Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review
Abstract
:1. Introduction
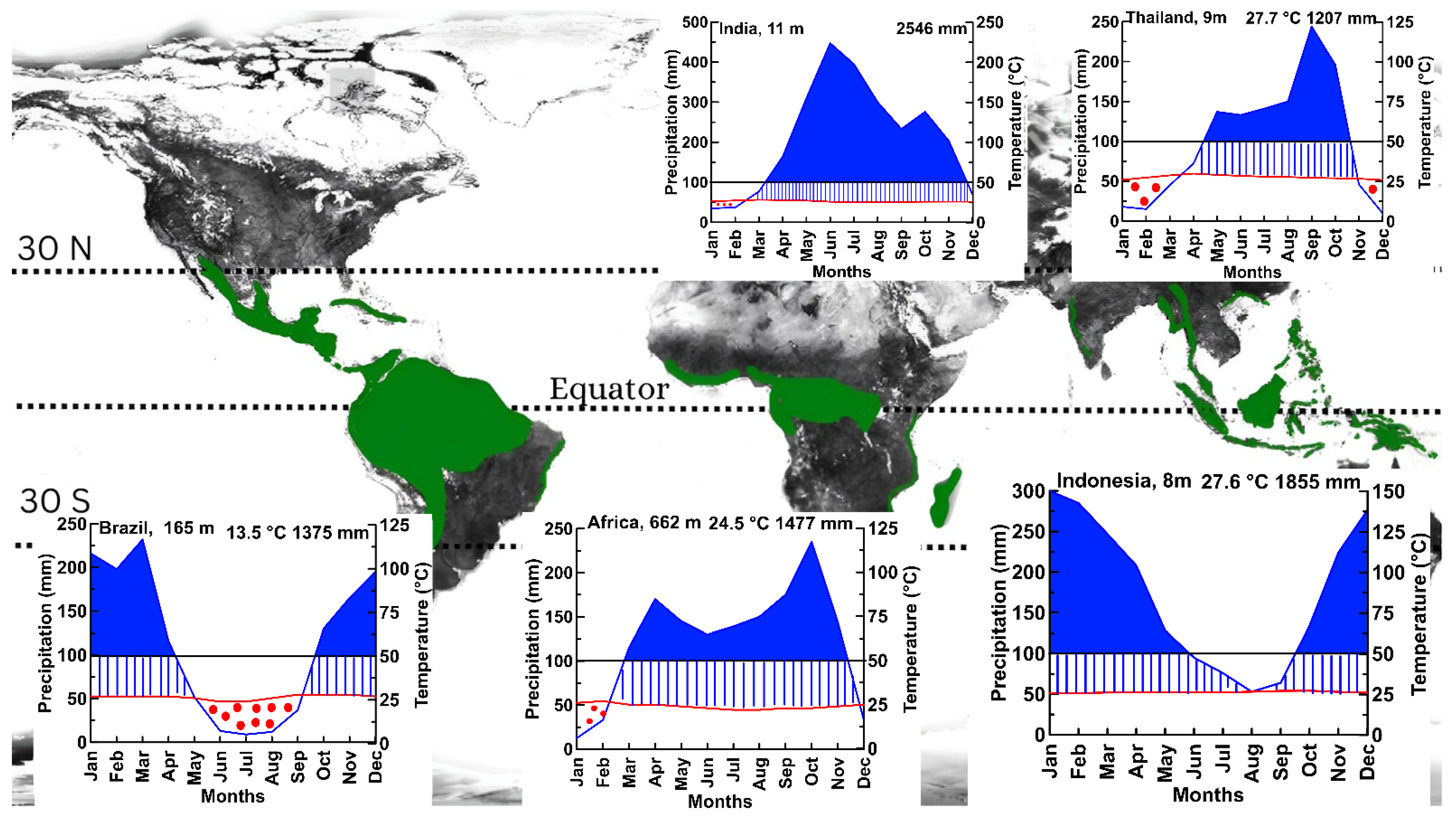
- A lack of distinct growth rings: In temperate regions, trees often produce annual growth rings that can be used to determine their age accurately. However, many tropical trees do not produce distinct annual rings, or they may produce rings that are difficult to distinguish, making it more challenging to accurately determine their age and track changes in growth over time [22].
- High species diversity: Tropical regions are known for their high species diversity, which can make it challenging to study specific tree species in depth. Researchers may study multiple species in order to obtain a comprehensive understanding of tree age and xylogenesis in a given region [23].
- Limited data availability: In many tropical regions, data on tree age and xylogenesis may be limited or unavailable, making it difficult for researchers to develop accurate models and identify patterns over time.
- Environmental factors: Tropical regions are subject to various environmental factors impacting tree growth, including temperature, rainfall, and soil nutrient availability. Understanding how these factors impact tree age and xylogenesis can be complex and may require long-term studies and advanced analytical tools [24].
2. South America
- Dendrochronology: the study of tree rings. It can provide information on the age and growth of trees. In tropical regions, where growth rings may not be as distinct, researchers may use other tree ring parameters such as tree ring width, density, and isotopic composition to infer past climate conditions and tree growth [38,39,41,73].
- Radiocarbon dating can be used to estimate the age of trees by measuring the amount of radiocarbon in the wood [72].
- Remote sensing: Remote sensing techniques, such as light detection and ranging (LiDAR), can be used to measure the size and shape of trees and their growth patterns and structural characteristics [74].
