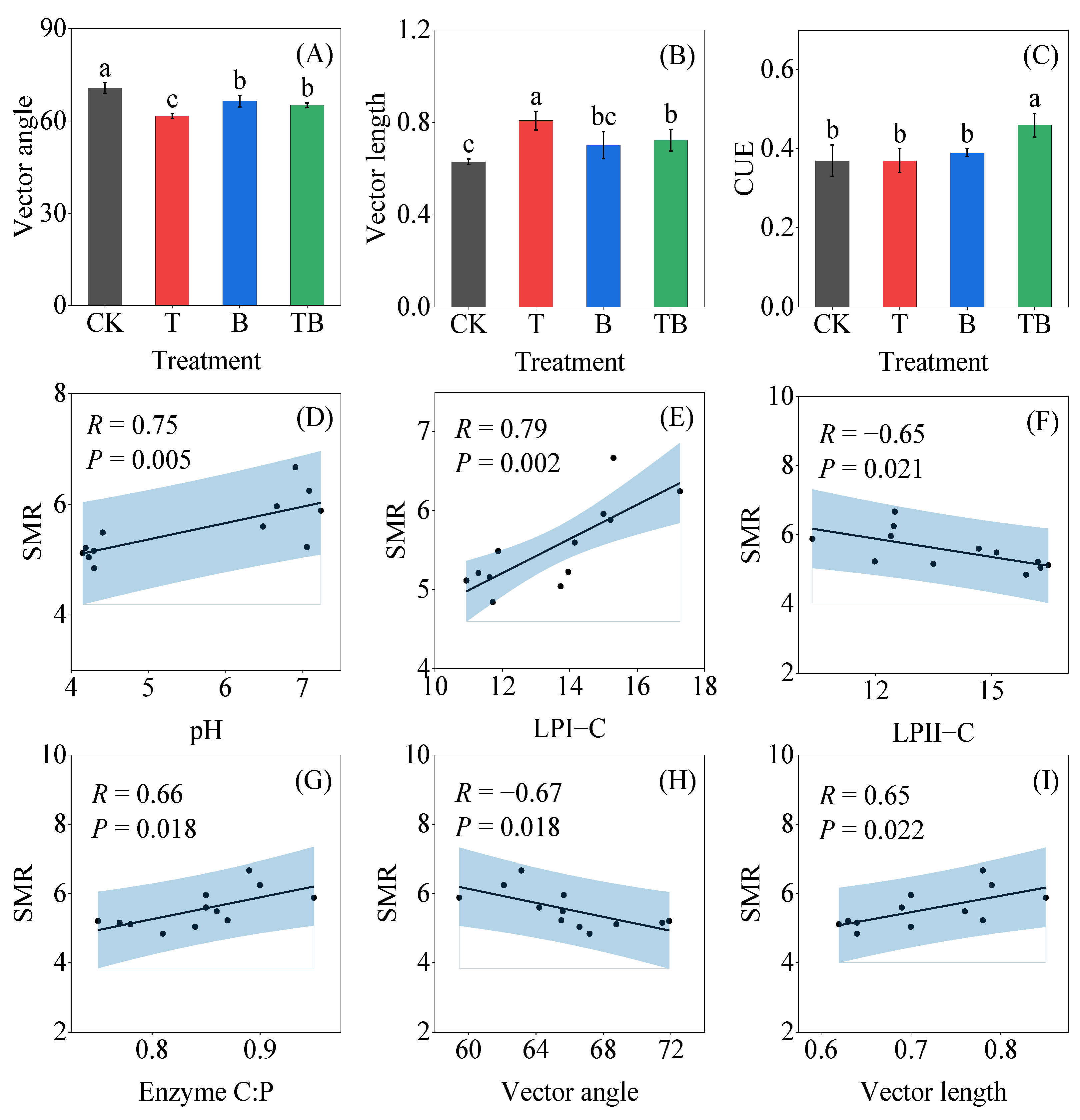
3.3. Soil Enzyme Activity and Stoichiometry
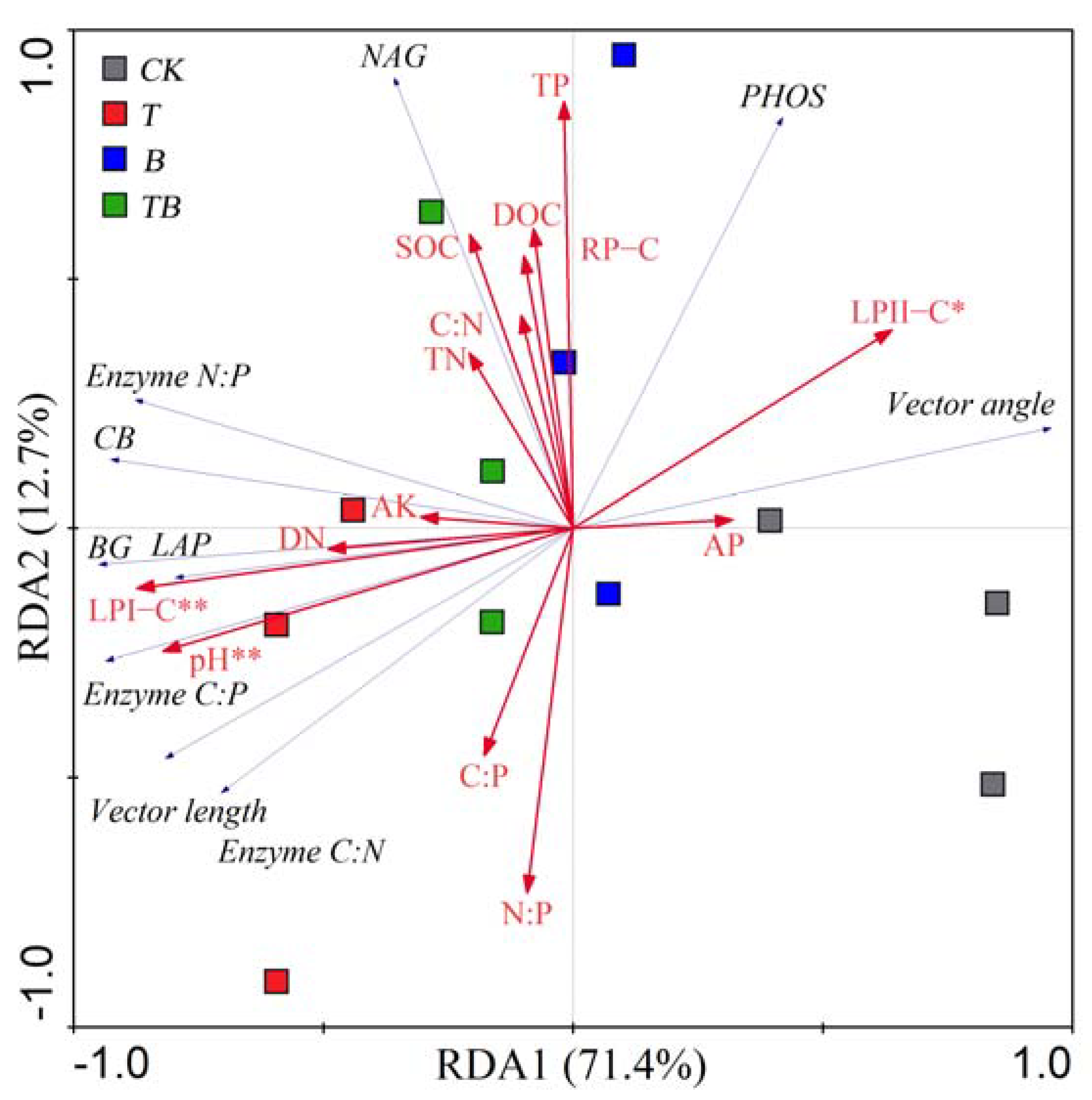
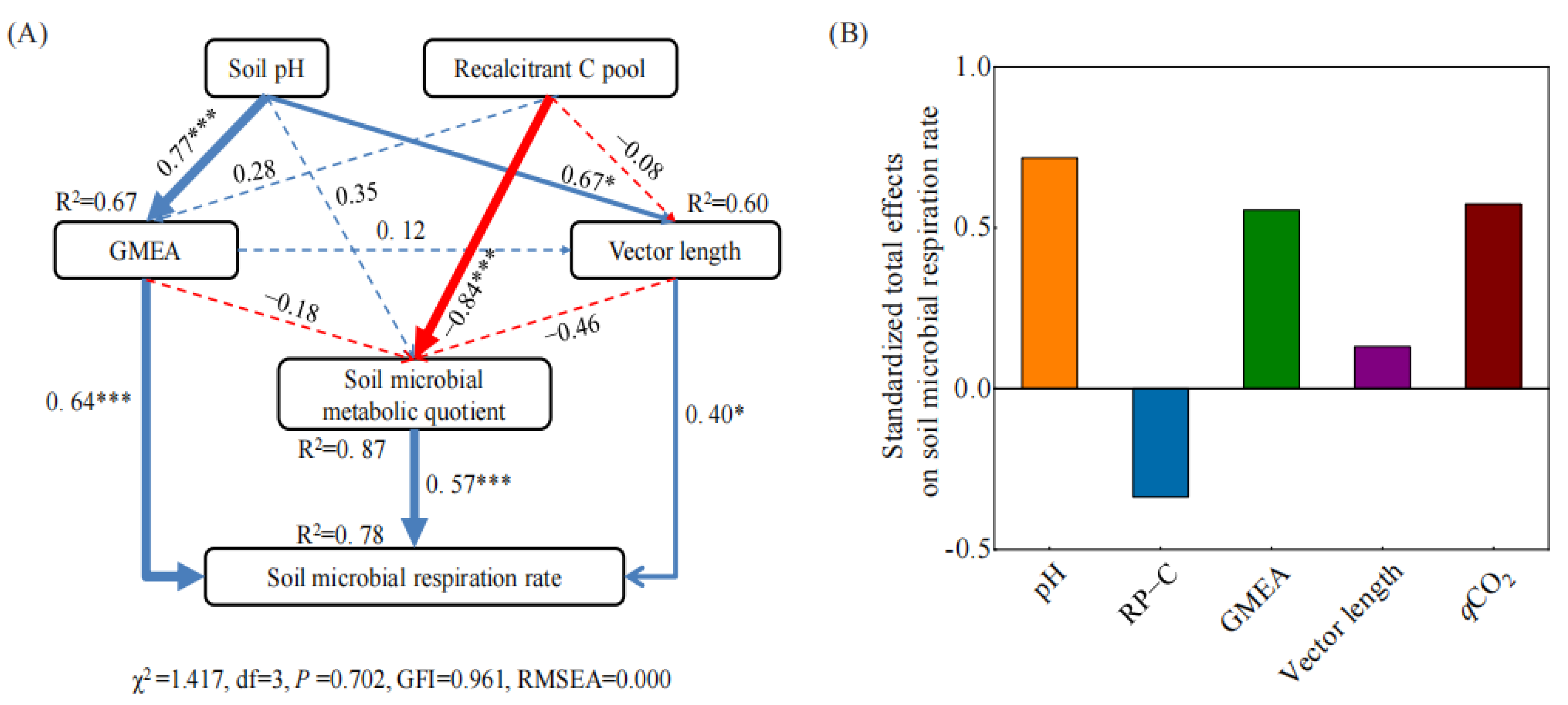
3.4. Factors Affecting Soil Enzyme Stoichiometry and Microbial Respiration Rate
4. Discussion
4.1. Biochar Combined with Oyster Shell Powder Attenuated the Stimulation of SOC Decomposition by Oyster Shell Powder Alone and Increased Soil C Recalcitrancy
4.2. Biochar Combined with Oyster Shell Powder Decreased the C-Degrading Enzyme Activity Relative to Oyster Shell Powder Alone
4.3. The Role of Soil pH and C Availability on Soil Enzyme Activity and Microbial CUE under Biochar and Oyster Shell Powder Addition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bonan, G.B. Forests and Climate Change: Forcings, Feedbacks, and the Climate Benefits of Forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, G.; Wang, E. Global subsoil organic carbon turnover times dominantly controlled by soil properties rather than climate. Nat. Commun. 2019, 10, 3688. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Liang, C.; Chen, J.; Li, Y.; Qin, H.; Fuhrmann, J.J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Qin, H.; Wang, H.; Strong, S.P.; Li, Y.; Xu, Q.; Wu, Q. Rapid soil fungal community response to intensive management in a bamboo forest developed from rice paddies. Soil Biol. Biochem. 2014, 68, 177–184. [Google Scholar] [CrossRef]
- Guo, K.; Zhao, Y.; Liu, Y.; Chen, J.; Wu, Q.; Ruan, Y.; Li, S.; Shi, J.; Zhao, L.; Sun, X.; et al. Pyrolysis temperature of biochar affects ecoenzymatic stoichiometry and microbial nutrient-use efficiency in a bamboo forest soil. Geoderma 2020, 363, 114162. [Google Scholar] [CrossRef]
- Dai, Z.; Zhang, X.; Tang, C.; Muhammad, N.; Wu, J.; Brookes, P.C.; Xu, J. Potential role of biochars in decreasing soil acidification-a critical review. Sci. Total Environ. 2016, 581–582, 601–611. [Google Scholar] [CrossRef]
- Li, G.; Kim, S.; Han, S.; Chang, H.; Du, D.; Son, Y. Precipitation affects soil microbial and extracellular enzymatic responses to warming. Soil Biol. Biochem. 2018, 120, 212–221. [Google Scholar] [CrossRef]
