The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Broth Culture Preparation and Collection of R. speratus Worker Termites
2.2. Cell Growth Curve of B. licheniformis PR2 and Production of Cuticle-Degrading Enzymes
2.3. Preparation of Crude Enzyme Fraction from B. licheniformis PR2 Bacterial Broth Culture
2.4. Anti-Termite Activity of B. licheniformis PR2 Broth Culture and Crude Enzymes on R. speratus Worker Termites
2.5. Effect of Treatments on the Morphological Changes in Cuticle of R. speratus Worker Termites
2.6. Statistical Analysis
3. Results
3.1. Cell Growth, Chitinase and Protease Production by B. licheniformis PR2
3.2. Anti-Termite Activity of B. licheniformis PR2 against R. speratus Worker Termites
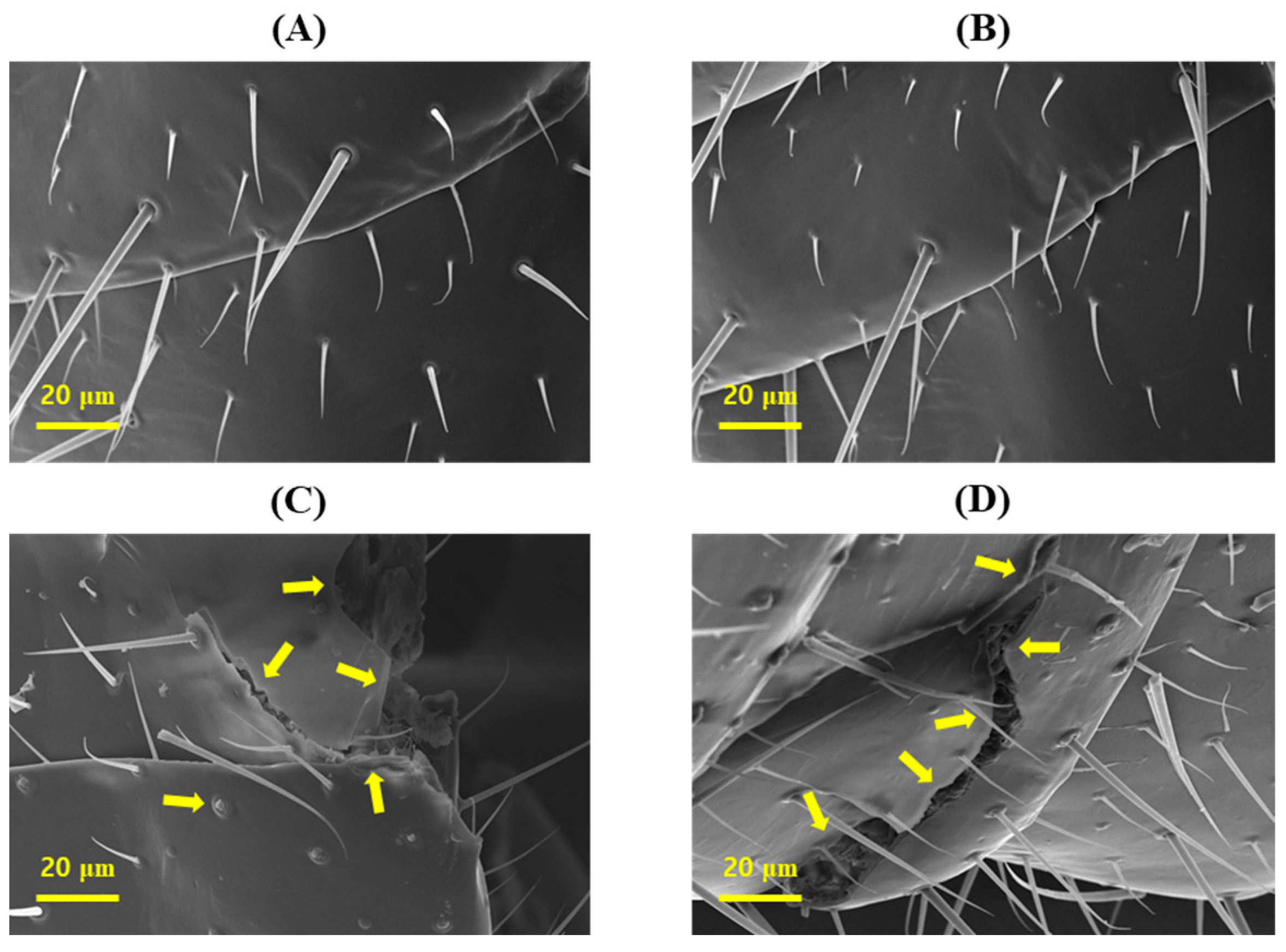
3.3. Morphological Cuticle Deformation of R. speratus Worker Termites after Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bignell, D.E.; Eggleton, P. Termites in ecosystems. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 363–387. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lim, I. Study of minimum passage size of subterranean termites (Reticulitermes speratus kyushuensis). J. Cult. Herit. Stud. (Munhwaje) 2020, 53, 188–197. [Google Scholar] [CrossRef]
- Moore, B.P. Biochemical studies in termites. In Biology of Termites; Krishna, K., Weesner, F.M., Eds.; Academic Press: New York, NY, USA, 1969; pp. 407–432. [Google Scholar]
- Traniello, J.F.A.; Leuthold, R.H. Behavior and ecology of foraging in termites. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 141–168. [Google Scholar] [CrossRef]
- Lainé, L.V.; Wright, D.J. The life cycle of Reticulitermes spp. (Isoptera: Rhinotermitidae): What do we know? Bull. Entomol. Res. 2003, 93, 267–278. [Google Scholar] [CrossRef]
- Engel, M.S.; Barden, P.; Riccio, M.L.; Grimaldi, D.A. Morphologically specialized termite castes and advanced sociality in the early cretaceous. Curr. Biol. 2016, 26, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Roisin, Y. Diversity and evolution of caste patterns. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 95–119. [Google Scholar] [CrossRef]
- Sindhu, S.S.; Rakshiya, Y.S.; Verma, M.K. Biological control of termites by antagonistic soil microorganisms. In Bioaugmentation, Biostimulation and Biocontrol; Singh, A., Parmar, N., Kuhad, R.C., Eds.; Springer: Berlin, Germany, 2011; pp. 261–309. [Google Scholar] [CrossRef]
- Eggleton, P.; Tayasu, I. Feeding groups, lifetypes and the global ecology of termites. Ecol. Res. 2001, 16, 941–960. [Google Scholar] [CrossRef]
- Eggleton, P. Global patterns of termite diversity. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 25–54. [Google Scholar] [CrossRef]
- Holt, J.A.; Lepage, M. Termites and soil properties. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 389–407. [Google Scholar] [CrossRef]