| Tree-Name/Information | Family | Location/Climate | Methods | Cambial/Wood Formation | References |
|---|---|---|---|---|---|
| Well-drained site Brosimum lactescens Dipteryx panamensis Goethalsia meiantha Hampea appendiculata Hymenolobium mesoamericanum, Laetia procera Pentaclethra macroloba Virola sebifera Swamp site Carapa guianensis Hernandia didymantha Pentaclethra macroloba Virola koschnyi | Moraceae Fabaceae Malvaceae Malvaceae Fabaceae Salicaceae Fabaceae Myristicaceae Meliaceae Hernandiaceae Fabaceae Myristicaceae | La Selva, Atlantic lowlands of northeastern Costa Rica; wet and weakly seasonal | Radial dendrometer | Not directly studied. They considered cambial dormancy whenever no growth was registered for several days. | [35] |
| Bombacopsis quinate Cordia apurensis Terminalia guianensis Sapium styllare Cedrela odorata Swietenia macrophylla Pterocarpus vernalis Pinus caribaea Tectona grandis | Bombacaceae Boraginaceae Combretaceae Euphorbiaceae Meliaceae Meliaceae Papilionaceae Pinaceae Verbenaceae | Semi-deciduous forest of the Reserva forestall de Caparo, Venezuela; a dry season with mean monthly rainfall of <50 mm from January to March; the mean annual precipitation is 1700 mm per year | Tree-ring analysis, dendrometer, cambial marking | Distinctive growth zones appeared, except Cordia apurensis, annual growth period was related to rainfall pattern. | [38] |
| 139 species (see Table S1) | South America | Dendrochronology | [39] | ||
Cordia alliodora Luehea candida Randia sp. Tabebuia ochracea T. rosea
Astronium graveolens Hymenaea courbaril Licania arborea Samanea saman Sideroxylon tempisque Simarouba glauca Swietenia macrophylla Thouinidium pentandrum
Chlorophora tinctoria Dalbergia retusa Diospyros nicaraguensis Guazuma ulmifolia Lonchocarpus minimiflorum Myrospermum frutescens Piscidia carthaginensis
Bursera simatuba Cochlospermum vitifolium Plumeria rubra Spondias purpurea | Rubiaceae Boraginaceae Tiliaceae Rubiaceae Bignoniaceae Bignoniaceae Caesalpinaceae Anacardiaceae Caesalpinaceae Chrysobalanaceae Mimosaceae Sapotaceae Simaroubaceae Meliaceae Sapindaceae Caesalpinaceae Moraceae Fabaceae Ebenaceae Sterculiaceae Fabaceae Fabaceae Fabaceae Bombaceae Burseraceae Cochlospermaceae Apocynaceae Anacardiaceae | Hacienda La Pacifica, Cañas, Guanacaste, Costa Rica, dry forest area | Vegetative phenology | Not directly studied. | [53] |
| Cedrela fissilis | Meliaceae | São Paulo State, Brazil | Wood formation, phenology | The active period coincides with the wet season, growth rings are marked by parenchyma band, and the large early wood vessels of the growth rings are formed. | [58] |
| Aphananthe monoica Plearanthodendron lindenii Psychotria costivenia | Cannabaceae Salicaceae Rubiaceae | Sub-tropical rainforest located in central Veracruz, Mexico; total annual rainfall was 2217.2 mm, the rainy season lasts 4 months (June–September); there is a short dry season (March and April) | 1 ha plot, measured diameter increment. Generalized canonical correlation analysis (GCCA), three thermo-hygrometer data loggers | Not directly studied. Cambial activity periods were associated with maximum temperature and day length. | [75] |
| Schizolobium parahyba | Fabaceae | School of Agronomic Science, Sào Paulo; the mean annual rainfall for 2002–2003 was 1399 mm, the mean annual air temperature was 20.2 °C | Wood block, phenology | The reduction in cambial activity to a minimum correlates with the dry season and leaf fall. The higher cambial activity correlates to the wet season. | [77] |
| Astronium graveolens Aspidosperma polyneuron Tabebuia serratifolia Zeyheria tuberculate Savia dictyocarpa Ocotea porosa Cariniana estrellensis Cariniana legalis Caesalpinia ferrea Copaifera langsdorffii Hymenaea courbaril Peltophorum dubium Schizolobium parahyba Anadenanthera macrocarpa Piptadenia gonoacantha Centrolobium tomentosum Dipteryx alata Myroxylon balsamum Platycyamus regnellii Colubrina glandulosa Balfourodendron riedelianum Esenbeckia leiocarpa Guazuma ulmifolia Aegiphila sellowiana | Anacardiaceae Apocynaceae Bignoniaceae Bignoniaceae Euphorbiaceae Lauraceae Lecythidaceae Lecythidaceae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Leguminosae Mimosaceae Mimosaceae Leguminosae. Leguminosae Rhamnaceae Rutaceae Rutaceae Sterculiaceae Verbenaceae | Southeast Brazil, semi-deciduous forest | Plant phenology observation, window method, and permanent dendrometer band | The semi-deciduous trees show a reduced rate of incremental growth during May and June (dry season) and faster rate during October and November (early rainy season). | [61] |
| Aphananthe monoica Pleuranthodendron lindenii Psychotria costivenia | Cannabaceae Salicaceae Rubiaceae | 1 ha, a subtropical rainforest located in central Veracruz, Mexico; total annual rainfall was 2217.2 mm, the rainy season lasts 4 months (June–September), There is a short dry season (March and April) | Phenology, radial growth | Leaf initiation, flowering, and vascular cambium activity were the most closely related simultaneous events during the summer (April–August). | [75] |
| Citharexylum myrianthum Senna multijuga | Verbenaceae Fabaceae | Rio Cachoeira Reserve in Antonina, Parana State, southern coast of Brazil | Stem growth, phenology | Not directly studied. | [68] |
| Swietenia macrophylla Hymenaea courbaril Parkia pendula | Meliaceae Fabaceae Fabaceae | A seasonal dry tropical forest in southeast Parà, Brazil | Vernier dendrometer, phenology | Not directly studied. | [63] |
| Cedrela odorata Swietenia macrophylla Carapa guianensis | Meliaceae Meliaceae Meliaceae | Manaus-Amazȏnas, Aripuanã-Mato Grosso, Santarem-Paral | Stem discs, cambium samples | S. macrophylla had cambial dormancy from September to November and cambial reactivation in May; in C. odorata, cambium divided from January to April, and was dormant from September to November; in C. guianensis, cambium dormancy was not consistent. | [56] |
| Cedrela odorata Swietenia macrophylla | Meliaceae Meliaceae | Rio Branco, Brazil | Tree ring, discs | S. macrophylla, cambial activity occurred throughout almost the whole year. C. odorata, cambial activity occurred in the rainy season from September of the previous year to June of the current year. | [57] |
