Abstract
Platycladus orientalis and Amorpha fruticosa are important pioneer species in arid and semi-arid regions, playing a crucial role in ecological restoration in water-limited areas. The purpose of this research was to examine how different watering and rewatering schedules influence the antioxidant enzyme activities and biochemical responses of P. orientalis and A. fruticosa. Five different water regimes (100%, 88%, 70%, 52%, and 40% of soil relative water content) were applied to seedlings for 15, 30, 45, and 60 days, after which they were rewatered. The levels of malondialdehyde (MDA), antioxidant enzyme activities (superoxide dismutase, peroxidase, and catalase), and osmotic solutes (soluble sugar and proline) were assessed in the leaves of P. orientalis and A. fruticosa. Measurements were taken at various growth stages, namely the initial, fast, and late stages, both during the drought period and after 2, 24, 48, and 72 h of rewatering. The results revealed that the malondialdehyde content in the leaves of P. orientalis and A. fruticosa increased with the level of stress. The activities of antioxidant enzymes and the levels of osmotic solutes in the leaves of P. orientalis and A. fruticosa exhibited varying enhancements as the water stress intensified. During the recovery phase, the antioxidant enzymes and soluble sugar content returned to the control level 72 h after rewatering at different growth stages. However, the proline content remained slightly elevated compared to the control plants. Overall, these findings suggest that the two plant species displayed minor differences in their responses to drought stress and rewatering in terms of antioxidant enzymes and other biochemical responses. This indicates their remarkable adaptability to severe drought conditions and their potential for rapid recovery after rewatering. These observations are highly significant for irrigation management strategies when establishing plantations in arid and semi-arid regions.
1. Introduction
Droughts are becoming more common as a result of global climate change and the unsustainable exploitation of water and soil resources caused by human activities [1]. Soil water scarcity directly affects nutrient uptake, affecting soil nutrient availability. This limitation hinders plant growth and development, which are crucial for young seedlings’ survival during ecological restoration projects in arid and semi-arid northern China [2]. On the other hand, plants have developed adaptive strategies to deal with drought stress by modifying their morphology, adjusting biochemical contents, and regulating antioxidant enzyme activities [3,4,5]. These mechanisms enable plants to survive in water-stressed environments. They engage in osmotic regulation to reduce cell osmotic potential and minimize water loss in order to maintain leaf tissue water content. They also boost antioxidant enzyme activity to combat reactive oxygen species-induced oxidative damage [6,7,8].
Moderate water stress may even help improve plant stress resistance, whereas excessive water deficit can be harmful to plants. Notably, plants can not only withstand a certain amount of drought, but they can also recover from water stress damage when rewatered. This remarkable resistance to adversity is a critical factor of their survival strategy [9,10,11]. Plants frequently experience alternating periods of drought and short-term rewatering in their natural environment [12,13]. Such changes in the water cycle can have significant impacts on plant growth and other key metabolic functions, potentially affecting both ecosystem productivity and agricultural yields. In arid and semi-arid areas, sporadic precipitation can pose a critical challenge to maintaining ecosystem stability and survival. For example, a small rainfall pulse can trigger a rapid response in desert ecosystems, inducing plant growth that helps ensure their survival. Wang et al. (2016) pointed out that rehydration can trigger compensatory impacts on plant growth and development [14]. However, plant recovery capacity varies, depending on factors such as plant species, drought intensity and duration, and growth cycle, resulting in differences in the rehydration process [5].
In recent years, China has implemented a series of ecosystem restoration programs aimed at improving fragile environments and restoring forests in previously deteriorated ecosystems. Platycladus orientalis and Amorpha fruticosa are the most commonly utilized species for afforestation in northern China, owing to their strong adaptability to local weather conditions and impressive growth performance [15,16]. An intriguing feature of P. orientalis is its remarkable longevity, with several individuals in the Shaanxi Province of China having lifespans of several thousand years. Additionally, A. fruticosa is a nitrogen-fixing species and frequently employed as a pioneer species for vegetation restoration in the region [17]. The effects of water stress on P. orientalis and A. fruticosa have been studied in depth in a number of reports [7,15,18,19]. Several of these reports note that these species can survive under these conditions by regulating their antioxidant enzyme activities and biochemical responses. While previous research has investigated the antioxidant enzymes and biochemical response mechanisms of plants under water stress [16,18,20], little is known about the complex recovery mechanisms mediated by antioxidants enzymes and biochemical processes in P. orientalis and A. fruticosa during their different growth stages under drought and subsequent rehydration.
Therefore, we examined antioxidant enzyme and biochemical responses in young seedlings of P. orientalis and A. fruticosa at three growth stages (initial, fast, and late) under different soil water conditions. We also evaluated their recovery after 2, 24, 48, and 72 h of rewatering. The study’s specific goals were to: (i) measure the levels of malondialdehyde and antioxidant enzyme activities in P. orientalis and A. fruticosa under drought stress and subsequent re-watering at different growth stages; and (ii) find the most cost-effective soil water level and stress duration for the growth and development of these two typical plants in arid and semi-arid areas.
2. Materials and Methods
2.1. Plant Material, Growth Conditions and Experimental Design
The study was carried out in a semi-controlled greenhouse setting at the Northwest A&F University in China. P. orientalis and A. fruticosa seedlings that were one year old were used in this study. Both species’ growth stages were classified into three stages: initial growth stage (mid-April to mid-June), fast growth stage (mid-June to mid-August), and late growth stage (mid-August to mid-October), denoted as IGS, FGS, and LGS, respectively. Ten kilograms of air-dried soil with a water retention capacity of 22.3% was placed in each pot (32 cm × 27 cm × 30 cm). In each container, two seedlings of each species were planted in March 2019. A randomized complete block design with three repetitions for each treatment was used. To reduce evaporation, each container’s soil surface was covered with 1.2 kg of small stones. The soil relative water content (SWC), which represents the ratio of soil water content to field capacity, was classified into five levels: well-watered (CK, 100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), and W4 (40% SWC). Treatments for drought duration were categorized as T1 (15 days), T2 (30 days), T3 (45 days), and T4 (60 days). Prior to beginning the drought stress, all containers were adequately watered for one month to allow the transplanted seedlings to establish. The plants were grown for six months to study their responses to drought stress and subsequent rewatering. The containers were watered every day using a weighing method to keep different soil water gradients for as long as was needed. When the desired duration of drought stress was reached, both species were rewatered to restore soil water content to control levels.
Both P. orientalis and A. fruticosa were subjected to the following treatments at each growth stage: (i) control treatment with well-watered plants maintained at 100% SWC; (ii) drought stress treatments, where water was withheld for 15, 30, 45, or 60 days and different soil water gradients (W1, W2, W3, W4) were achieved by weighing the pots; and (iii) rehydration treatment, where plants were rewatered after being subjected to drought stress for 15, 30, 45, or 60 days, and leaf samples were collected at 2, 24, 48, and 72 h after rewatering.
2.2. Physiological and Biochemical Traits Measurement
The contents of malondialdehyde (MDA), antioxidant enzyme activities, and osmotic solutes in fresh leaves were analyzed at the end of the drought stress period (at 15, 30, 45, and 60 days after drought stress) and at 2, 24, 48, and 72 h after rewatering at each growth stage. For preparing the crude extracts, 0.5 g of fresh leaves from three replicates in each treatment were homogenized in a pre-chilled mortar and pestle using 10 mL of 50 mmol L−1 phosphate buffer (pH 7.8) containing 1% of soluble polyvinyl pyrrolidone (PVP). The homogenates were then centrifuged at 10,000× g for 20 min at 4 °C, and the resulting supernatants were used for enzyme activity and biochemical assays. The content of MDA was determined using 2-thiobarbituric acid (TBA) following the protocol described by Wang et al. (2014) [21]. Briefly, 2 mL extract solution was added to 2 mL of 0.5% TBA (dissolved with 15% trichloroacetic acid) and incubated in a water bath at 95 °C for 30 min. After centrifugation (10,000× g for 10 min), the MDA content was determined as mmol g−1 FW (fresh weight) by measuring the absorbance at wavelengths of 450 nm, 532 nm, and 600 nm using a spectrophotometer.
The activities of superoxide dismutase (SOD, EC 1.15.1.1), peroxidase (POD, EC 1.11.1.7), and catalase (CAT, EC 1.11.1.6) in the leaves of selected plant species were measured following the protocols by Roy et al. (2020) [22], Ekmekci and Terzioglu (2005) [23] and Beers and Sizer (1952) [24], respectively.
The total soluble sugars (SS) content was assessed by anthrone-H2SO4 method [25], and a standard curve was constructed using glucose at different concentrations. The proline (Pro) content was assessed using the sulphosalicylic acid method, and the optical density was recorded at 520 nm using a spectrophotometer, as described by Bates et al. (1973) [26].
2.3. Data Analysis
The means and standard deviations (SDs) of three replicates for each treatment are presented in the following figures. In order to conduct the statistical analyses, SPSS 17 (SPSS Inc., Chicago, IL, USA) was used. To determine statistically significant differences, multiple comparisons were performed using the least significant difference (LSD) test at a significance level of p < 0.05. Origin 8.5 was used to create all graphics.