- Su, N.-Y.; Scheffrahn, R.H. Termites as pests of buildings. In Termites: Evolution, Sociality, Symbioses, Ecology; Abe, T., Bignell, D.E., Higashi, M., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 437–453. [Google Scholar] [CrossRef]
- Buczkowski, G.; Bertelsmeier, C. Invasive termites in a changing climate: A global perspective. Ecol. Evol. 2017, 7, 974–985. [Google Scholar] [CrossRef]
- Ghaly, A.; Edwards, S. Termite damage to buildings: Nature of attacks and preventive construction methods. Am. J. Eng. Appl. Sci. 2011, 4, 187–200. [Google Scholar] [CrossRef]
- Indrayani, Y.; Setyawati, D.; Maryani, Y.; Simangunsong, B. A termite attack on rubber plantation on peat soil: Level of damage and identification of pest species. IOP Conf. Ser. Earth Environ. Sci. 2022, 976, 012006. [Google Scholar] [CrossRef]
- Park, I.K.; Shin, S.C. Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2005, 53, 4388–4392. [Google Scholar] [CrossRef]
- Ahmad, F.; Fouad, H.; Liang, S.Y.; Hu, Y.; Mo, J.C. Termites, and Chinese agricultural system: Applications and advances in integrated termite management and chemical control. Insect Sci. 2021, 28, 2–20. [Google Scholar] [CrossRef]
- Lee, J.M.; Kim, Y.H.; Hong, J.Y.; Lim, B.; Park, J.H. Exploration of preservatives that inhibit wood feeding by inhibiting termite intestinal enzyme activity. J. Korean Wood Sci. Technol. 2020, 48, 376–392. [Google Scholar] [CrossRef]
- Im, I.G.; Cha, H.S.; Kang, W.C.; Lee, S.B.; Han, G.S. The status of damage and monitoring of subterranean termite (Reticulitermes spp.) (Blattodea: Rhinotermitidae) for wooden cultural heritage in Korea. J. Conserv. Sci. 2021, 37, 191–208. [Google Scholar] [CrossRef]
- Han, S.H.; Lee, K.S.; Chung, Y.J. Characteristic of termite inhabits in South Korea and the control. Conserv. Stud. 1998, 19, 133–158. [Google Scholar]
- Kim, S.H.; Chung, Y.J. Ingestion toxicity of fipronil on Reticulitermes speratus kyushuensis (Isoptera: Rhinotermitidae) and its applicability as a termite bait. J. Korean Wood Sci. Technol. 2017, 45, 159–167. [Google Scholar] [CrossRef]
- Lee, S.; Im, I.; Kim, S. A history of termite control and improvements to prevent termites in wooden architectural heritage. J. Cult. Herit. Stud. (Munhwaje) 2021, 54, 194–215. [Google Scholar] [CrossRef]
- Lemus, R.; Abdelghani, A. Chlorpyrifos: An unwelcome pesticide in our homes. Rev. Environ. Health 2000, 15, 421–433. [Google Scholar] [CrossRef]
- Gordon, J.M.; Velenovsky IV, J.F.; Chouvenc, T. Subterranean termite colony elimination can be achieved even when only a small proportion of foragers feed upon a CSI bait. J. Pest Sci. 2022, 95, 1207–1216. [Google Scholar] [CrossRef]