| Citharexylum myrianthum Schizolobium parahyba Senna multijuga Virola bicuhyba Handroanthus serratifolius Cabralea canjerana Cariniana estrellensis Inga edulis Inga marginata Myrsine coriacea | Verbenaceae Fabaceae Fabaceae Myristicaceae Bignoniaceae Meliaceae Lecythidaceae Fabaceae Fabaceae Primulaceae | The wet areas of the Atlantic Forest; an average rainfall of 1778 mm, no dry season and rare occurrence of frosts; the average temperature was 20.8 degrees Celsius | Permanent dendrobands | The girth increments of the 10 species were, in general, weakly or not related to rainfall, but strongly and positively related to temperature and day length. | [70] |
| Kielmeyera grandiflora | Calophyllaceae | In the Cerrado sensu stricto, Botucatu, Sào Paulo, Brazil | Bud and leaf phenology, wood block | The cambium was dormant in May, during the rainy season. Photoperiod and temperature may be important in controlling the growth of K. grandiflora. | [66] |
| Cordiera concolor | Rubiaceae | Botanical Garden of the Uni, Estadual Paulista (UNESP) | growth dynamics of an evergreen shrubby species | The cambium is dormant during the rainy season. Cambial activity was positively related to day length, and although it occurred in the rainy season. | [64] |
3. Tropical and Subtropical Africa
4. Southwest Asia
5. Southeast Asia
6. Future Research
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Alessandro, S. Non-linear dynamics of population and natural resources: The emergence of different patterns of development. Ecol. Econ. 2007, 62, 473–481. [Google Scholar] [CrossRef]
- Wagner, A.O.; Lackner, N.; Mutschlechner, M.; Prem, E.M.; Markt, R.; Illmer, P. Biological pretreatment strategies for second-generation lignocellulosic resources to enhance biogas production. Energies 2018, 11, 1797. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef]
- Brooks, T.M.; Mittermeier, R.A.; Da Fonseca, G.A.; Gerlach, J.; Hoffmann, M.; Lamoreux, J.F.; Mittermeier, C.G.; Pilgrim, J.D.; Rodrigues, A.S. Global biodiversity conservation priorities. Science 2006, 313, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, Y.; Zhou, L.; Li, B.; Luo, Y. An imperative need for global change research in tropical forests. Tree Physiol. 2013, 33, 903–912. [Google Scholar] [CrossRef]
- Artaxo, P.; Hansson, H.C.; Machado, L.A.T.; Rizzo, L.V. Tropical forests are crucial in regulating the climate on Earth. PLoS Clim. 2022, 1, e0000054. [Google Scholar] [CrossRef]
- Crowther, T.W.; Glick, H.B.; Covey, K.R.; Bettigole, C.; Maynard, D.S.; Thomas, S.M.; Smith, J.R.; Hintler, G.; Duguid, M.C.; Amatulli, G. Mapping tree density at a global scale. Nature 2015, 525, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Stahle, D.; Mushove, P.; Cleaveland, M.; Roig, F.; Haynes, G. Management implications of annual growth rings in Pterocarpus angolensis from Zimbabwe. For. Ecol. Manag. 1999, 124, 217–229. [Google Scholar] [CrossRef]
- Plomion, C.; Leprovost, G.; Stokes, A. Wood formation in trees. Plant Physiol. 2001, 127, 1513–1523. [Google Scholar] [CrossRef]
- Smith, C.R.; De Leo, F.C.; Bernardino, A.F.; Sweetman, A.K.; Arbizu, P.M. Abyssal food limitation, ecosystem structure and climate change. Trends Ecol. Evol. 2008, 23, 518–528. [Google Scholar] [CrossRef]
- Walter, H.; Lieth, H. Klimadiagramm-Weltatlas; VEB Gustav Fischer Verlag: Jena, Germany, 1967. [Google Scholar]
- Wright, S.J. Tropical forests in a changing environment. Trends Ecol. Evol. 2005, 20, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Deb, J.; Phinn, S.; Butt, N.; McAlpine, C. Climate change impacts on tropical forests: Identifying risks for tropical Asia. J. Trop. For. Sci. 2018, 30, 182–194. [Google Scholar]
- Wang, G.; Mang, S.L.; Riehl, B.; Huang, J.; Wang, G.; Xu, L.; Huang, K.; Innes, J. Climate change impacts and forest adaptation in the Asia–Pacific region: From regional experts’ perspectives. J. For. Res. 2019, 30, 277–293. [Google Scholar] [CrossRef]
- Zimmerman, B.L.; Kormos, C.F. Prospects for sustainable logging in tropical forests. Bioscience 2012, 62, 479–487. [Google Scholar]
- Carvalho, E.A., Jr.; Mendonça, E.N.; Martins, A.; Haugaasen, T. Effects of illegal logging on Amazonian medium and large-sized terrestrial vertebrates. For. Ecol. Manag. 2020, 466, 118105. [Google Scholar] [CrossRef]
- Murphy, B.P.; Bowman, D.M. What controls the distribution of tropical forest and savanna? Ecol. Lett. 2012, 15, 748–758. [Google Scholar] [CrossRef]
- Feeley, K.J.; Joseph Wright, S.; Nur Supardi, M.; Kassim, A.R.; Davies, S.J. Decelerating growth in tropical forest trees. Ecol. Lett. 2007, 10, 461–469. [Google Scholar] [CrossRef]
- Chambers, J.Q.; Asner, G.P.; Morton, D.C.; Anderson, L.O.; Saatchi, S.S.; Espírito-Santo, F.D.; Palace, M.; Souza, C. Regional ecosystem structure and function: Ecological insights from remote sensing of tropical forests. Trends Ecol. Evol. 2007, 22, 414–423. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Jørgensen, H.B. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 2010, 98, 754–763. [Google Scholar] [CrossRef]
- Putz, F.E.; Blate, G.M.; Redford, K.H.; Fimbel, R.; Robinson, J. Tropical forest management and conservation of biodiversity: An overview. Conserv. Biol. 2001, 15, 7–20. [Google Scholar] [CrossRef]
- Worbes, M.; Herawati, H.; Martius, C. Tree growth rings in tropical peat swamp forests of Kalimantan, Indonesia. Forests 2017, 8, 336. [Google Scholar] [CrossRef]
- Wright, J.S. Plant diversity in tropical forests: A review of mechanisms of species coexistence. Oecologia 2002, 130, 1–14. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Baker, P.J.; Groenendijk, P.; Schippers, P.; van der Sleen, P.; Vlam, M.; Sterck, F. Tropical forests and global change: Filling knowledge gaps. Trends Plant Sci. 2013, 18, 413–419. [Google Scholar] [CrossRef]
- Waisel, Y.; Fahn, A. The Effects of Environment on Wood Formation and Cambial Activity in Robina pseudacacia L. New Phytol. 1965, 64, 436–442. [Google Scholar] [CrossRef]
- Baas, P.; Vetter, R.E. Growth Rings in Tropical Trees; Rijksherbarium: Leiden, The Netherlands, 1989. [Google Scholar]