3. Results
3.1. Effects of Drought Stress and Rewatering on Lipid Peroxidation
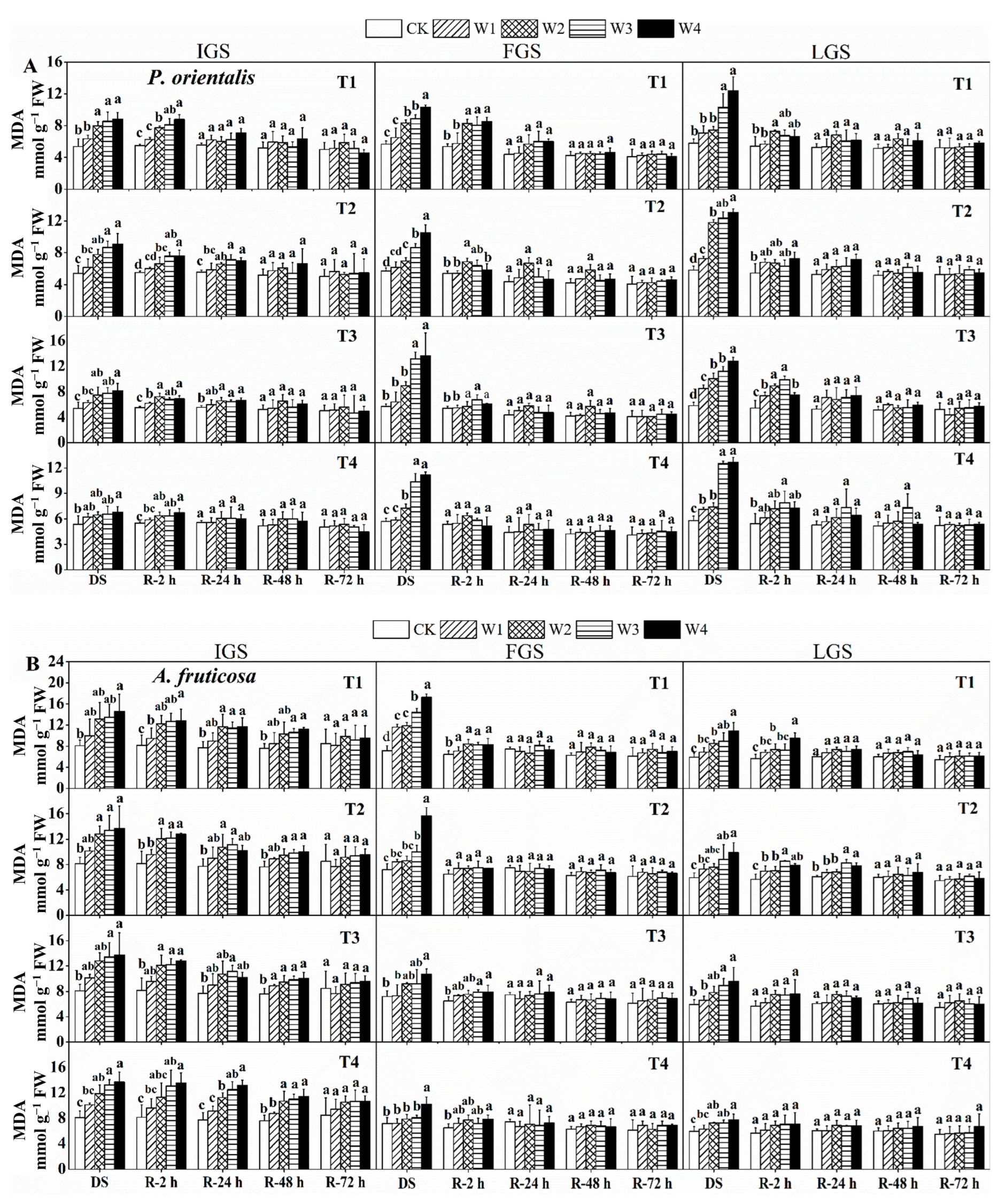
The MDA concentration of P. orientalis and A. fruticosa seedlings increased as drought stress intensified (Figure 1). In all treatments of drought stress, W4 significantly increased the MDA content of both afforestation species at each growth stage. During the initial growth stage (IGS) and the late growth stage (LGS), the MDA content of P. orientalis was highest in T2 under W4, measuring 9.08 and 13.0 mmol g−1 fresh weight (FW), respectively.
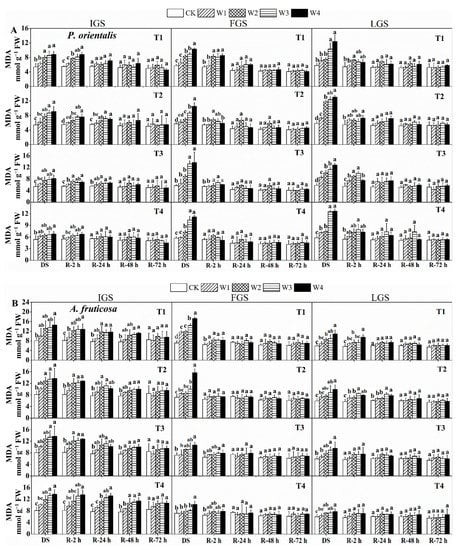
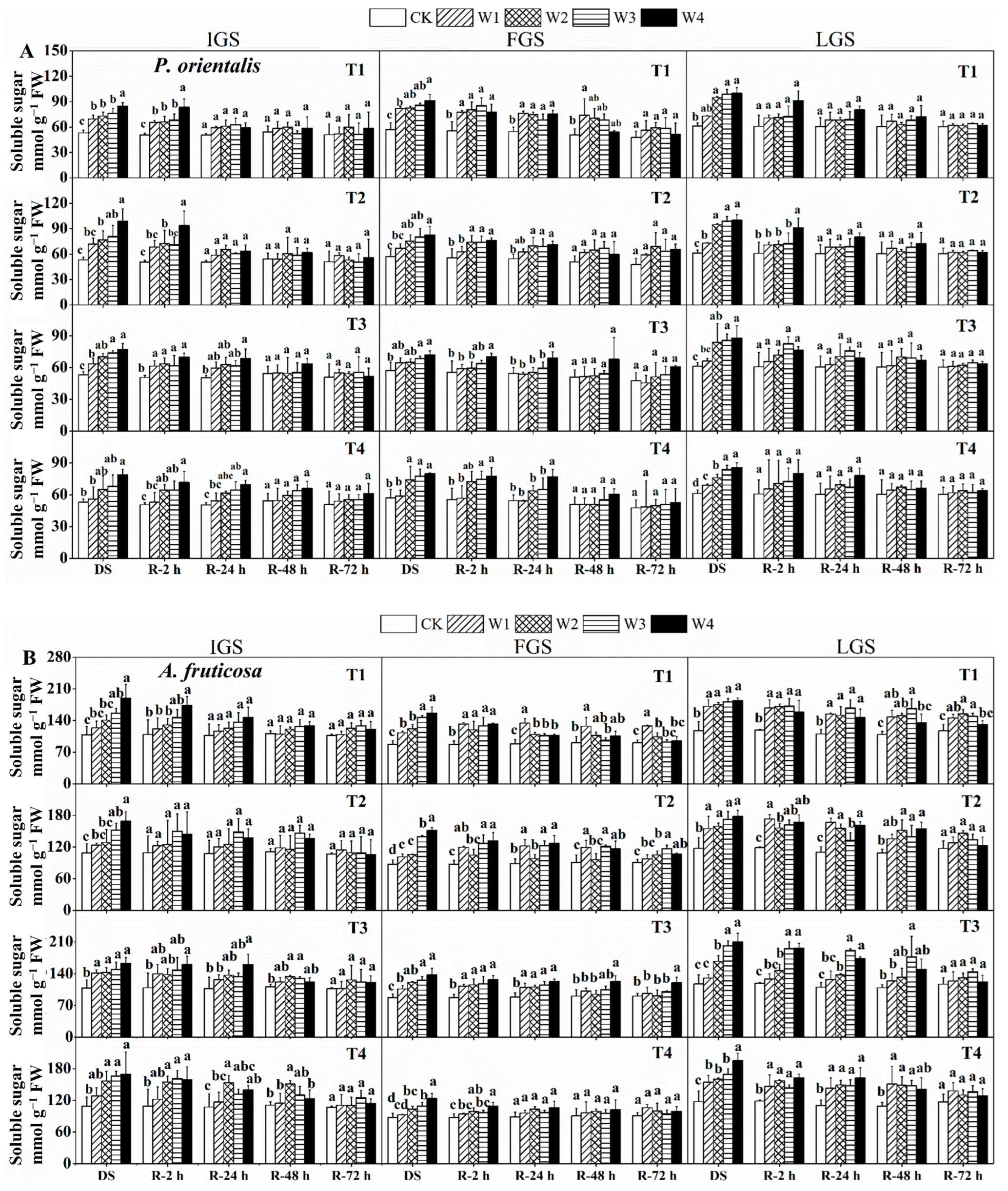
Figure 1.
The effect of drought stress and rewatering on malondialdehyde (MDA) levels in the leaves of P. orientalis (A) and A. fruticosa (B) seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. Different letters denote significant differences (p < 0.05) observed among the drought treatments.