- Lu, M.; Shi, X.; Feng, Q.; Zhang, M.; Guo, Y.; Dong, X.; Guo, R. Modification of oyster shell powder by humic acid for ammonium removal from aqueous solutions and nutrient retention in soil. J. Environ. Chem. Eng. 2021, 9, 106708. [Google Scholar] [CrossRef]
- Yang, X.; Huang, Y.; Liu, K.; Zheng, C. Effects of oyster shell powder on leaching characteristics of nutrients in low-fertility latosol in South China. Environ. Sci. Pollut. R 2022, 29, 56200–56214. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, D.K.; Ali, M.A.; Kim, P.J. Effects of oyster shell on soil chemical and biological properties and cabbage productivity as a liming materials. Waste Manag. 2008, 28, 2702–2708. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, P.; Yang, C.; Lu, S. Effects of oyster shell powder and lime on availability and forms of phosphorus, and enzyme activity in acidic paddy soil. Environ. Sci. 2022, 1–13, 5224–5233. (In Chinese) [Google Scholar]
- Coonan, E.C.; Kirkby, C.A.; Kirkegaard, J.A.; Amidy, M.R.; Strong, C.L.; Richardson, A.E. Lime and nutrient addition affects the dynamics and fractions of soil carbon in a short-term incubation study with 13C-labeled wheat straw. Soil Sci. 2019, 184, 43–51. [Google Scholar] [CrossRef]
- Grover, S.P.; Butterly, C.R.; Wang, X.; Tang, C. The short-term effects of liming on organic carbon mineralisation in two acidic soils as affected by different rates and application depths of lime. Biol. Fertil. Soils 2017, 53, 431–443. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science, Technology and Implementation; Taylor and Francis: Abingdon, UK, 2015; pp. 2–20. [Google Scholar]
- Chen, J.; Li, S.; Liang, C.; Xu, Q.; Li, Y.; Qin, H.; Fuhrmann, J.J. Response of microbial community structure and function to short-term biochar amendment in an intensively managed bamboo (Phyllostachys praecox) plantation soil: Effect of particle size and addition rate. Sci. Total Environ. 2017, 574, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sun, X.; Zheng, J.; Zhang, X.; Liu, X.; Bian, R.; Li, L.; Cheng, K.; Zheng, J.; Pan, G. Biochar amendment changes temperature sensitivity of soil respiration and composition of microbial communities 3 years after incorporation in an organic carbon-poor dry cropland soil. Biol. Fertil. Soils 2018, 54, 175–188. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, K.; Zhao, Y.; Li, S.; Wu, Q.; Liang, C.; Sun, X.; Xu, Q.; Chen, J.; Qin, H. Change in composition and function of microbial communities in an acid bamboo (Phyllostachys praecox) plantation soil with the addition of three different biochars. For. Ecol. Manag. 2020, 473, 118336. [Google Scholar] [CrossRef]
- Singh, R.; Singh, P.; Singh, H.; Raghubanshi, A.S. Impact of sole and combined application of biochar, organic and chemical fertilizers on wheat crop yield and water productivity in a dry tropical agro-ecosystem. Biochar 2019, 1, 229–235. [Google Scholar] [CrossRef]
- Agegnehu, G.; Srivastava, A.K.; Bird, M.I. The role of biochar and biochar-compost in improving soil quality and crop performance: A review. Appl. Soil Ecol. 2017, 119, 156–170. [Google Scholar] [CrossRef]
- Wu, Q.; Lian, R.; Bai, M.; Bao, J.; Liu, Y.; Li, S.; Liang, C.; Qin, H.; Chen, J.; Xu, Q. Biochar co-application mitigated the stimulation of organic amendments on soil respiration by decreasing microbial activities in an infertile soil. Biol. Fertil. Soils 2021, 57, 793–807. [Google Scholar] [CrossRef]
- Luo, Y.; Wan, S.; Hui, D.; Wallace, L.L. Acclimatization of soil respiration to warming in a tall grass prairie. Nature 2001, 413, 622–625. [Google Scholar] [CrossRef]
- Chen, J.; Elsgaard, L.; van Groenigen, K.J.; Olesen, J.E.; Liang, Z.; Jiang, Y.; Laerke, P.E.; Zhang, Y.; Luo, Y.; Huanggate, B.A.; et al. Soil carbon loss with warming: New evidence from carbon-degrading enzymes. Glob. Change Biol. 2020, 26, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Dou, Y.; Wang, B.; Xue, Z.; Wang, Y.; An, S.; Chang, S. Deciphering factors driving soil microbial life-history strategies in restored grasslands. iMeta 2022, 2, e66. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 2010, 42, 391–404. [Google Scholar] [CrossRef]
- Nannipieri, P.; Trasar, C.C.; Dick, R.P. Soil enzyme activity: A brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol. Fertil. Soils 2018, 54, 11–19. [Google Scholar] [CrossRef]