- Sheppard, P.R. Dendroclimatology: Extracting climate from trees. Wiley Interdiscip. Rev. Clim. Chang. 2010, 1, 343–352. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Saurer, M.; Siegwolf, R.; Knorre, A.A.; Prokushkin, A.S.; Churakova, O.V.; Fonti, M.V.; Büntgen, U. Long-term ecological consequences of forest fires in the continuous permafrost zone of Siberia. Environ. Res. Lett. 2020, 15, 034061. [Google Scholar] [CrossRef]
- Boulanger, Y.; Arseneault, D.; Morin, H.; Jardon, Y.; Bertrand, P.; Dagneau, C. Dendrochronological reconstruction of spruce budworm (Choristoneura fumiferana) outbreaks in southern Quebec for the last 400 years. Can. J. For. Res. 2012, 42, 1264–1276. [Google Scholar] [CrossRef]
- Preechamart, S.; Pumijumnong, N.; Bräuning, A.; Muangsong, C.; Cai, B.; Payomrat, P.; Buajan, S.; Wang, F.; Li, M. Tree-ring oxygen isotope chronology of teak log coffins in northwestern Thailand and its relationship with Pacific Decadal Oscillation and El Niño-Southern Oscillation. Quat. Int. 2022, 629, 81–92. [Google Scholar] [CrossRef]
- Root, T.L.; Price, J.T.; Hall, K.R.; Schneider, S.H.; Rosenzweig, C.; Pounds, J.A. Fingerprints of global warming on wild animals and plants. Nature 2003, 421, 57–60. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Songtrirat, P.; Buajan, S.; Preechamart, S.; Chareonwong, U.; Muangsong, C. Climate control of cambial dynamics and tree-ring width in two tropical pines in Thailand. Agric. For. Meteorol. 2021, 303, 108394. [Google Scholar] [CrossRef]
- Uggla, C.; Magel, E.; Moritz, T.; Sundberg, B. Function and dynamics of auxin and carbohydrates during earlywood/latewood transition in Scots pine. Plant Physiol. 2001, 125, 2029–2039. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-W.; Eckstein, D.; Jalkanen, R.; Rickebusch, S.; Schmitt, U. Estimating the onset of cambial activity in Scots pine in northern Finland by means of the heat-sum approach. Tree Physiol. 2008, 28, 105–112. [Google Scholar] [CrossRef]
- Breitsprecher, A.; Bethel, J. Stem-growth periodicity of trees in a tropical wet forest of Costa Rica. Ecology 1990, 71, 1156–1164. [Google Scholar] [CrossRef]
- Rossi, S.; Morin, H.; Deslauriers, A. Causes and correlations in cambium phenology: Towards an integrated framework of xylogenesis. J. Exp. Bot. 2012, 63, 2117–2126. [Google Scholar] [CrossRef] [PubMed]
- Worbes, M. Growth rings, increment and age of trees in inundation forests, savannas and a mountain forest in the Neotropics. IAWA J. 1989, 10, 109–122. [Google Scholar] [CrossRef]
- Worbes, M. Annual growth rings, rainfall-dependent growth and long-term growth patterns of tropical trees from the Caparo Forest Reserve in Venezuela. J. Ecol. 1999, 87, 391–403. [Google Scholar] [CrossRef]
- Worbes, M. One hundred years of tree-ring research in the tropics–A brief history and an outlook to future challenges. Dendrochronologia 2002, 20, 217–231. [Google Scholar] [CrossRef]
- Suzuki, E. Diversity in specific gravity and water content of wood among Bornean tropical rainforest trees. Ecol. Res. 1999, 14, 211–224. [Google Scholar] [CrossRef]
- Worbes, M. How to measure growth dynamics in tropical trees a review. IAWA J. 1995, 16, 337–351. [Google Scholar] [CrossRef]
- Ohashi, S.; Okada, N.; Nobuchi, T.; Siripatanadilok, S.; Veenin, T. Detecting invisible growth rings of trees in seasonally dry forests in Thailand: Isotopic and wood anatomical approaches. Trees 2009, 23, 813–822. [Google Scholar] [CrossRef]
- Norstrom, E.; Holmgren, K.; Morth, C.-M. Rainfall-driven variations in δ13C composition and wood anatomy of Breonadia salicina trees from South Africa between AD 1375 and 1995. S. Afr. J. Sci. 2005, 101, 162–168. [Google Scholar]
- Fichtler, E.; Helle, G.; Worbes, M. Stable-carbon isotope time series from tropical tree rings indicate a precipitation signal. Tree-Ring Res. 2010, 66, 35–49. [Google Scholar] [CrossRef]
- Van Der Sleen, P.; Groenendijk, P.; Vlam, M.; Anten, N.P.; Boom, A.; Bongers, F.; Pons, T.L.; Terburg, G.; Zuidema, P.A. No growth stimulation of tropical trees by 150 years of CO2 fertilization but water-use efficiency increased. Nat. Geosci. 2015, 8, 24–28. [Google Scholar] [CrossRef]
- Nock, C.A.; Baker, P.J.; Wanek, W.; Leis, A.; Grabner, M.; Bunyavejchewin, S.; Hietz, P. Long-term increases in intrinsic water-use efficiency do not lead to increased stem growth in a tropical monsoon forest in western Thailand. Glob. Chang. Biol. 2011, 17, 1049–1063. [Google Scholar] [CrossRef]
- Dansgaard, W. Stable isotopes in precipitation. Tellus 1964, 16, 436–468. [Google Scholar] [CrossRef]
- Tharammal, T.; Bala, G.; Noone, D. Impact of deep convection on the isotopic amount effect in tropical precipitation. J. Geophys. Res. Atmos. 2017, 122, 1505–1523. [Google Scholar] [CrossRef]
- Villegas, P.; Paredes, V.; Betancur, T.; Taupin, J.D.; Toro, L.E. Groundwater evolution and mean water age inferred from hydrochemical and isotopic tracers in a tropical confined aquifer. Hydrol. Process. 2018, 32, 2158–2175. [Google Scholar] [CrossRef]
- Slik, J.F.; Arroyo-Rodríguez, V.; Aiba, S.-I.; Alvarez-Loayza, P.; Alves, L.F.; Ashton, P.; Balvanera, P.; Bastian, M.L.; Bellingham, P.J.; Van Den Berg, E. An estimate of the number of tropical tree species. Proc. Natl. Acad. Sci. USA 2015, 112, 7472–7477. [Google Scholar] [CrossRef]
- Hubbell, S.P. Estimating the global number of tropical tree species, and Fisher’s paradox. Proc. Natl. Acad. Sci. USA 2015, 112, 7343–7344. [Google Scholar] [CrossRef]
- Borchert, R. Climatic periodicity, phenology, and cambium activity in tropical dry forest trees. IAWA J. 1999, 20, 239–247. [Google Scholar] [CrossRef]
- Borchert, R.; Robertson, K.; Schwartz, M.D.; Williams-Linera, G. Phenology of temperate trees in tropical climates. Int. J. Biometeorol. 2005, 50, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, D.C.; de Carvalho, C.R.; Davidson, E.A.; Jipp, P.H.; Lefebvre, P.A.; Negreiros, G.H.; da Silva, E.D.; Stone, T.A.; Trumbore, S.E.; Vieira, S. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 1994, 372, 666–669. [Google Scholar] [CrossRef]
- Schöngart, J.; Piedade, M.T.F.; Ludwigshausen, S.; Horna, V.; Worbes, M. Phenology and stem-growth periodicity of tree species in Amazonian floodplain forests. J. Trop. Ecol. 2002, 18, 581–597. [Google Scholar] [CrossRef]