In the fast growth stage (FGS), the MDA content of P. orientalis at T3 of W4 was 2.40 times higher compared to the control. For A. fruticosa seedlings in all three growth stages, the MDA content of W4 reached its highest values across all drought durations, exhibiting 1.80, 2.40, and 1.83 times higher levels compared to the control at T1, respectively.
The MDA content in the two tree species gradually decreased during the recovery phase, but the rate at which it recovered differed slightly depending on the duration of post-rehydration. For P. orientalis (Figure 1A), after 2 h of rewatering, the MDA content in the IGS under the W1 treatment nearly reached the control level. MDA content in both T1 and T4 treatments was similar to the control 24 h after rewatering. At 48 h after rewatering, the MDA content in all drought stress treatments reached a level close to the control. The MDA content in the FGS rapidly decreased after 2 h of rewatering, but it was restored to the control level in the T4 treatment. At 24 h after rewatering, the MDA content decreased to the control level and then gradually decreased with increasing post-rehydration time, showing no significant difference among the stressed plants. The MDA content in the LGS decreased to a level identical to the control in the W1 treatment 2 h after rewatering. After 24 h of rewatering, the MDA content in each drought treatment returned to the control level and then gradually decreased. There was no significant difference between the water-stressed and control treatments 48 and 72 h after rewatering. After rewatering, the MDA concentration in A. fruticosa in the IGS steadily decreased, and, after 72 h, it tended to reach a level similar to the control (Figure 1B). MDA content in the FGS decreased rapidly in T1 and T2, but slowly in T3 and T4. MDA content was comparable to the control in the T2 treatment 2 h after rewatering. In all stress treatments, MDA content recovered to the control level 24 h after rewatering. MDA content changes in the LGS were similar to those in the IGS of A. fruticosa. At 24 h after rewatering, the MDA content in all treated plants nearly returned to the control level, then slightly decreased and remained relatively stable. These findings show that the response of MDA content to drought stress differed between the two tree species, but it could be restored to control levels after 72 h of watering.
3.2. Effects of Drought Stress and Rewatering on Antioxidant Enzyme Activities
3.2.1. SOD Activity
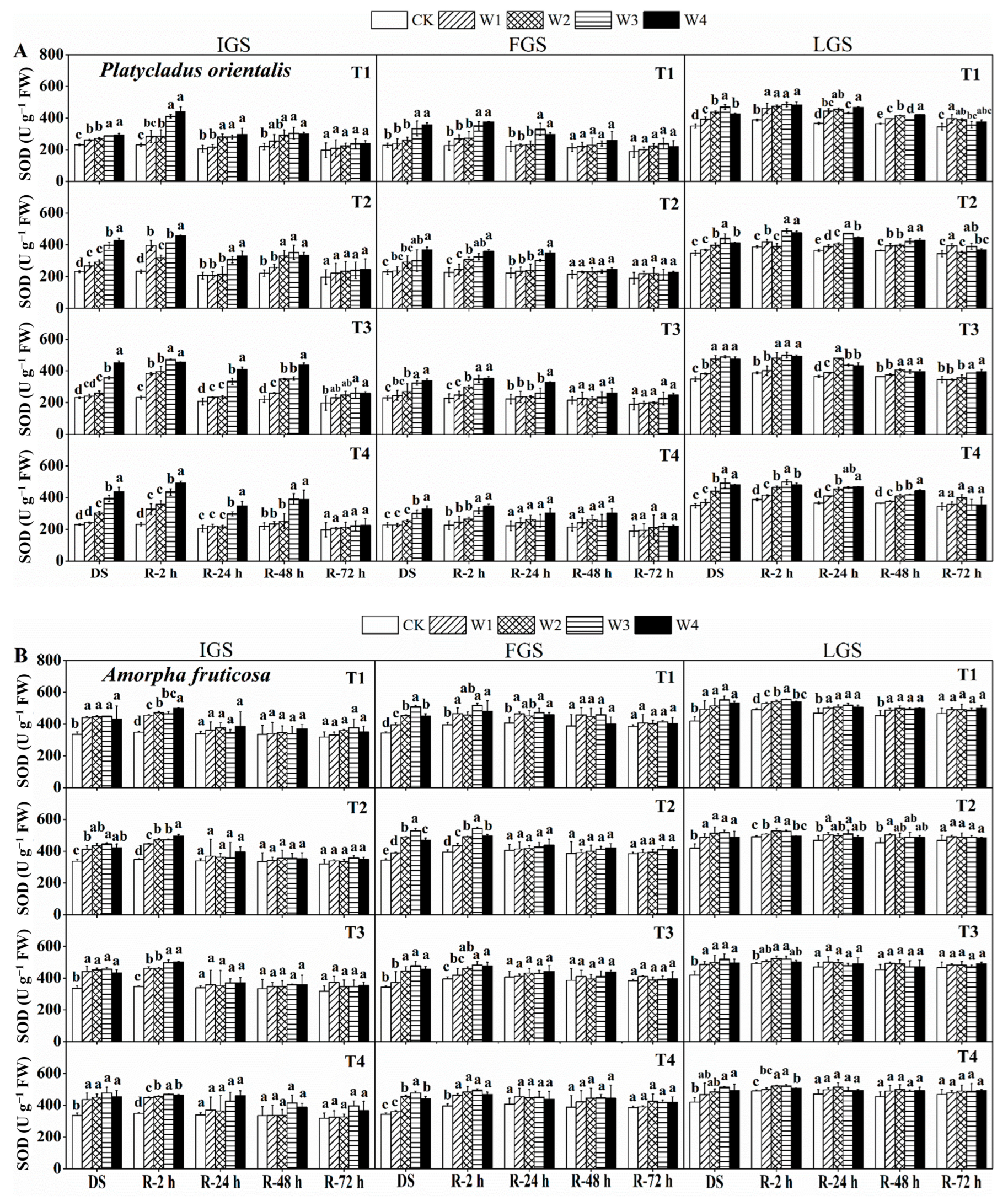
The effect of drought stress on SOD activity was examined in P. orientalis and A. fruticosa at three growth stages (Figure 2A,B). With increasing drought stress, the SOD activity of P. orientalis in the IGS and FGS gradually increased, whereas in the LGS it initially increased and then slightly decreased. The SOD activity of A. fruticosa leaves in all three growth stages exhibited a similar trend to that observed in P. orientalis in the LGS. The W3 and W4 treatments resulted in a significant increase in SOD activity in both species. Maximum SOD activity was measured in T3 and T2 of the P. orientalis IGS and FGS under treatment W4, with values 1.95 and 1.61 times higher than the control, respectively. When P. orientalis was treated with W3 and T4, its SOD activity reached a peak (1.41 times that of the control) in the LGS. Maximum SOD activity was observed in the IGS, FGS, and LGS of A. fruticosa under treatment W3, combined with T4, T2, and T4, respectively. After two hours of re-watering, the SOD activity of both tree species increased and then gradually decreased. However, at different times, the response of the two species to rewatering varied. In the case of P. orientalis (Figure 2A), the SOD activity of T1 treated with W1 in the IGS was comparable to the control two hours after rewatering. After rewatering, the SOD activity initially decreased, then increased slightly, and then decreased again at various time intervals. We observed that SOD activity returned to the control level after 72 h of rewatering, except for the SOD activity of P. orientalis at T3 grown in W3 and W4, which remained significantly higher than the control. SOD activity initially increased in the P. orientalis FGS and then decreased after rewatering. At 2 h after rewatering, the SOD activities of treatments W1 and W2 had essentially returned to the control level, whereas the SOD activities of treatments W3 and W4 were significantly higher than the control.
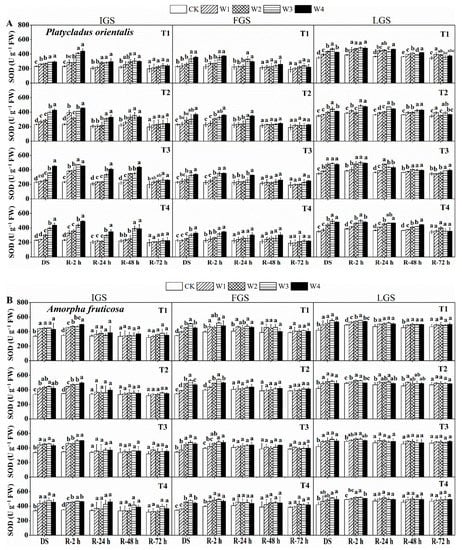
Figure 2.
The effect of drought stress and rewatering on superoxide dismutase (SOD) activity in the leaves of P. orientalis (A) and A. fruticosa (B) seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. Different letters denote significant differences (p < 0.05) observed among the drought treatments.
AT 24 h after rewatering, SOD activity in T4 could reach the same level as the control. In all drought stress treatments, SOD activity decreased to a level close to the control 48 h after rewatering, and remained relatively stable 72 h after rewatering. In the LGS, the rate of SOD activity recovery was slower than in the IGS and FGS, and did not reach the control level, even after 72 h. At all three stages of growth for A. fruticosa (Figure 2B), the SOD activity recovery trend was almost the same. During the IGS, SOD activity was back to normal 24 h after rewatering, and it stayed that way. During the FGS, 24 h after rewatering, SOD activity returned to the control level in all drought stress treatments except for A. fruticosa grown in T1, where it did not completely return to the control level. The activity of SOD in T3 and T4 in the LGS recovered to the control level 24 h after rewatering. After 72 h of getting water again, the drought-stressed plants’ SOD activity was back to normal. In all stages of growth, drought stress and watering had different effects on the SOD activity of two different plant species. The ability of the IGS and FGS of two tree species to recover SOD activity was slightly faster than that of the LGS.