- He, B.; Ni, Y.; Jin, Y.; Fu, Z. Pesticides-induced energy metabolic disorders. Sci. Total Environ. 2020, 729, 139033. [Google Scholar] [CrossRef]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Mahmood, I.; Imadi, S.R.; Shazadi, K.; Gul, A.; Hakeem, K.R. Effects of pesticides on environment. In Plant, Soil and Microbes; Hakeem, K., Akhtar, M., Abdullah, S., Eds.; Springer: Cham, Switzerland, 2016; pp. 253–269. [Google Scholar]
- Gargouri, B.; Yousif, N.M.; Bouchard, M.; Fetoui, H.; Fiebich, B.L. Inflammatory and cytotoxic effects of bifenthrin in primary microglia and organotypic hippocampal slice cultures. J. Neuroinflamm. 2018, 15, 159. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef] [PubMed]
- Osbrink, W.L.; Lax, A.R.; Brenner, R.J. Insecticide susceptibility in Coptotermes formosanus and Reticulitermes virginicus (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2001, 94, 1217–1228. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.S.; Sharma, R.; Parween, T.; Patanjali, P.K. Pesticide contamination and human health risk factor. In Modern Age Environmental Problems and Their Remediation; Oves, M., Zain, K.M., Ismail, I.M.I., Eds.; Springer: Cham, Switzerland, 2016; pp. 49–68. [Google Scholar] [CrossRef]
- Rakshiya, Y.S.; Verma, M.K.; Sindhu, S.S. Efficacy of antagonistic soil bacteria in management of subterranean termites (Isoptera). Res. Environ. Life Sci. 2016, 9, 949–955. [Google Scholar]
- Jabeen, F.; Hussain, A.; Manzoor, M.; Younis, T.; Rasul, A.; Qazi, J.I. Potential of bacterial chitinolytic, Stenotrophomonas maltophilia, in biological control of termites. Egypt. J. Biol. Pest Control 2018, 28, 86. [Google Scholar] [CrossRef]
- Choi, S.-I.; Ajuna, H.B.; Won, S.-J.; Choub, V.; Kim, C.-W.; Moon, J.-H.; Ahn, Y.S. The insecticidal potential of Bacillus velezensis CE 100 against Dasineura jujubifolia Jiao & Bu (Diptera: Cecidomyiidae) larvae infestation and its role in the enhancement of yield and fruit quality of jujube (Zizyphus jujuba Miller var. inermis Rehder). Crop Prot. 2023, 163, 106098. [Google Scholar] [CrossRef]
- Moon, J.-H.; Won, S.-J.; Choub, V.; Choi, S.-I.; Ajuna, H.B.; Ahn, Y.S. Biological control of fall webworm larva (Hyphantria cunea Drury) and growth promotion of poplar seedlings (Populus×canadensis Moench) with Bacillus licheniformis PR2. For. Ecol. Manag. 2022, 525, 120574. [Google Scholar] [CrossRef]
- Ajuna, H.B.; Kim, I.; Han, Y.S.; Maung, C.E.H.; Kim, K.Y. Aphicidal activity of Bacillus thuringiensis strain AH-2 against cotton aphid (Aphis gossypii). Entomol. Res. 2021, 51, 151–160. [Google Scholar] [CrossRef]