- Dünisch, O.; Bauch, J.; Gasparotto, L. Formation of increment zones and intraannual growth dynamics in the xylem of Swietenia macrophylla, Carapa guianensis, and Cedrela odorata (Meliaceae). IAWA J. 2002, 23, 101–119. [Google Scholar] [CrossRef]
- Dünisch, O.; Montóia, V.R.; Bauch, J. Dendroecological investigations on Swietenia macrophylla King and Cedrela odorata L. (Meliaceae) in the central Amazon. Trees 2003, 17, 244–250. [Google Scholar] [CrossRef]
- Marcati, C.R.; Angyalossy, V.; Evert, R.F. Seasonal variation in wood formation of Cedrela fissilis (Meliaceae). IAWA J. 2006, 27, 199–211. [Google Scholar] [CrossRef]
- Bräuning, A.; Volland-Voigt, F.; Burchardt, I.; Ganzhi, O.; Nauss, T.; Peters, T. Climatic control of radial growth of Cedrela montana in a humid mountain rainforest in southern Ecuador. Erdkunde 2009, 64, 337–345. [Google Scholar] [CrossRef]
- Rodríguez-Ramírez, E.C.; Ferrero, M.E.; Acevedo-Vega, I.; Crispin-DelaCruz, D.B.; Ticse-Otarola, G.; Requena-Rojas, E.J. Plastic adjustments in xylem vessel traits to drought events in three Cedrela species from Peruvian Tropical Andean forests. Sci. Rep. 2022, 12, 21112. [Google Scholar] [CrossRef]
- Lisi, C.S.; Fo, M.T.; Botosso, P.C.; Roig, F.A.; Maria, V.R.; Ferreira-Fedele, L.; Voigt, A.R. Tree-ring formation, radial increment periodicity, and phenology of tree species from a seasonal semi-deciduous forest in southeast Brazil. IAWA J. 2008, 29, 189–207. [Google Scholar] [CrossRef]
- Ferrero, M.E.; Villalba, R.; Rivera, S.M. An assessment of growth ring identification in subtropical forests from northwestern Argentina. Dendrochronologia 2014, 32, 113–119. [Google Scholar] [CrossRef]
- Grogan, J.; Schulze, M. The impact of annual and seasonal rainfall patterns on growth and phenology of emergent tree species in Southeastern Amazonia, Brazil. Biotropica 2012, 44, 331–340. [Google Scholar] [CrossRef]
- de Lara, N.O.T.; Marcati, C.R. Cambial dormancy lasts 9 months in a tropical evergreen species. Trees 2016, 30, 1331–1339. [Google Scholar] [CrossRef]
- Bhalerao, R.P.; Fischer, U. Environmental and hormonal control of cambial stem cell dynamics. J. Exp. Bot. 2017, 68, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Bosio, F.; Rossi, S.; Marcati, C.R. Periodicity and environmental drivers of apical and lateral growth in a Cerrado woody species. Trees 2016, 30, 1495–1505. [Google Scholar] [CrossRef]
- Aloni, R.; Schwalm, K.; Langhans, M.; Ullrich, C.I. Gradual shifts in sites of free-auxin production during leaf-primordium development and their role in vascular differentiation and leaf morphogenesis in Arabidopsis. Planta 2003, 216, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Marques, R.; Botosso, P.; Marques, M. Stem growth and phenology of two tropical trees in contrasting soil conditions. Plant Soil 2012, 354, 269–281. [Google Scholar] [CrossRef]
- Valdez-Hernández, M.; Andrade, J.L.; Jackson, P.C.; Rebolledo-Vieyra, M. Phenology of five tree species of a tropical dry forest in Yucatan, Mexico: Effects of environmental and physiological factors. Plant Soil 2010, 329, 155–171. [Google Scholar] [CrossRef]
- Shimamoto, C.Y.; Botosso, P.C.; Amano, E.; Marques, M.C. Stem growth rhythms in trees of a tropical rainforest in Southern Brazil. Trees 2016, 30, 99–111. [Google Scholar] [CrossRef]
- Volland-Voigt, F.; Bräuning, A.; Ganzhi, O.; Peters, T.; Maza, H. Radial stem variations of Tabebuia chrysantha (Bignoniaceae) in different tropical forest ecosystems of southern Ecuador. Trees 2011, 25, 39–48. [Google Scholar] [CrossRef]
- Baker, J.C.; Santos, G.M.; Gloor, M.; Brienen, R.J. Does Cedrela always form annual rings? Testing ring periodicity across South America using radiocarbon dating. Trees 2017, 31, 1999–2009. [Google Scholar] [CrossRef]
- Roel-Valdes, J.; Arizo-Luque, V.; Ronda-Perez, E. Epidemiology of occupationally-caused carpal tunnel syndrome in the province of Alicante, Spain 1996–2004. Rev. Esp. Salud Publica 2006, 80, 395–409. [Google Scholar]
- Xiao, X.; Hagen, S.; Zhang, Q.; Keller, M.; Moore, B., III. Detecting leaf phenology of seasonally moist tropical forests in South America with multi-temporal MODIS images. Remote Sens. Environ. 2006, 103, 465–473. [Google Scholar] [CrossRef]
- Yáñez-Espinosa, L.; Terrazas, T.; López-Mata, L. Phenology and radial stem growth periodicity in evergreen subtropical rainforest trees. IAWA J. 2010, 31, 293–307. [Google Scholar] [CrossRef]
- Lavrič, M.; Eler, K.; Ferlan, M.; Vodnik, D.; Gričar, J. Chronological sequence of leaf phenology, xylem and phloem formation and sap flow of Quercus pubescens from abandoned karst grasslands. Front. Plant Sci. 2017, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Marcati, C.R.; Milanez, C.R.D.; Machado, S.R. Seasonal development of secondary xylem and phloem in Schizolobium parahyba (Vell.) Blake (Leguminosae: Caesalpinioideae). Tree 2008, 22, 3–12. [Google Scholar] [CrossRef]
- Sintayehu, D.W. Impact of climate change on biodiversity and associated key ecosystem services in Africa: A systematic review. Ecosyst. Health Sustain. 2018, 4, 225–239. [Google Scholar] [CrossRef]
- Leal Filho, W.; Azeiteiro, U.M.; Balogun, A.-L.; Setti, A.F.F.; Mucova, S.A.; Ayal, D.; Totin, E.; Lydia, A.M.; Kalaba, F.K.; Oguge, N.O. The influence of ecosystems services depletion to climate change adaptation efforts in Africa. Sci. Total Environ. 2021, 779, 146414. [Google Scholar] [CrossRef] [PubMed]
- Krepkowski, J.; Bräuning, A.; Gebrekirstos, A.; Strobl, S. Cambial growth dynamics and climatic control of different tree life forms in tropical mountain forest in Ethiopia. Trees 2011, 25, 59–70. [Google Scholar] [CrossRef]
- Krepkowski, J.; Bräuning, A.; Gebrekirstos, A. Growth dynamics and potential for cross-dating and multi-century climate reconstruction of Podocarpus falcatus in Ethiopia. Dendrochronologia 2012, 30, 257–265. [Google Scholar] [CrossRef]
- Couralet, C.; Sterck, F.J.; Sass-Klaassen, U.; Van Acker, J.; Beeckman, H. Species-specific growth responses to climate variations in understory trees of a Central African rain forest. Biotropica 2010, 42, 503–511. [Google Scholar] [CrossRef]