3.2.2. POD Activity
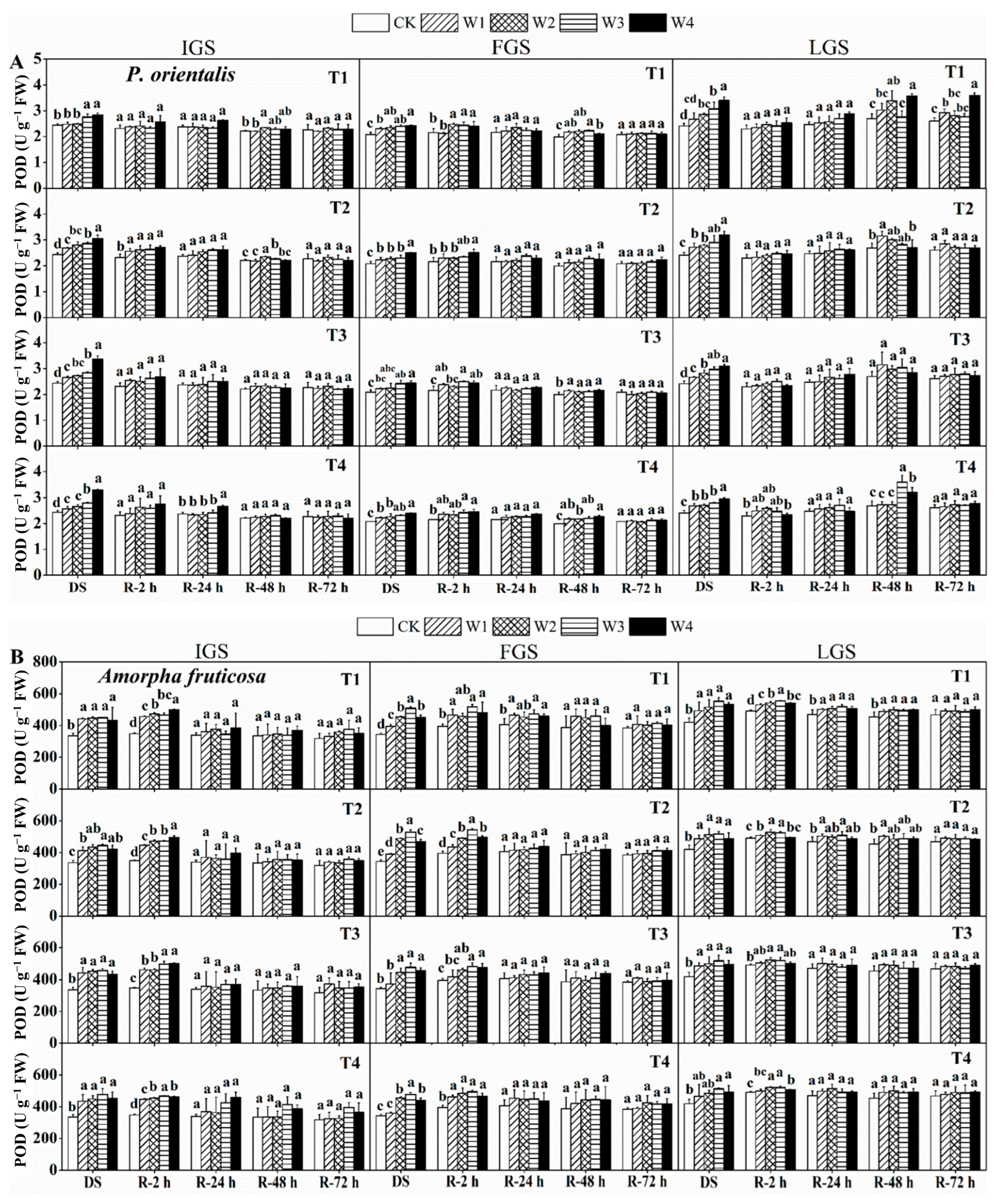
The POD (peroxidase) activity between the two chosen species under the drought and rewatering treatments showed a slight variation, as shown in Figure 3. The POD activity of P. orientalis gradually increased with increasing levels of drought stress, whereas the POD activity of A. fruticosa initially increased and then slightly decreased. The highest POD activity of P. orientalis was found in treatments T3, T2, and T1 under W4 in the IGS, FGS, and LGS. These treatments had POD activity levels that were 1.38, 1.20, and 1.41 times higher than the control, respectively. For A. fruticosa, treatments T2, T1, and T1 under W3 had maximum POD activity in the IGS, FGS, and LGS, which was 1.96, 1.94, and 2.05 times higher than the control, respectively.
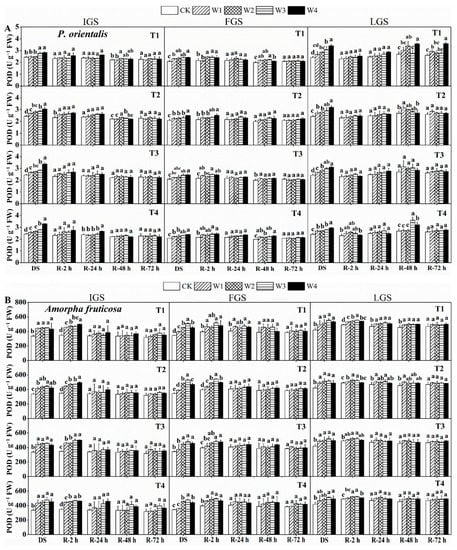
Figure 3.
The effect of drought stress and rewatering on peroxidase (POD) activity in the leaves of P. orientalis (A) and A. fruticosa (B) seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. Different letters denote significant differences (p < 0.05) observed among the drought treatments.
The two afforestation species’ responses to rewatering in terms of POD activity varied as well. In the case of P. orientalis (Figure 3A), POD activity showed an initial decrease, followed by an increase and then a subsequent decrease, in the IGS; a slight initial increase followed by a decrease in the FGS; and an initial decrease, followed by an increase and a subsequent decrease, in the LGS. Following rewetting, the recovery of POD activity in the various stages of P. orientalis varied. After 72 h of rewatering, POD activity in the IGS and FGS returned to the control level. While the POD activity in treatment T1 under both W1 and W4 was still significantly higher than the control level in the LGS, only the POD activity in treatments T2, T3, and T4 of drought stress returned to the control level.
The changes in POD activity in the IGS of A. fruticosa (Figure 3B) were similar to those seen in the LGS of P. orientalis, showing a pattern of an initial decrease, slight increase, and subsequent decrease. Similar to the P. orientalis IGS, the changes in POD activity in the FGS showed a downward trend. POD activity in the LGS initially rose and then fell. After 72 h of rewatering, all of the drought stress treatments on A. fruticosa seedlings in the IGS, FGS, and LGS reached POD activity levels that were relatively close to the control, with the exception of treatments T1 and T2 of the LGS. These findings suggest that the IGS and FGS of the two tree species had a slightly higher rate of POD activity recovery than the LGS.