- Kalha, C.; Singh, P.; Kang, S.; Hunjan, M.; Gupta, V.; Sharma, R. Entomopathogenic viruses and bacteria for insect–pest control. In Integrated Pest Management: Current Concepts and Ecological Perspective; Abrol, D.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 225–244. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, L.; Dong, G.; Chen, F. Isolation and identification of entomopathogenic fungi and an evaluation of their actions against the larvae of the fall webworm, Hyphantria cunea (Drury) (Lepidoptera: Arctiidae). Biocontrol 2020, 65, 101–111. [Google Scholar] [CrossRef]
- Leger, R.S.; Cooper, R.M.; Charnley, A.K. Cuticle-degrading enzymes of entomopathogenic fungi: Cuticle degradation in vitro by enzymes from entomopathogens. J. Invertebr. Pathol. 1986, 47, 167–177. [Google Scholar] [CrossRef]
- Bordereau, C.; Andersen, S.O. Structural cuticular proteins in termite queens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1978, 60, 251–256. [Google Scholar] [CrossRef]
- Ye, C.; Song, Z.; Wu, T.; Zhang, W.; Saba, N.U.; Xing, L.; Su, X. Endocuticle is involved in caste differentiation of the lower termite. Curr. Zool. 2021, 67, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Mahapatro, G.K. Can insecticide resistance be developed in termites? Curr. Sci. 2017, 112, 1097–1098. [Google Scholar] [CrossRef]
- Kakkar, G.; Chouvenc, T.; Su, N.-Y. Postecdysis sclerotization of mouthparts of the formosan subterranean termites (Isoptera: Rhinotermitidae). J. Econ. Entomol. 2016, 109, 792–799. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Lee, U.; Park, Y.S.; Maung, C.E.H.; Ajuna, H.B.; Ahn, Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases production of jujube fruit under field conditions. Horticulturae 2021, 7, 49. [Google Scholar] [CrossRef]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]
- Ghorbel-Frikha, B.; Sellami-Kamoun, A.; Fakhfakh, N.; Haddar, A.; Manni, L.; Nasri, M. Production and purification of a calcium-dependent protease from Bacillus cereus BG1. J. Ind. Microbiol. Biotechnol. 2005, 32, 186–194. [Google Scholar] [CrossRef]
- Choub, V.; Ajuna, H.B.; Won, S.-J.; Moon, J.-H.; Choi, S.-I.; Maung, C.E.H.; Kim, C.-W.; Ahn, Y.S. Antifungal activity of Bacillus velezensis CE 100 against anthracnose disease (Colletotrichum gloeosporioides) and growth promotion of walnut (Juglans regia L.) trees. Int. J. Mol. Sci. 2021, 22, 10438. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vincent, J.F. Arthropod cuticle: A natural composite shell system. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1311–1315. [Google Scholar] [CrossRef]
- Vincent, J.F.; Wegst, U.G. Design and mechanical properties of insect cuticle. Arthropod Struct. Dev. 2004, 33, 187–199. [Google Scholar] [CrossRef]
- Moussian, B. The arthropod cuticle. In Arthropod Biology and Evolution: Molecules, Development, Morphology; Minelli, A., Boxshall, G., Fusco, G., Eds.; Springer: Berlin, Germany, 2013; pp. 171–196. [Google Scholar] [CrossRef]