- Cui, Y.; Moorhead, D.L.; Wang, X.; Xu, M.; Wang, X.; Wei, X.; Zhu, Z.; Ge, T.; Peng, S.; Zhu, B.; et al. Decreasing microbial phosphorus limitation increases soil carbon release. Geoderma 2022, 419, 115868. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad, S.J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Lauber, C.L.; Weintraub, M.N.; Ahmed, B.; Allison, S.D.; Crenshaw, C.; Contosta, A.R.; Cusack, D.; Frey, S.; Gallo, M.E.; et al. Stoichiometry of soil enzyme activity at global scale. Ecol. Lett. 2008, 11, 1252–1264. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Rinkes, Z.L.; Sinsabaugh, R.L.; Weintraub, M.N. Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: Informing enzyme based decomposition models. Front. Microbiol. 2013, 4, 223. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.T.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Biol. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Cui, Y.; Bing, H.; Fang, L.; Jiang, M.; Shen, G.; Yu, J.; Wang, X.; Zhu, H.; Wu, Y.; Zhang, X. Extracellular enzyme stoichiometry reveals the carbon and phosphorus limitations of microbial metabolisms in the rhizosphere and bulk soils in alpine ecosystems. Plant Soil 2019, 458, 1–14. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, C.; Shao, S.; Chen, J.; Qin, H.; Xu, Q. Linkages of litter and soil C:N:P stoichiometry with soil microbial resource limitation and community structure in a subtropical broadleaf forest invaded by Moso bamboo. Plant Soil 2021, 465, 473–490. [Google Scholar] [CrossRef]
- Yuan, Y.; Li, Y.; Mou, Z.; Kuang, L.; Wu, W.; Zhang, J.; Wang, F.; Hui, D.; Peñuelas, J.; Sardans, J.; et al. Phosphorus addition decreases microbial residual contribution to soil organic carbon pool in a tropical coastal forest. Glob. Change Biol. 2020, 27, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhang, S.; Wang, X.; Xu, X.; Ai, C.; Liang, G.; Zhu, P.; Zhou, W. Enzymatic stoichiometry reveals phosphorus limitation-induced changes in the soil bacterial communities and element cycling: Evidence from a long-term field experiment. Geoderma 2022, 426, 116124. [Google Scholar] [CrossRef]