3.2.3. CAT Activity
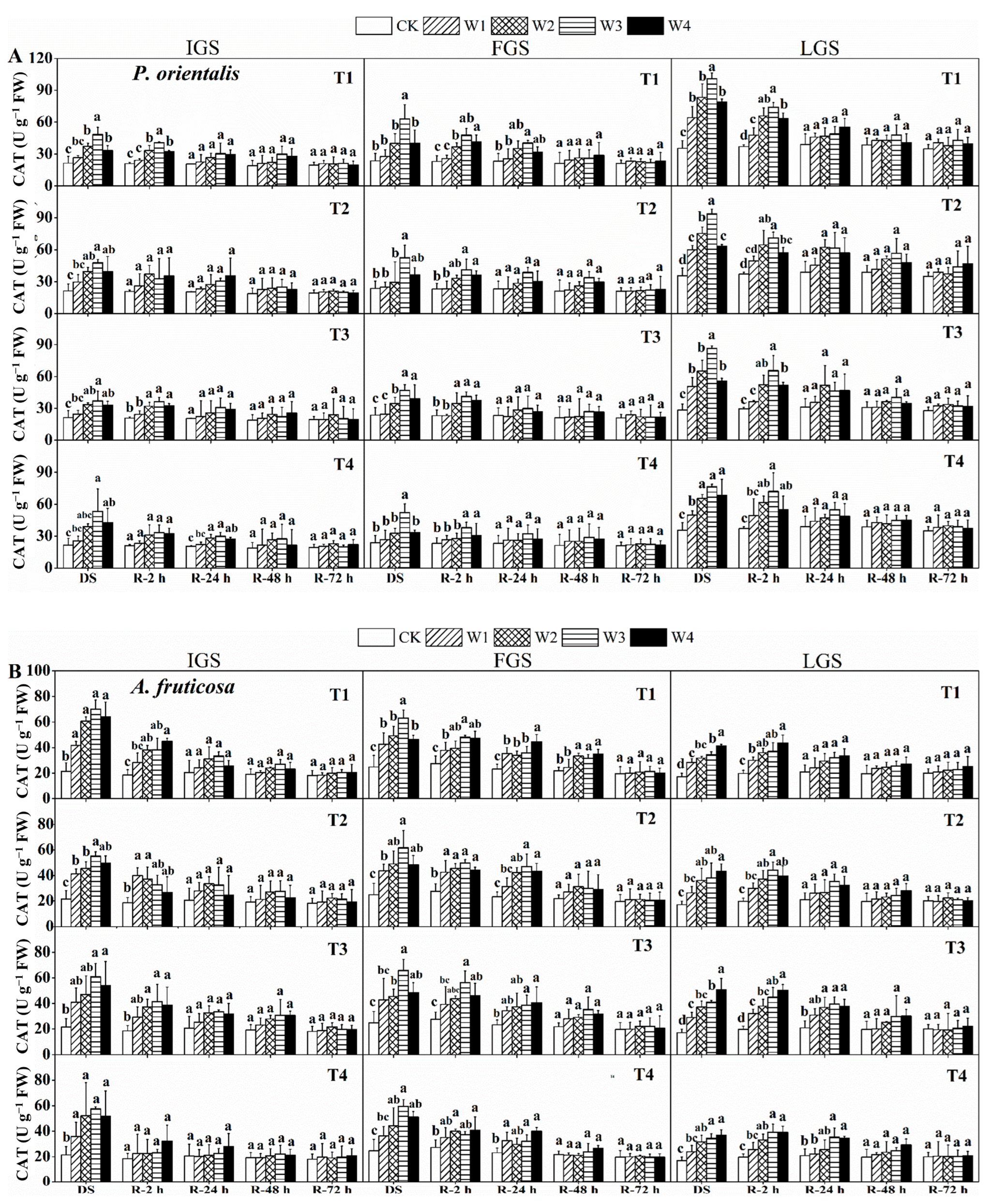
As shown in Figure 4, the CAT activity of both tree species increased initially and then decreased slightly as SWC decreased, with the exception of A. fruticosa in LGS, which showed a gradually increasing trend. P. orientalis had the highest CAT activity in T4, T1, and T3 under W3 in the IGS, FGS, and LGS, which were 2.50, 2.66, and 3.05 times higher than the control treatment, respectively. Similarly, in the IGS and FGS, the highest CAT activity was observed in T1 and T3 under W3, which were 3.27 and 2.65 times greater than the control treatment, respectively. The highest CAT activity in the LGS was observed in T3 under W4 for A. fruticosa seedlings, with a value of 50.72 U g−1 FW.
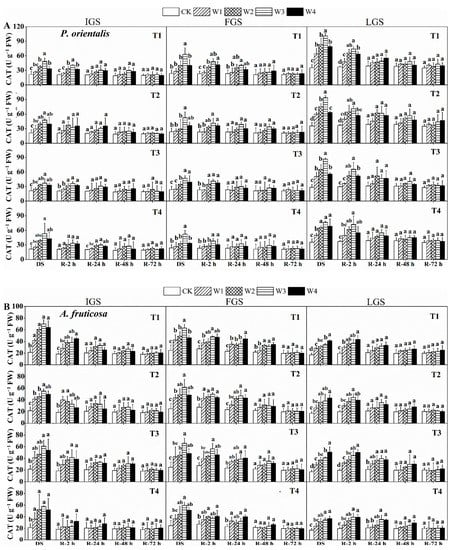
Figure 4.
The effect of drought stress and rewatering on catalase (CAT) activity in the leaves of P. orientalis (A) and A. fruticosa (B) seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. Different letters denote significant differences (p < 0.05) observed among the drought treatments.
The two tree species’ leaves responded differently to rewatering in terms of CAT activity. The CAT activity in all growth stages of P. orientalis and in the IGS and FGS of A. fruticosa decreased over time after rewatering. However, in the LGS of A. fruticosa, CAT activity first increased and then decreased. After two hours of rewatering, the CAT activity in T2 and T4 of P. orientalis (Figure 4A) in the IGS was almost identical to that of the control. In all drought stress treatments, with the exception of T4 under W2, W3, and W4, the CAT activity decreased almost to the control level after 24 h of rewatering. At 48 and 72 h after rewatering, CAT activity was not significantly different. With the exception of T1 under W3 after 24 h of rewatering, the CAT activity in drought stress treatments in FGS returned to the control level. After 48 h of rewatering, the changes in CAT activity were comparable to those of P. orientalis in IGS. After 24 h of rewatering in LGS, P. orientalis’ CAT activity essentially reached the control level, and then showed a downward trend, but there was no discernible difference from the control.
During rehydration, there were some variations in CAT activity for A. fruticosa (Figure 4B) at various growth stages. After two hours of rewatering in the IGS, the CAT activity in T4 began to resemble the control level. After 24 h of rewatering, the CAT activity decreased to the control level in all drought stress treatments and then remained largely stable. After 48 h of rewatering in the FGS, the CAT activity of A. fruticosa decreased to the control level (apart from T1 under W2, W3, and W4) and then remained largely stable. After 24 h of rewatering in the LGS, the CAT activity in T1 and T2 returned to the control level. The CAT activity of all treatments was essentially similar to the control treatment after 48 h of rewatering, and it then remained largely stable. The findings show that, after 72 h of rewatering, the CAT activity of the two tree species had nearly returned to the control level.
3.3. Effects of Drought and Rewatering on Osmotic Solutes
3.3.1. Soluble Sugar Content
Soluble sugar (SS) content response strategies in our selected seedlings at different growth stages varied with changes in soil water content (SWC) (Figure 5). As SWC decreased, the SS content of both tree species’ leaves increased. The SS content of P. orientalis seedlings gradually increased with decreasing SWC during the IGS, and it was significantly higher in T1, T2 (except for W1 treatment), T3, and T4 (except for W1, W2, and W3 treatments) compared to the control under drought stress. SS content increased significantly in W3 and W4 treatments during the FGS, with T1, T2, T3, and T4 under W4 treatment showing 1.60, 1.45, 1.26, and 1.40 times higher SS content than the control, respectively. The SS content in T2 and T4 under W1, as well as T3 under W1 and W2, was higher than the control. Except for T1 and T3 under W1, drought stress significantly increased the SS content in P. orientalis leaves during the late growth stage (LGS).
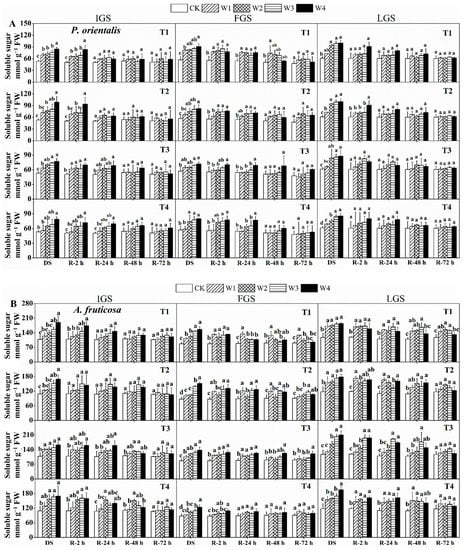
Figure 5.
The effect of drought stress and rewatering on soluble sugar content in the leaves of P. orientalis (A) and A. fruticosa (B) seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. Different letters denote significant differences (p < 0.05) observed among the drought treatments.
In the IGS, the SS contents of drought stress treatments (except for T1 and T2 under W1 and W2 treatments, and T4 under W1) were significantly higher than the control for A. fruticosa. Except for T4 in W1 (in FGS) and T3 in W1 (in LGS), the SS content increased significantly under drought stress treatments in the FGS and LGS.
The SS content of both species gradually decreased after rewatering at different time points, but the recovery rate was slightly different. Compared to the control, the SS content in the IGS and FGS in P. orientalis seedlings (Figure 5A) could generally recover to the control level 48 h after rewatering, with the exception of T1 in W1 of FGS. After 24 h of rewatering, the SS content in the LGS quickly recovered to the control level. The recovery rate of SS content in A. fruticosa (Figure 5B) was slightly slower than in P. orientalis. After 2 h of rewatering in the IGS, the SS content in T2 reached the same level as the control. The SS content in T1 recovered to the control level 24 h after rewatering and then followed a similar trend as the drought stress treatment for T2. The SS content with drought stress for T3 and T4 was still higher than the control 48 h after rewatering. After 72 h of rewatering, the SS content in all treatments had essentially returned to the control level.
After 24 h of rewatering, the SS content in T4 in the FGS recovered to the control level and then decreased slightly. The SS content of the treated plants had not returned to the control level 72 h after rewatering. The SS content in T3 under W1 was higher in the LGS at 2 and 24 h after rewatering than in the control. The SS content was decreasing 48 h after rewatering. Except for T1 under W1, W2, and W3, with higher SS content, it could be restored to the control level after 72 h of re-watering.