- Trouet, V.; Mukelabai, M.; Verheyden, A.; Beeckman, H. Cambial growth season of brevi-deciduous Brachystegia spiciformis trees from south central Africa restricted to less than four months. PLoS ONE 2012, 7, e47364. [Google Scholar] [CrossRef] [PubMed]
- MARTIN, D.; MOSS, J. Age determination of Acacia tortilis (Forsk.) Hayne from northern Kenya. Afr. J. Ecol. 1997, 35, 266–277. [Google Scholar] [CrossRef]
- Prins, H.H.; van der Jeugd, H.P. Herbivore population crashes and woodland structure in East Africa. J. Ecol. 1993, 81, 305–314. [Google Scholar] [CrossRef]
- Andersen, G.L.; Krzywinski, K. Longevity and growth of Acacia tortilis; insights from 14 C content and anatomy of wood. BMC Ecol. 2007, 7, 1–14. [Google Scholar] [CrossRef]
- Gebrekirstos, A.; Mitlöhner, R.; Teketay, D.; Worbes, M. Climate–growth relationships of the dominant tree species from semi-arid savanna woodland in Ethiopia. Trees 2008, 22, 631–641. [Google Scholar] [CrossRef]
- Van Camp, J.; Hubeau, M.; Van den Bulcke, J.; Van Acker, J.; Steppe, K. Cambial pinning relates wood anatomy to ecophysiology in the African tropical tree Maesopsis eminii. Tree Physiol. 2018, 38, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Steppe, K.; Sterck, F.; Deslauriers, A. Diel growth dynamics in tree stems: Linking anatomy and ecophysiology. Trends Plant Sci. 2015, 20, 335–343. [Google Scholar] [CrossRef]
- O’Brien, M.J.; Leuzinger, S.; Philipson, C.D.; Tay, J.; Hector, A. Drought survival of tropical tree seedlings enhanced by non-structural carbohydrate levels. Nat. Clim. Chang. 2014, 4, 710–714. [Google Scholar] [CrossRef]
- Battipaglia, G.; Zalloni, E.; Castaldi, S.; Marzaioli, F.; Cazzolla-Gatti, R.; Lasserre, B.; Tognetti, R.; Marchetti, M.; Valentini, R. Long tree-ring chronologies provide evidence of recent tree growth decrease in a central African tropical forest. PLoS ONE 2015, 10, e0120962. [Google Scholar] [CrossRef]
- Dié, A.; Kitin, P.; Kouamé, F.N.G.; Van den Bulcke, J.; Van Acker, J.; Beeckman, H. Fluctuations of cambial activity in relation to precipitation result in annual rings and intra-annual growth zones of xylem and phloem in teak (Tectona grandis) in Ivory Coast. Ann. Bot. 2012, 110, 861–873. [Google Scholar] [CrossRef]
- Venugopal, N.; KV, K. Seasonal pattern of cell division in the vascular cambium of some tropical timber trees. Cytologia 1994, 59, 323–332. [Google Scholar] [CrossRef]
- Rao, K.; Rajput, K.S. Seasonal behaviour of vascular cambium in teak (Tectona grandis) growing in moist deciduous and dry deciduous forests. IAWA J. 1999, 20, 85–93. [Google Scholar] [CrossRef]
- Pumijumnong, N. Dendrochrnologie mit Teak (Tectona grandis L.) in Nord-Thailand. Jahrringbildung—Chronol.—Klimasignal. Diss. Univ. Hambg. 1995, 104. [Google Scholar]
- Buajan, S.; Pumijumnong, N.; Songtrirat, P.; Muangsong, C. Relationship of cambial activity and xylem production in Teak (Tectona geandis) to phenology and climate variable in North western Thailand. J. Trop. For. Sci. 2023, 35, 141–156. [Google Scholar]
- Nanda, A.; Suresh, H.S.; Krishnamurthy, Y.L. Phenology of a tropical dry deciduous forest of Bhadra wildlife sanctuary, southern India. Ecol. Process. 2014, 3, 1–12. [Google Scholar] [CrossRef]
- Singh, N.D.; Venugopal, N. Cambial activity and annual rhythm of xylem production of Pinus kesiya Royle ex. Gordon (Pinaceae) in relation to phenology and climatic factors growing in sub-tropical wet forest of North East India. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 198–204. [Google Scholar] [CrossRef]
- Swaminathan, C.; Anbarasu, M. Response of cambium growth to mechanical injuries in tree species. J. Trop. For. Sci. 2022, 34, 170–175. [Google Scholar] [CrossRef]
- Nobuchi, T.; Ogata, Y.; Siripatanadilok, S. Seasonal characteristics of wood formation in Hopea odorata and Shorea henryana. IAWA J. 1995, 16, 361–369. [Google Scholar] [CrossRef]
- Begum, S.; Nakaba, S.; Oribe, Y.; Kubo, T.; Funada, R. Induction of cambial reactivation by localized heating in a deciduous hardwood hybrid poplar (Populus sieboldii × P. grandidentata). Ann. Bot. 2007, 100, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Geiger, F. Anatomische Untersuchungen über Die Jahresringbildung von Tectona Grandis; Borntraeger: Beeville, TX, USA, 1915. [Google Scholar]
- Coster, C. Zur Anatomie und Physiologie der Zuwachszonen-und Jahresringbildung in den Tropen. Ph.D Thesis, Wageningen University, Wageningen, The Netherlands, Brill, Leiden, The Netherlands, 1927. [Google Scholar]
- Rahman, M.H.; Nugroho, W.D.; Nakaba, S.; Kitin, P.; Kudo, K.; Yamagishi, Y.; Begum, S.; Marsoem, S.N.; Funada, R. Changes in cambial activity are related to precipitation patterns in four tropical hardwood species grown in Indonesia. Am. J. Bot. 2019, 106, 760–771. [Google Scholar] [CrossRef]
- Sass, U.; Killmann, W.; Eckstein, D. Wood formation in two species of Dipterocarpaceae in peninsular Malaysia. IAWA J. 1995, 16, 371–384. [Google Scholar] [CrossRef]
- Fujii, T. Growth periodicity in relation to the xylem development in three Shorea spp.(Dipterocarpaceae) growing in Sarawak. In Tree-Ring Anal.; Wimmer, R., Vetter, R.E., Eds.; CABI Publishing: Oxon, UK, 1999; pp. 169–183. [Google Scholar]
- Wang, K.H.; Hamzah, M.Z. Different cambial activities in response to climatic factors of three Malaysian rainforest Shorea species with different stem diameters. Trees 2018, 32, 1519–1530. [Google Scholar] [CrossRef]
- Nabeshima, E.; Kubo, T.; Hiura, T. Variation in tree diameter growth in response to the weather conditions and tree size in deciduous broad-leaved trees. For. Ecol. Manag. 2010, 259, 1055–1066. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Dixon, S.L. Environmental factors influence plant vascular system and water regulation. Plants 2019, 8, 65. [Google Scholar] [CrossRef]
- Begum, S.; Kudo, K.; Rahman, M.H.; Nakaba, S.; Yamagishi, Y.; Nabeshima, E.; Nugroho, W.D.; Oribe, Y.; Kitin, P.; Jin, H.-O. Climate change and the regulation of wood formation in trees by temperature. Trees 2018, 32, 3–15. [Google Scholar] [CrossRef]
- Wang, K.H.; Hamzah, M.Z. Annual wood formation of tropical pioneer species related to stem diameters. J. Wood Sci. 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Palakit, K.; Siripatanadilok, S.; Lumyai, P.; Duangsathaporn, K. Leaf phenology and wood formation of white cedar trees Melia azedarach L. and their responses to climate variability. Songklanakarin J. Sci. Technol. 2018, 40, 61–68. [Google Scholar]