- Lu, R.K. Analytical Methods for Soil and Agro-Chemistry; China Agricultural Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Rovira, P.; Vallejo, V.R. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma 2002, 107, 109–141. [Google Scholar] [CrossRef]
- Liu, W.; Xu, W.; Hong, J.; Wan, S. Interannual variability of soil microbial biomass and respiration in responses to topography, annual burning and N addition in a semiarid temperate steppe. Geoderma 2010, 158, 259–267. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microb. 2003, 69, 3593–3599. [Google Scholar] [CrossRef]
- Situ, G.; Zhao, Y.; Zhang, L.; Yang, X.; Chen, D.; Li, S.; Wu, Q.; Xu, Q.; Chen, J.; Qin, H. Linking the chemical nature of soil organic carbon and biological binding agent in aggregates to soil aggregate stability following biochar amendment in a rice paddy. Sci. Total Environ. 2022, 847, 157460. [Google Scholar] [CrossRef]
- Saiya-Cork, K.R.; Sinsabaugh, R.L.; Zak, D.R. The effects of long-term nitrogen deposition on extracellular enzyme activity in an Acer saccharum forest soil. Soil Biol. Biochem. 2002, 34, 1309–1315. [Google Scholar] [CrossRef]
- Chen, J.; Chen, D.; Xu, Q.; Fuhrmann, J.; Li, L.; Pan, G.; Li, Y.; Qin, H.; Liang, C.; Sun, X. Organic carbon quality, composition of main microbial groups, enzyme activities, and temperature sensitivity of soil respiration of an acid paddy soil treated with biochar. Biol. Fertil. Soils 2019, 55, 185–197. [Google Scholar] [CrossRef]
- Rousk, J.; Baathe, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar]
- Noah, F.; Benjamin, P.; Joshua, P.; Robert, B. Predicting the temperature dependence of microbial respiration in soil: A continental-scale analysis. Glob. Biogeochem. Cycles 2006, 20, B3026. [Google Scholar]
- Lu, W.; Ding, W.; Zhang, J.; Li, Y.; Luo, J.; Bolan, N.; Xie, Z. Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: A negative priming effect. Soil Biol. Biochem. 2014, 76, 12–21. [Google Scholar] [CrossRef]