3.3.2. Proline Content
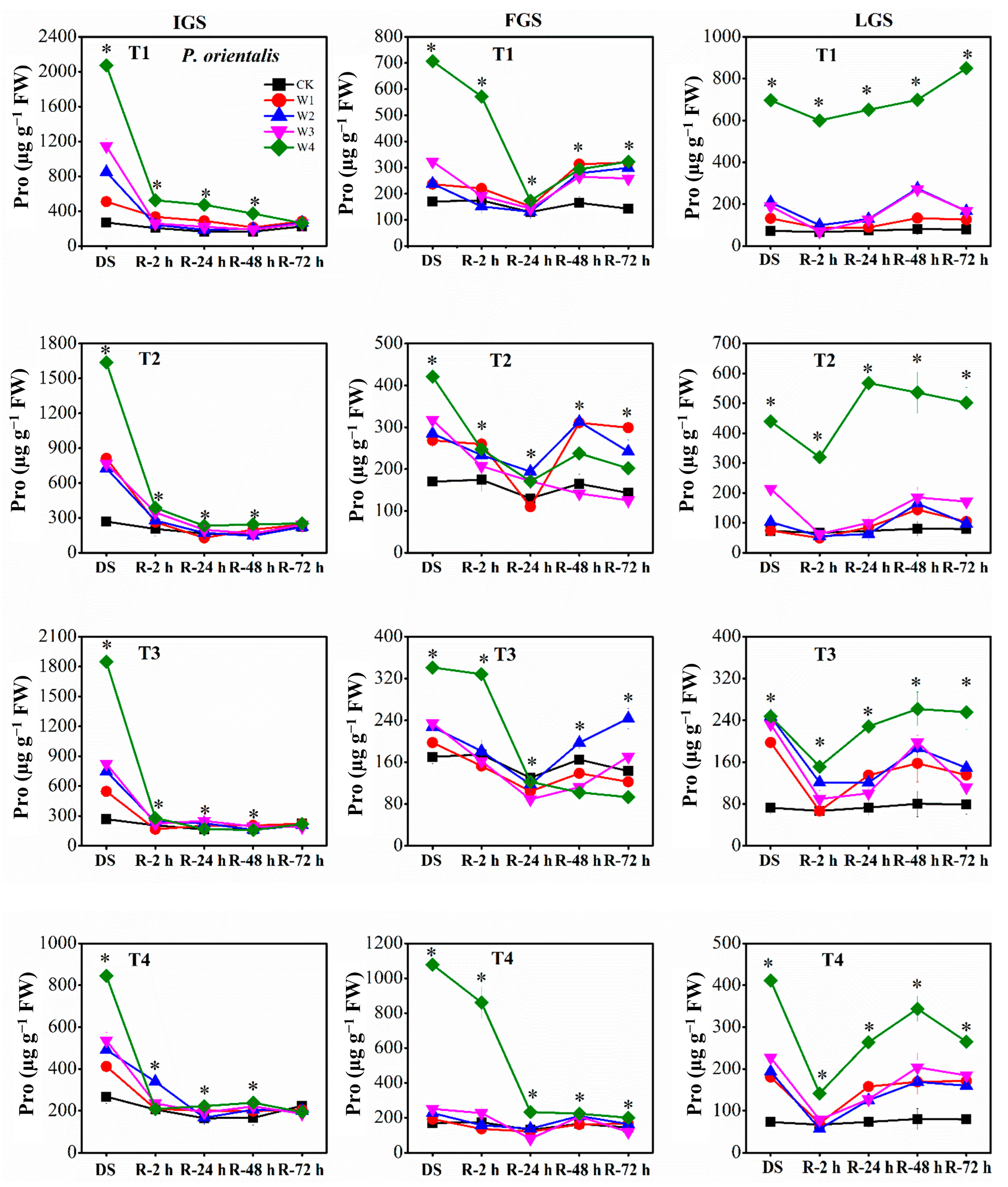
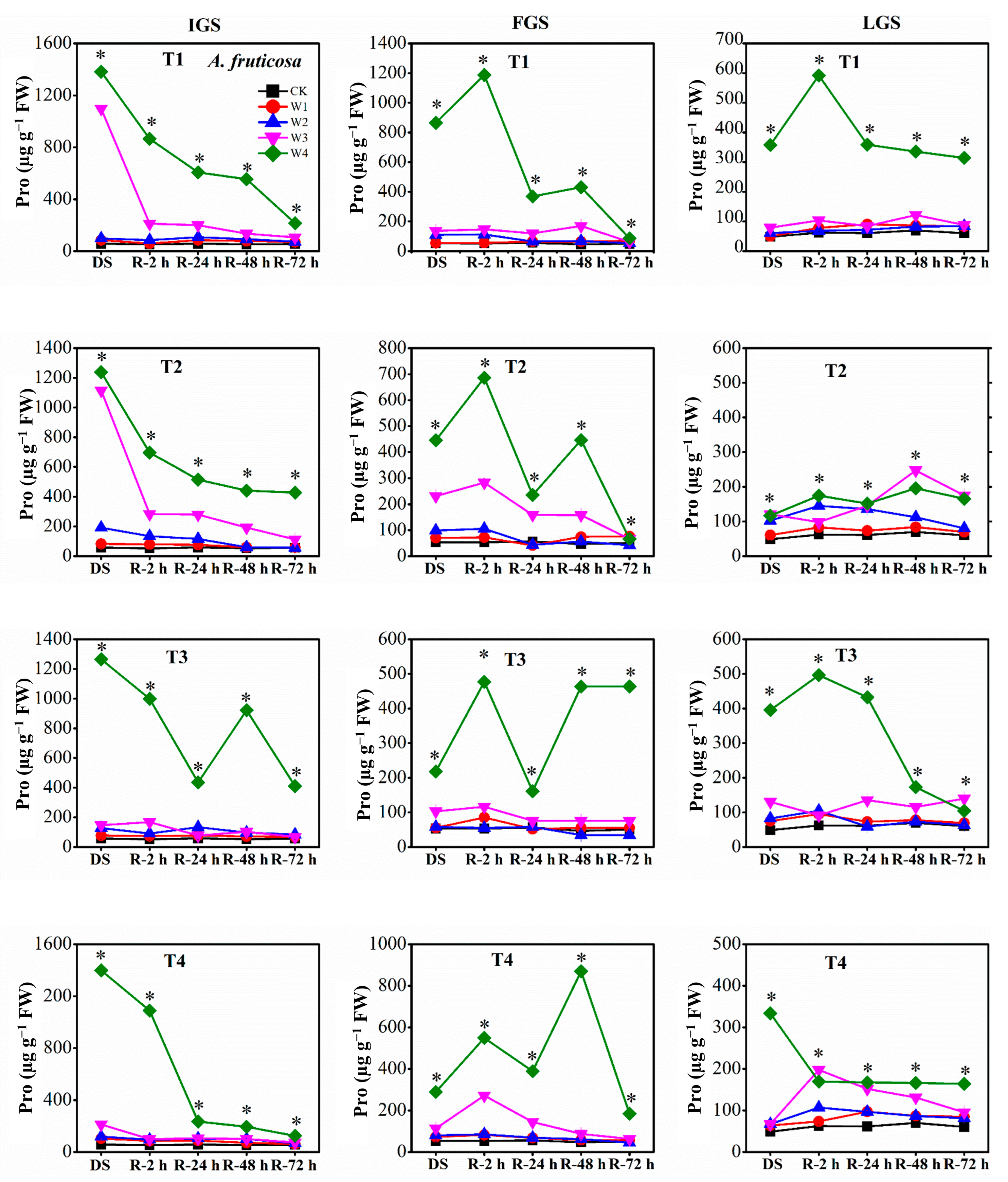
Our findings revealed that the proline (Pro) content of our experimental seedlings increased with increasing levels of drought stress throughout all growth stages. Specifically, the Pro content of W2, W3, and W4 treatments was significantly greater than that of the control (Figure 6). The Pro content of P. orientalis reached its maximum levels in T1, T4, and T1 under W4 in the IGS, FGS, and LGS, exhibiting 7.75, 6.34, and 9.56 times the control level, respectively. In contrast, the Pro content in the IGS, FGS, and LGS was highest in T4, T1, and T3 under W4 for A. fruticosa, with Pro levels 23.97, 16.19, and 8.12 times higher than the control level, respectively.
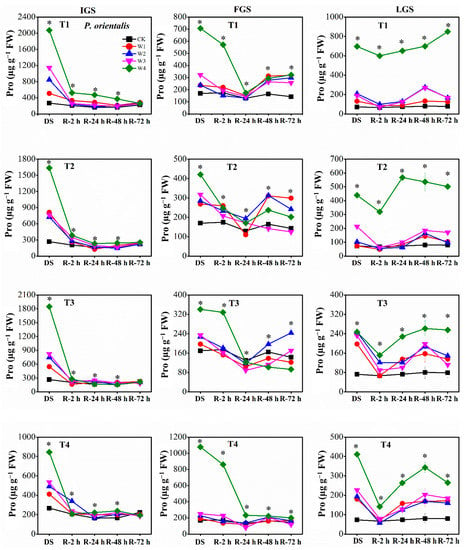
Figure 6.
The effect of drought stress and rewatering on proline (Pro) content in leaves of P. orientalis seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. * Significant differences among different drought stress treatments (p < 0.05).