- Gibbs, A. Physical properties of insect cuticular hydrocarbons: Model mixtures and lipid interactions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1995, 112, 667–672. [Google Scholar] [CrossRef]
- Mitov, M.; Soldan, V.; Balor, S. Observation of an anisotropic texture inside the wax layer of insect cuticle. Arthropod Struct. Dev. 2018, 47, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, C.K.; Schrank, A.; Vainstein, M.H. Regulation of extracellular chitinases and proteases in the entomopathogen and acaricide Metarhizium anisopliae. Curr. Microbiol. 2003, 46, 205–210. [Google Scholar] [CrossRef]
- Stoykov, Y.M.; Pavlov, A.I.; Krastanov, A.I. Chitinase biotechnology: Production, purification, and application. Eng. Life Sci. 2015, 15, 30–38. [Google Scholar] [CrossRef]
- Fang, W.; Feng, J.; Fan, Y.; Zhang, Y.; Bidochka, M.J.; Leger, R.J.S.; Pei, Y. Expressing a fusion protein with protease and chitinase activities increases the virulence of the insect pathogen Beauveria bassiana. J. Invertebr. Pathol. 2009, 102, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Mótyán, J.A.; Tóth, F.; Tőzsér, J. Research applications of proteolytic enzymes in molecular biology. Biomolecules 2013, 3, 923–942. [Google Scholar] [CrossRef]
- Zukowski, J.; Su, N.-Y. Survival of termites (Isoptera) exposed to various levels of relative humidity (RH) and water availability, and their RH preferences. Fla. Entomol. 2017, 100, 532–538. [Google Scholar] [CrossRef]
- Zukowski, J.; Su, N.-Y. A comparison of morphology among four termite species with different moisture requirements. Insects 2020, 11, 262. [Google Scholar] [CrossRef]
- Fu, R.; Luo, J.; Feng, K.; Lu, X.; Tang, F. Termite-killing components in Serratia marcescens (SM1). J. For. Res. 2020, 32, 1739–1744. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, J.-H.; Ajuna, H.B.; Won, S.-J.; Choub, V.; Choi, S.-I.; Yun, J.-Y.; Hwang, W.J.; Park, S.W.; Ahn, Y.S. The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae). Forests 2023, 14, 1000. https://doi.org/10.3390/f14051000
Moon J-H, Ajuna HB, Won S-J, Choub V, Choi S-I, Yun J-Y, Hwang WJ, Park SW, Ahn YS. The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae). Forests. 2023; 14(5):1000. https://doi.org/10.3390/f14051000
Chicago/Turabian StyleMoon, Jae-Hyun, Henry B. Ajuna, Sang-Jae Won, Vantha Choub, Su-In Choi, Ju-Yeol Yun, Won Joung Hwang, Sang Wook Park, and Young Sang Ahn. 2023. "The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae)" Forests 14, no. 5: 1000. https://doi.org/10.3390/f14051000
APA StyleMoon, J.-H., Ajuna, H. B., Won, S.-J., Choub, V., Choi, S.-I., Yun, J.-Y., Hwang, W. J., Park, S. W., & Ahn, Y. S. (2023). The Anti-Termite Activity of Bacillus licheniformis PR2 against the Subterranean Termite, Reticulitermes speratus kyushuensis Morimoto (Isoptera: Rhinotermitidae). Forests, 14(5), 1000. https://doi.org/10.3390/f14051000