- Babujia, L.C.; Hungria, M.; Franchini, J.C.; Brookes, P.C. Microbial biomass and activity at various soil depths in a Brazilian oxisol after two decades of no-tillage and conventional tillage. Soil Biol. Biochem. 2010, 42, 2174–2181. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Chen, X.; Tan, M.; Duan, Z.; Wu, Z.; Li, X.; Fan, X. Response of soil enzyme activity to long-term restoration of desertified land. CATENA 2015, 133, 64–70. [Google Scholar]
- Foster, E.J.; Hansen, N.; Wallenstein, M.; Cotrufo, F. Biochar and manure amendments impact soil nutrients and microbial enzymatic activities in a semi-arid irrigated maize cropping system. Agric. Ecosyst. Environ. 2016, 233, 404–414. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of biochar on carbon fractions and enzyme activity of red soil. CATENA 2014, 121, 214–221. [Google Scholar]
- Elzobair, K.A.; Stromberger, M.E.; Ippolito, J.A.; Lentz, R.D. Contrasting effects of biochar versus manure on soil microbial communities and enzyme activities in an Aridisol. Chemosphere 2016, 142, 145–152. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, K.; Liu, J.; Chen, Y.; Han, L. Changes in soil properties and CO2 emissions after biochar addition: Role of pyrolysis temperature and aging. Sci. Total Environ. 2022, 839, 156333. [Google Scholar] [CrossRef]
- Niu, Y.; Chai, R.; Jin, G.; Wang, H.; Tang, C.; Zhang, Y. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Bian, R.; Cheng, K.; Zheng, J. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from Southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Ge, T.; Luo, Y.; Liu, S.; Xu, X.; Tong, C.; Shibistova, O.; Guggenberger, G.; Wu, J. Microbial stoichiometric flexibility regulates rice straw mineralization and its priming effect in paddy soil. Soil Biol. Biochem. 2018, 121, 67–76. [Google Scholar] [CrossRef]
- Jones, D.L.; Cooledge, E.C.; Hoyle, F.C.; Griffiths, R.I.; Murphy, D.V. pH and exchangeable aluminum are major regulators of microbial energy flow and carbon use efficiency in soil microbial communities. Soil Biol. Biochem. 2019, 138, 107584. [Google Scholar] [CrossRef]

| Soil Chemical Property | Treatment | |||
|---|---|---|---|---|
| CK | T | B | TB | |
| pH | 4.21 ± 0.07 c | 7.08 ± 0.17 a | 4.31 ± 0.09 c | 6.74 ± 0.29 b |
| SOC (g kg−1) | 29.42 ± 1.90 b | 29.87 ± 2.09 b | 35.56 ± 1.47 a | 35.20 ± 2.04 a |