The Pro content of the two afforestation species showed a decreasing trend as the duration of rewatering grew during the rehydration process. The ability of Pro to recover, however, varied slightly between the two species of trees. For P. orientalis (Figure 6), the Pro content in the IGS decreased gradually over time, after a rapid initial decline within 2 h of rewatering. The Pro content of all treatments had returned to the control level 72 h after rewatering. At 2 h, 24 h, and 48 h after rewatering, P. orientalis seedlings in the FGS displayed various patterns in terms of Pro content. The Pro content had not yet completely returned to the control level 72 h after rewatering, indicating a significant lag effect of rewatering after drought stress. With increasing rewatering time in the LGS, the Pro content initially decreased, then marginally increased, and finally decreased once more, with the exception of the Pro content in T1 under W4. The Pro content had not entirely returned to the control level 72 h after rewatering. In contrast, the Pro content of A. fruticosa (Figure 7) decreased slowly in the IGS after a rapid initial decline, with the exception of the Pro content in T3 under W4 after 48 h of rewatering. While the Pro contents of W3 and W4 in all drought stress treatments remained significantly higher than the control level at 72 h after rewatering, the Pro contents of T2 under W1 and W2, and T4 under W1, began to approach the control level. After rewatering in the FGS, the Pro content of W1, W2, and W3 initially increased and then gradually decreased, whereas the Pro content of W4 initially increased, then decreased, then slightly increased, and finally decreased. Except for the Pro content in T3 and T4 under W2, T1 under W2, and T4 under W3, the Pro content had not yet fully returned to the control level 72 h after rewatering. Overall, the Pro content in the LGS initially increased slightly before decreasing after rewatering. Except for the Pro content of A. fruticosa in T3 under W2, the Pro content decreased but remained higher than the control level at 72 h after rewatering.
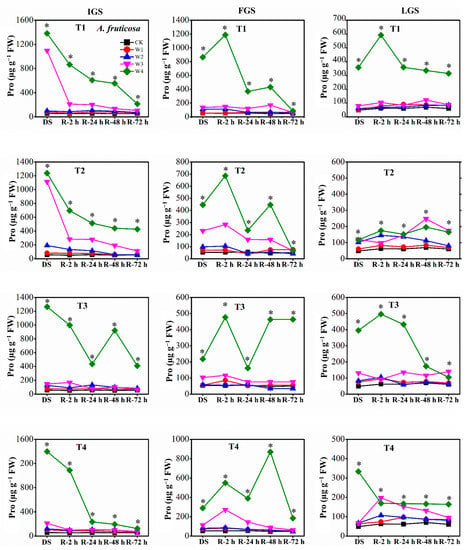
Figure 7.
The effect of drought stress and rewatering on proline (Pro) content in leaves of A. fruticosa seedlings. IGS, FGS, and LGS represent the initial, fast, and late growth stages, respectively. CK (100% SWC), W1 (88% SWC), W2 (70% SWC), W3 (52% SWC), W4 (40% SWC), T1 (15 days of drought stress), T2 (30 days), T3 (45 days), and T4 (60 days) indicate different soil water gradients and stress duration, respectively. DS refers to drought stress, while R-2 h, R-24 h, R-48 h, and R-72 h indicate time intervals after rewatering. Data are presented as mean ± SD, n = 3. * Significant differences among different drought stress treatments (p < 0.05).
4. Discussion
Revegetation is an essential component of ecosystem restoration and plays a significant role in mitigating the effects of drought. Drought is a significant environmental stressor that can negatively impact plant survival, growth, and ecosystem health as a whole [20,27]. Therefore, understanding how different plant species react to drought and how well they can recover after being rewatered is crucial for the success of revegetation projects. Recent research has concentrated on two tree species, P. orientalis and A. fruticosa, because of their capacity to adapt to and withstand drought stress. P. orientalis is a coniferous tree that is also called Chinese thuja or Oriental arborvitae. It grows in arid and semi-arid areas. A. fruticosa, also known as false indigo bush, is a deciduous shrub that is well-known for its drought tolerance. Both species possess unique drought-resistant characteristics, making them ideal candidates for reestablishing vegetation in arid and drought-prone areas.
Plants in arid and semiarid regions often experience periods of drought followed by rehydration as part of the normal cycle of growth and development. Due to structural and functional changes brought on by drought stress, malondialdehyde (MDA), a byproduct of lipid peroxidation, accumulates in plant cell membranes. MDA is a physiological indication of cell membrane damage, representing the severity of membrane impairment [28]. Our study found that the MDA content in the leaves of these two tree species increased as water deficit and drought duration increased, with W4 conditions being particularly notable. Excessive production of reactive oxygen species (ROS) has been linked to oxidative stress, which in turn has been linked to drought-induced lipid peroxidation of cell membranes [4].
In response to changes in the external environment brought on by drought stress and rewatering, plants display a variety of adaptive responses that involve morphological, physiological, biochemical, and molecular mechanisms, as well as changes in gene expression [27,29]. The antioxidant enzyme system and osmotic regulation system in plants play crucial roles in mitigating the effects of drought [3]. In a previous study, Goodarzian Ghahfarokhi et al. (2015) [30] reported that changes in the activity of antioxidant enzymes, such as superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), at different growth stages contribute to a plant’s resistance against excessive soil moisture stress. Furthermore, osmoprotectants, such as proline (Pro) and soluble sugars (SS), have been identified as key components in osmotic adjustment, improving drought resistance in a variety of plant species [31,32]. In our study, we found that SOD, POD, and CAT activities, together with Pro and SS contents, increased with both the duration and intensity of water stress, which supports previous findings [17,19] that P. orientalis and A. fruticosa have the ability to adapt to drought stress. However, the W4 treatment resulted in notable decreases in the activities of antioxidant enzymes and the contents of the two osmotic solutes. This is also in accordance with Liu et al. (2017) [33], who stated that water stress significantly influenced the antioxidant enzyme system of Hippophae rhamnoides; the activities of antioxidant enzymes (SOD, POD and CAT) of seedlings increased at first and then decreased with the increase of drought, and the antioxidant enzyme system was severely impaired under severe water stress (soil relative water content < 38.9%).
Different antioxidant and osmoregulation system recovery capacities were observed between the two tree species following rewatering. When plants that have endured a period of drought are rewatered, it takes them some time to adjust to the new conditions, but they soon mount a swift and immediate response to the availability of water. The activation of antioxidant defenses and repair mechanisms plays a crucial role in the prevention of oxidative damage and the restoration of cellular homeostasis [34]. As a result, MDA levels continue to decline, resulting in non-significant changes in MDA levels at 24, 48, and 72 h after rewatering. The proline content of P. orientalis in the later growth stage did not fully recover to control levels, whereas in other growth stages of P. orientalis and all growth stages of A. fruticosa, it fully returned to normal at 72 h after rewatering. In all cases, the W4 treatment showed significantly higher proline content than the control. These results show that the capacity to recover after rewatering varies depending on the species and growth stage. However, drought-resistant plants can recover quickly after a few days of normal watering, even compensating for lost growth during the drought period [35].
Indeed, research on rewatering plants after a drought has shown a variety of compensatory effects [14]. Improved growth and development are not the only changes that occur in plants after being rewatered; antioxidant enzyme activities and osmotic solute contents also shift [36]. Upon being re-watered, both P. orientalis and A. fruticosa showed a rapid increase in antioxidant enzyme activities and osmotic regulation systems, indicating that the oxidative stress brought on by drought had been effectively mitigated. The species that were chosen showed strong sensitivity and the potential to quickly recover after receiving more water. However, the indexes that were measured during the rewatering period showed that the two species were slightly different in certain respects. Overall, drought-resistant plants can recover quickly after a few hours to days of normal watering, and in some cases, they can even increase growth to compensate for the losses incurred during the drought period. Our findings highlight the various drought and compensatory effects observed in the two tree species, indicating that the extent of recovery is affected by factors such as plant species, growth stage, drought intensity, and drought duration.
5. Conclusions
In this study, we examined how P. orientalis and A. fruticosa seedlings responded to water stress and subsequent rewatering at various growth stages. Our research demonstrated how well both species were able to adapt to drought stress. Both species demonstrated a notable ability to recover after rewatering through the efficient regulation of antioxidant enzyme activities and biochemical processes. It is important to note that the two species’ recovery capacities differed slightly in terms of osmoregulation compounds and antioxidant enzyme activities. However, normal function was successfully restored in both species within a relatively short time frame of 24 to 72 h after rewatering, similar to the control group. However, the W4 treatment (40% soil water content), where some exceptions were noted, led to an intriguing observation. These exceptions implied that water content below this level might potentially be detrimental to plants, highlighting the significance of maintaining at least a certain level of soil moisture for plant growth and recovery. Overall, our results highlight the remarkable drought stress tolerance of P. orientalis and A. fruticosa seedlings, as well as their potential to recover from such stressors via regulatory mechanisms. These insights contribute to our understanding of plant resilience and have implications for future drought mitigation and sustainable agricultural practices.