- Palakit, K.; Lumyai, P.; Duangsathaporn, K. Periodic growth of Afzelia xylocarpa (Kurz) Craib and Lagerstroemia duperreana Pierre ex Gagnep. and their responses to climatic variability. Agric. Nat. Resour. 2021, 55, 282–291. [Google Scholar]
- Vlam, M.; Baker, P.J.; Bunyavejchewin, S.; Zuidema, P.A. Temperature and rainfall strongly drive temporal growth variation in Asian tropical forest trees. Oecologia 2014, 174, 1449–1461. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Danpradit, S.; Tadang, N.; Buajan, S.; Muangsong, C. Cambial activity and radial growth dynamics of three tropical tree species at Chang Island, Thailand. J. Trop. For. Sci. 2019, 31, 404–414. [Google Scholar] [CrossRef]
- Tanaka, K.; Tantasirin, C.; Suzuki, M. Interannual variation in leaf expansion and outbreak of a teak defoliator at a teak stand in northern Thailand. Ecol. Appl. 2011, 21, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- Pumijumnong, N.; Buajan, S. Seasonal cambial activity of five tropical tree species in central Thailand. Trees 2013, 27, 409–417. [Google Scholar] [CrossRef]
- Pumijumnong, N.; Wanyaphet, T. Seasonal cambial activity and tree-ring formation of Pinus merkusii and Pinus kesiya in Northern Thailand in dependence on climate. For. Ecol. Manag. 2006, 226, 279–289. [Google Scholar] [CrossRef]

| Tree-Name/Information | Family | Location | Methods | Cambial/Wood Formation | References |
|---|---|---|---|---|---|
| Pinus patula Podocarpus falcatus Prunus africana Celtis africana | Pinaceae Podocarpaceae Rosaceae Cannabaceae | Munessa-Shashamene Forest, Ethiopian | Cambium activity (microcores), dendrometer | Lack of water availability during the long dry season induced cambial dormancy. Evergreen tree species were able to initiate wood formation during the short rainy season, while growth in the deciduous broadleaved species started in the long rainy season. | [80] |
| Podocarpus falcatus | Podocarpaceae | Munessa-Shashamene Forest, Ethiopian | Dendrochronology, stem disks, radiocarbon dating | Permanent growing leads to irregular wood formation | [81] |
| Pterocarpus angolensis | Fabaceae | Western Zimbabwe | Dendrochronology | The annual rings of P. angolensis are tightly synchronized with the seasonality of rainfall in western Zimbabwe. | [8] |
| Acacia tortilis | Fabaceae | Marsabit district, northern Kenya | Discs | Not directly studied. | [84] |
| Acalypha fruticose Ocimum suave Gardenia jovisionanius Acacia tortilis Justicia cordata Cordia ovalis Acacia sieberiana Cassia didymobotrya Balanites aegyptiaca Phyllanthus sepialis Cordia sinensis Lippia javanica Capparis farinose Dovyalis xanthocarpa Kigelia africana | Euphorbiaceae Labiatae Rubiaceae Fabaceae Acanthaceae Boraginaceae Fabaceae Fabaceae Zygophyllaceae Euphorbiaceae Boraginaceae Verbenaceae Capparaceae Flacourtiaceae Bignoniaceae | Northern Tanzania, lake Manyara National Park | Plot, diameter, growth rate | Not directly studied. | [85] |
| Acacia tortilis | Fabaceae | Hyper-arid eastern desert of Egypt | Dendrochronology, carbon-14 dating | Not directly studied. | [86] |
| Acacia senegal Acacia seyal Acacia tortilis Balanities aegyptiaca | Fabaceae Fabaceae Fabaceae Zygophyllaceae | Abernosa, Ethiopian | Dendrochronology | All three species show a positive correlation between tree rings and rainfall data. | [87] |
| Tectona grandis | Lamiaceae | Ivory Coast | Wood block | Dormant cambium occurred during the main dry season from October to February. The most active cambium and radial growth occurred during the peak of the rainy season in June. | [92] |
| Maesopsis eminii | Rhamnaceae | Seedling from Uganda transplant in Ghent, Belgium | Cambial pinning, dendrometer, micro-CT, sap flow | Wood growth completely stopped during the drought period. | [88] |
| Tree-Name/Information | Family | Location | Methods | Cambial/Wood Formation | References |
|---|---|---|---|---|---|
| Deciduous trees Albizzia lebbeck Dalbergia sissoo Tectona grandis Terminalia crenulata Evergreen trees Calophyllum inophyllum Mangifera indica Morinda tinctoria | Fabaceae Fabaceae Lamiaceae Combretaceae Calophyllaceae Anacardiaceae Rubiaceae | Tropical tree in India | Wood formation at stem twigs of 0.5 cm | A. Lebbeck, D. sissoo, and T. crenulata cambium is reactive twice a year. In T. grandis, cambium is reactive once a year (April–May). In C. inophyllum, M. indica, and M. tinctoria, cambium is active throughout the year. | [93] |
| Tectona grandis | Lamiaceae | Moist deciduous forest (MDF) in Waghai region of Dangs Forest and dry deciduous forest (DDF) in Pavagadh | wood block | In MDF, cambial cell activity peaked in August and September and ceased in October. In DDF, cambial cell activity peaked in July and August and ceased in November. Maximum radial growth in trees of both forests occurred during the monsoon season. | [94] |
| Acrocarpus fraxinifolius Adina cordifolia Albizia odoratissima Anogeissus latifolia Bombax malabaricum Dalbergia latifolia Diospyros montana Ficus benghalensis Ficus infectoria Ficus tsiela Kigelia pinnata Lagerstroemia lanceolate Lannea coromandelica Melia dubia Mitragyna parviflora Pterocarpus marsupium Schleichera oleosa Syzygium cumini Tectona grandis Terminalia bellerica Terminalia paniculata Terminalia tomentosa | Fabaceae Rubiaceae Fabaceae Combretaceae Malvaceae Fabaceae Ebenaceae Moraceae Moraceae Moraceae Bignoniaceae Lythraceae Anacardiaceae Meliaceae Rubiaceae Fabaceae Sapindaceae Myrtaceae Lamiaceae Combretaceae Combretaceae Combretaceae | Bhadra wildlife sanctuary, Karnataka, Central western Ghats, southern India | Tree phenology | Not directly studied. Leafing and flowing activity occur in the summer or premonsoon. Fruiting patterns occur during the monsoon to post monsoon. | [97] |
| Pinus kesiya | Pinaceae | Laitkor protected forest of Meghalaya, Shillong | Twig samples 1.5–2 cm in diameter | Cambium reactivation and dormancy occur after the sprouting of new leaves in mid-February, simultaneously with cone formation. | [98] |