| DOC (mg kg−1) | 946.38 ± 27.78 b | 941.84 ± 34.13 b | 1035.37 ± 28.39 a | 1049.43 ± 29.57 a |
| TN (g kg−1) | 3.04 ± 0.17 a | 3.11 ± 0.30 a | 3.10 ± 0.20 a | 3.30 ± 0.12 a |
| DN (mg kg−1) | 80.67 ± 5.91c | 104.61 ± 8.68 b | 73.20 ± 8.90 c | 130.40 ± 12.17 a |
| TP (g kg−1) | 1.09 ± 0.11 a | 1.05 ± 0.19 a | 1.20 ± 0.20 a | 1.24 ± 0.03 a |
| AP (mg kg−1) | 204.57 ± 14.35 a | 184.95 ± 15.24 a | 204.62 ± 22.18 a | 208.26 ± 15.30 a |
| AK (mg kg−1) | 592.55 ± 26.02 a | 611.16 ± 46.43 a | 593.49 ± 16.55 a | 596.26 ± 60.45 a |
| C:N | 9.67 ± 0.44 b | 9.64 ± 1.01 b | 11.50 ± 0.25 a | 10.68 ± 0.66 ab |
| C:P | 27.20 ± 4.05 a | 28.82 ± 4.20 a | 30.22 ± 4.69 a | 28.39 ± 1.24 a |
| N:P | 2.82 ± 0.47 a | 3.00 ± 0.41 a | 2.63 ± 0.42 a | 2.66 ± 0.14 a |
| Soil Chemical Property | Explains | Pseudo-F | p-Value |
|---|---|---|---|
| pH | 50% | 9.82 | 0.002 ** |
| SOC | 7% | 0.80 | 0.436 |
| DOC | 7% | 0.71 | 0.458 |
| TN | 6% | 0.67 | 0.512 |
| DN | 18% | 2.22 | 0.112 |
| TP | 9% | 1.03 | 0.352 |
| AP | 8% | 0.83 | 0.434 |
| AK | 7% | 0.73 | 0.456 |
| C:N | 4% | 0.42 | 0.662 |
| C:P | 5% | 0.52 | 0.588 |
| N:P | 8% | 0.82 | 0.434 |
| LPI−C | 55% | 12.29 | 0.002 ** |
| LPII−C | 32% | 4.66 | 0.010 * |
| RP−C | 5% | 0.50 | 0.624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, H.; Yuan, G.; Liu, Y.; Yu, J.; Li, S.; Wu, Q.; Qin, H.; Chen, J. Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation. Forests 2023, 14, 853. https://doi.org/10.3390/f14040853
Ji H, Yuan G, Liu Y, Yu J, Li S, Wu Q, Qin H, Chen J. Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation. Forests. 2023; 14(4):853. https://doi.org/10.3390/f14040853
Chicago/Turabian StyleJi, Haonan, Gensheng Yuan, Yang Liu, Jinzhu Yu, Songhao Li, Qifeng Wu, Hua Qin, and Junhui Chen. 2023. "Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation" Forests 14, no. 4: 853. https://doi.org/10.3390/f14040853
APA StyleJi, H., Yuan, G., Liu, Y., Yu, J., Li, S., Wu, Q., Qin, H., & Chen, J. (2023). Short-Term Effects of Bamboo Biochar and Oyster Shell Powder on Soil Organic Carbon Fraction, Microbial Respiration, and Enzymatic Stoichiometry in a Lei Bamboo Plantation. Forests, 14(4), 853. https://doi.org/10.3390/f14040853