Author Contributions
Conceptualization, S.F. and J.W.; methodology, S.F.; software, S.F.; validation, J.W.; formal analysis, S.F., A.S., R.R. and Y.H.; investigation, S.F., A.S., R.R., J.W. and Y.H.; resources, J.W.; data curation, S.F., A.S. and R.R.; writing—original draft preparation, S.F.; writing—review and editing, S.F., S.S., A.S., R.R. and J.W.; visualization, S.F., A.S., R.R., J.W. and Y.H.; supervision, J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31670713), Nation Key R&D Program of China (2017YFC0504402), Doctoral Research Foundation Project of Xianyang Vocational and Technical College (2021BK05) and Shaanxi Province Science and Technology Co-ordination Innovation Project (2016KTCL03-18).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trenberth, K.E.; Dai, A.; Van Der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Chang. 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Feild Crops Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Roy, R.; Mostofa, M.G.; Wang, J.; Fornara, D.; Sarker, T.; Zhang, R. Revegetation intervention of drought-prone coal-mined spoils using Caragana korshinskii under variable water and nitrogen-phosphorus resources. Agric. Water Manag. 2021, 246, 106712. [Google Scholar] [CrossRef]
- Roy, R.; Wang, J.; Mostofa, M.G.; Fornara, D. Optimal water and fertilizer applications improve growth of Tamarix chinensis in a coal mine degraded area under arid conditions. Physiol. Plant. 2021, 172, 371–390. [Google Scholar] [CrossRef]
- Shimpl, F.C.; Ferreira, M.J.; Jaquetti, R.K.; Martins, S.C.V.; de Carvalho Gonçalves, J.F. Physiological responses of young Brazil nut (Bertholletia excelsa) plants to drought stress and subsequent rewatering. Flora Morphol. Distrib. Funct. Ecol. Plants 2019, 252, 10–17. [Google Scholar] [CrossRef]
- Merwad, A.R.M.A.; Desoky, E.S.M.; Rady, M.M. Response of Water Deficit-Stressed Vigna Unguiculata Performances to Silicon, Proline or Methionine Foliar Application. Sci. Hortic. 2018, 228, 132–144. [Google Scholar] [CrossRef]
- Yan, W.; Zhong, Y.; Shangguan, Z. Rapid response of the carbon balance strategy in Robinia pseudoacacia and Amorpha fruticosa to recurrent drought. Environ. Exp. Bot. 2017, 138, 46–56. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, J.; Yang, N. Growth responses, antioxidant enzyme activities and lead accumulation of Sophora japonica and Platycladus orientalis seedlings under Pb and water stress. Plant Growth Regul. 2015, 75, 383–389. [Google Scholar] [CrossRef]
- Cao, X.; Jia, J.; Zhang, C.; Li, H.; Liu, T.; Jiang, X.; Polle, A.; Peng, C.; Luo, Z. Bin Anatomical, physiological and transcriptional responses of two contrasting poplar genotypes to drought and re-watering. Physiol. Plant. 2014, 151, 480–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, S.; Cao, B.; Cao, D.; Leng, G.; Li, H.; Yin, L.; Shan, L.; Deng, X. Genotypic variation in growth and physiological response to drought stress and re-watering reveals the critical role of recovery in drought adaptation in maize seedlings. Front. Plant Sci. 2016, 6, 1–15. [Google Scholar] [CrossRef]
- Xu, Z.; Zhou, G.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef]
- Hamerlynck, E.P.; Smith, B.S.; Sheley, R.L.; Svejcar, T.J. Compensatory Photosynthesis, Water-Use Efficiency, and Biomass Allocation of Defoliated Exotic and Native Bunchgrass Seedlings. Rangel. Ecol. Manag. 2016, 69, 206–214. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Chen, X.; Fu, J.; Zhang, Y. Metabolic and growth responses of maize to successive drought and re-watering cycles. Agric. Water Manag. 2016, 172, 62–73. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, J.J.; Sun, R.H.; Hou, X.G.; Zhao, W.; Shi, J.; Zhang, Y.F.; Qi, L.; Li, X.L.; Dong, P.H.; et al. Correlation of the corn compensatory growth mechanism after post-drought rewatering with cytokinin induced by root nitrate absorption. Agric. Water Manag. 2016, 166, 77–85. [Google Scholar] [CrossRef]
- Roy, R.; Mostofa, M.G.; Wang, J.; Sikdar, A.; Sarker, T. Improvement of growth performance of Amorpha fruticosa under contrasting regime of water and fertilizer in coal-contaminated spoils using response surface methodology. BMC Plant Biol. 2020, 20, 1–15. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, X.; Ma, W.; Song, J.; Rahman, S.U.; Wang, J.; Zhang, Y. Morphological and physiological responses to cyclic drought in two contrasting genotypes of Catalpa bungei. Environ. Exp. Bot. 2017, 138, 77–87. [Google Scholar] [CrossRef]
- Yan, W.; Zheng, S.; Zhong, Y.; Shangguan, Z. Contrasting dynamics of leaf potential and gas exchange during progressive drought cycles and recovery in Amorpha fruticosa and Robinia pseudoacacia. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Wang, L.; Dai, Y.; Sun, J.; Wan, X. Differential hydric deficit responses of Robinia pseudoacacia and Platycladus orientalis in pure and mixed stands in northern China and the species interactions under drought. Trees-Struct. Funct. 2017, 31, 2011–2021. [Google Scholar] [CrossRef]
- Aliarab, A.; Vazifekhah, E.O.; Sadati, S.E. Effect of soil moisture content and nitrogen fertilizer on survival, growth and some physiological characteristics of Platycladus orientalis seedlings. J. For. Sci. 2020, 66, 511–523. [Google Scholar] [CrossRef]
- Ben Hamed, S.; Lefi, E.; Chaieb, M. Physiological responses of Pistacia vera L. versus Pistacia atlantica Desf. to water stress conditions under arid bioclimate in Tunisia. Sci. Hortic. 2016, 203, 224–230. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, F.; Wang, G.; Zhang, G.; Wang, Y.; Chen, X.; Mao, Z. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensis Rehd. seedlings under replant conditions. Sci. Hortic. 2014, 175, 9–15. [Google Scholar] [CrossRef]
- Roy, R.; Wang, J.; Golam, M.; Fornara, D.; Sikdar, A.; Sarker, T.; Wang, X.; Shah, M. Fine-tuning of soil water and nutrient fertilizer levels for the ecological restoration of coal-mined spoils using Elaeagnus angustifolia. J. Environ. Manag. 2020, 270, 110855. [Google Scholar] [CrossRef] [PubMed]
- Ekmekci, Y.; Terzioglu, S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic. Biochem. Physiol. 2005, 83, 69–81. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of Hydrogen Peroxide. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Joseph, H.R. The determination of sugar in blood and spinal fluid with anthrone reagent. J. Biol. Chem. 1955, 212, 335–343. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Khoyerdi, F.F.; Shamshiri, M.H.; Estaji, A. Changes in some physiological and osmotic parameters of several pistachio genotypes under drought stress. Sci. Hortic. 2016, 198, 44–51. [Google Scholar] [CrossRef]
- Kong, X.; Wei, B.; Gao, Z.; Zhou, Y.; Shi, F.; Zhou, X.; Zhou, Q.; Ji, S. Changes in Membrane Lipid Composition and Function Accompanying Chilling Injury in Bell Peppers. Plant Cell Physiol. 2018, 59, 167–178. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Goodarzian Ghahfarokhi, M.; Mansurifar, S.; Taghizadeh-Mehrjardi, R.; Saeidi, M.; Jamshidi, A.M.; Ghasemi, E. Effects of drought stress and rewatering on antioxidant systems and relative water content in different growth stages of maize (Zea mays L.) hybrids. Arch. Agron. Soil Sci. 2015, 61, 493–506. [Google Scholar] [CrossRef]
- Roy, R.; Mahboob, M.G.; Arena, C.; Kader, M.A.; Sultana, S.; Hasan, A.K.; Wang, J.; Sarker, T.; Zhang, R.; Barmon, M. The Modulation of Water, Nitrogen, and Phosphorous Supply for Growth Optimization of the Evergreen Shrubs Ammopiptanthus mongolicus for Revegetation Purpose. Front. Plant Sci. 2021, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Nú˜nez-Delgado, A.; Sultana, S.; Wang, J.; Munir, A.; Battaglia, M.L.; Sarker, T.; Seleiman, M.F.; Barmon, M.; Zhang, R. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J. Environ. Manag. 2021, 295, 113076. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, R.; Zhang, G.; Guo, J.; Dong, Z. Effects of soil drought on photosynthetic traits and antioxidant enzyme activities in Hippophae rhamnoides seedlings. J. For. Res. 2017, 28, 255–263. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; Da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer Science & Business Media: Berlin, Germany, 2012; pp. 261–315. ISBN 9789400722200. [Google Scholar]
- Couchoud, M.; Salon, C.; Girodet, S.; Jeudy, C.; Vernoud, V.; Prudent, M. Pea Efficiency of Post-drought Recovery Relies on the Strategy to Fine-Tune Nitrogen Nutrition. Front. Plant Sci. 2020, 11, 204. [Google Scholar] [CrossRef]
- Abid, M.; Ali, S.; Qi, L.K.; Zahoor, R.; Tian, Z.; Jiang, D.; Snider, J.L.; Dai, T. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2018, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).