| Albizzia lebbek Azadirachta indica Delonix regia Lannea coromandelica Madhuca longifolia Pongamia glabra Peltophorum pterocarpum Terminalia catappa Senna siamea Syzygium cumini Terminalia arjuna | Fabaceae Meliaceae Fabaceae Anacardiaceae Sapotaceae Fabaceae Fabaceae Combretaceae Fabaceae Myrtaceae Combretaceae | Natural forest, India, maximum temperature is 35.12 °C, and minimum temperature is 18.46 °C; the mean annual rainfall is 979.00 mm. | Wood incision | Not directly studied. This study concluded that when a tree was wounded, the cambium divided more rapidly horizontally than vertically to cover the wound and that L. coromandelica had a cambial recovery growth of 73%. | [99] |
| Tree Name (Family) | Family | Location | Method | Cambial/Wood Formation | References |
|---|---|---|---|---|---|
| Tectona grandis | Lamiaceae | Java, Indonesia | Wood anatomy, dendrochronology | Teak annual ring width positively correlated with rainfall. | [102] |
| Tectona grandis | Lamiaceae | Java, Indonesia | Wood anatomy, dendrochronology | Teak annual ring width positively correlated with rainfall. | [103] |
| Acacia mangium Eucalyptus urophylla Neolamarkia cadamba Tectona grandis | Fabaaceae Myrtaceae Rubiaceae Lamiaceae | Wanagama 1 Forest, Faculty of Forestry, Universitas Gadjah Mada, Yogyakarta, Indonesia | Wood block | Cambium of four tree species is reactive during the rainy season and stops dividing when there is no rain. | [104] |
| Shorea patoiensis Shorea pinanga Shorea dasyphylla | Dipterocarpaceae | Sarawak, east Malaysia | Wood block | No dormancy of cambial period of three species. Climate factors, such as increase in precipitation and sunshine, may be one of the causes of boundary formation, but the growth rhythm is inconsistent. | [106] |
| Shorea leprosula Shorea acuminate Shorea parvifolia | Dipterocarpaceae | Lowland dipterocarpaceae secondary rain forest, Ayer Hitam Forest Reserve in the state of Selangor in Peninsular Malaysia | Wood block | The active cambium of Shorea trees was detected in months with high relative humidity and low vapor pressure deficit. The Shorea species with different stem diameters have different growth strategies and thus react differently to climate factors. | [107] |
| Macaranga gigantean Endospermum diadenum | Euphorbiaceae | The forest lowland Dipterocarp rainforest, Ayer Hitam Forest Reserve, Salangor, Malaysia | An aluminum ban, wood block | There is no significant correlation between mean number of cambium and enlarging cell layers with monthly total rainfall, monthly mean relative humidity, vapor pressure deficit and day length. | [111] |
| Hopea odorata Shorea henryana | Dipterocarpaceae | Subtropical Thailand | Pinning method | The radial growth in Hopea and Shorea was more related to the amount of rainfall than the temperature. In Hopea, more wood was formed in the rainy season than in the dry season. However, the amount of radial growth was not always proportional to the rainfall. The authors proposed that one of the reasons is low light intensity on rainy days, and another possible reason is the effect of soil moisture content. | [100] |
| Pinus merkusii Pinus kesiya | Pinaceae | Chiangmai, Thailand | Wood block, dendrochronology | Soil moisture influenced the cambial activity of both pine species. The amount of rainfall in different months influenced the annual ring width of both pine species. | [118] |
| Hopea pierrei, Cleidion spiciflorum, Tetrameles nudiflora | Dipterocarpaceae Euphorbiaceae Tetramelaceae | Chang Island, Thailand | Wood block | The cambium of T. nudiflora appeared active year-long and was most active at the end of August. Cambial activity for H. pierrei and C. spiciflorum was highest at the end of June and July, respectively. | [115] |
| Tectona grandis | Lamiaceae | Mae Mo, Lampang, Thailand | Leaf area index, soil moisture | Not directly studied. Teak leaf expansion began in late March, lasting to early May. Soil moisture data indicated that leaf expansion occurred after increases in soil moisture at depths of 0.1–0.4 m. | [116] |
| Tetrameles nudiflora, Magnolia bailionii, Canarium euphyllum, Toona ciliata, Spondias axillaris | Tetramelaceae Magnoliaceae Burseraceae Meliaceae Anacardiaceae | Kao Yai National Park, Thailand | Wood block | The cambium of T. nudiflora and M. baillonii was most active when rainfall peaked in June, whereas the cambium of C. euphyllum, T. ciliate, and S. axillaris was most active in March. | [117] |
| Pinus latteri Pinus kesiya | Pinaceae | Chiangmai, Thailand; the average rainfall was 1119 mm, with monthly mean temperature ranging from 27 to 29 °C. | Dendrochronology, wood block | Monthly rainfall, relative humidity, soil moisture, monthly minimum temperature, and monthly mean temperature were positively related to the number of cambial cell layers and cambial zone width. The amount of rainfall and relative humidity in April and May had positive effects on the tree-ring width of both pine species. | [32] |
| Tectona grandis | Lamiaceae | Maesaring, Maehongson, Northern Thailand | Dendrochronology, wood block | The activation of cambial cells started in May, during the rainy season, and ended in October (late rainy season). | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pumijumnong, N.; Muangsong, C.; Buajan, S.; Songtrirat, P.; Chatwatthana, R.; Chareonwong, U. Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review. Forests 2023, 14, 1025. https://doi.org/10.3390/f14051025
Pumijumnong N, Muangsong C, Buajan S, Songtrirat P, Chatwatthana R, Chareonwong U. Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review. Forests. 2023; 14(5):1025. https://doi.org/10.3390/f14051025
Chicago/Turabian StylePumijumnong, Nathsuda, Chotika Muangsong, Supaporn Buajan, Piyarat Songtrirat, Rattanakorn Chatwatthana, and Uthai Chareonwong. 2023. "Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review" Forests 14, no. 5: 1025. https://doi.org/10.3390/f14051025
APA StylePumijumnong, N., Muangsong, C., Buajan, S., Songtrirat, P., Chatwatthana, R., & Chareonwong, U. (2023). Factors Affecting Cambial Growth Periodicity and Wood Formation in Tropical Forest Trees: A Review. Forests, 14(5), 1025. https://doi.org/10.3390/f14051025






