Effect of Changes in Throughfall on Soil Respiration in Global Forest Ecosystems: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Quality Control
2.2. Data Analysis
3. Results
3.1. Overall Pattern of Soil CO2 Emission by TF Manipulation
3.2. Relationship between lnRCO2 Values and Changes in Throughfall
3.3. Effect of TF Manipulation on Soil Properties
3.4. Relationships between lnRCO2 Values and Environmental Variables
4. Discussion
4.1. Throughfall Effects on Forest Soil CO2 Emissions Vary with Climate Types
4.2. Throughfall Effects on Soil CO2 Emissions Vary with Forest Types and Soil Properties
5. Conclusions and Future Perspectives
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Forest Resources Assessment 2015: How Are the World’s Forests Changing? Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Cathcart, J.F.; Kline, J.D.; Tilton, D.M. Carbon storage and Oregon’s land-use planning program. J. For. 2007, 105, 167–172. [Google Scholar]
- Zaehle, S.; Bondeau, A.; Carter, T.R.; Cramer, W.; Erhard, M.; Prentice, I.C.; Reginster, I.; Rounsevell, M.D.A.; Sitch, S.; Smith, B.; et al. Projected changes in terrestrial carbon storage in Europe under climate and land-use change, 1990–2100. Ecosystems 2007, 10, 380–401. [Google Scholar] [CrossRef]
- Law, B.E.; Ryan, M.G.; Anthoni, P.M. Seasonal and annual respiration of a ponderosa pine ecosystem. Glob. Chang. Biol. 1999, 5, 169–182. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grunwald, T.; et al. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Bolstad, P.V.; Davis, K.J.; Martin, J.; Cook, B.D.; Wang, W. Component and whole-system respiration fluxes in northern deciduous forests. Tree Physiol. 2004, 24, 493–504. [Google Scholar] [CrossRef]
- Cox, P.M.; Betts, R.A.; Jones, C.D.; Spall, S.A.; Totterdell, I.J. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 2000, 408, 184–187. [Google Scholar] [CrossRef]
- Valentini, R.; Matteucci, G.; Dolman, A.J.; Schulze, E.D.; Rebmann, C.; Moors, E.J.; Granier, A.; Gross, P.; Jensen, N.O.; Pilegaard, K.; et al. Respiration as the main determinant of carbon balance in European forests. Nature 2000, 404, 861–865. [Google Scholar] [CrossRef]
- Lal, R.; Kimble, J.; Levine, E.; Stewart, B. Soils and Global Change; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Schimel, D.S.; House, J.I.; Hibbard, K.A.; Bousquet, P.; Ciais, P.; Peylin, P.; Braswell, B.H.; Apps, M.J.; Baker, D.; Bondeau, A.; et al. Recent patterns and mechanisms of carbon exchange by terrestrial ecosystems. Nature 2001, 414, 169–172. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef]
- Wentz, F.J.; Ricciardulli, L.; Hilburn, K.; Mears, C. How much more rain will global warming bring? Science 2007, 317, 233–235. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, L.; Nie, Y.; Fu, Y.; Du, Z.; Shao, J.; Zheng, Z.; Wang, X. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016, 228, 70–81. [Google Scholar] [CrossRef]
- Houghton, R.A. Balancing the global carbon budget. Annu. Rev. Earth Planet. Sci. 2007, 35, 313–347. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Wunderlich, S.; Borken, W.; Kitzler, B.; Zechmeister-Boltenstern, S.; Jandl, R. Soil respiration under climate change: Prolonged summer drought offsets soil warming effects. Glob. Chang. Biol. 2012, 18, 2270–2279. [Google Scholar] [CrossRef]
- Mazza, G.; Amorini, E.; Cutini, A.; Manetti, M.C. The influence of thinning on rainfall interception by Pinus pinea L. in Mediterranean coastal stands (Castel Fusano-Rome). Ann. For. Sci. 2011, 68, 1323–1332. [Google Scholar] [CrossRef]
- Cattânio, J.; Davidson, E.; Nepstad, D.; Verchot, L.; Ackerman, I. Unexpected results of a pilot throughfall exclusion experiment on soil emissions of CO2, CH4, N2O, and NO in eastern Amazonia. Biol. Fertil. Soils 2002, 36, 102–108. [Google Scholar]
- Borken, W.; Savage, K.; Davidson, E.A.; Trumbore, S.E. Effects of experimental drought on soil respiration and radiocarbon efflux from a temperate forest soil. Glob. Chang. Biol. 2006, 12, 177–193. [Google Scholar] [CrossRef]
- Sotta, E.D.; Veldkamp, E.; Schwendenmann, L.; Guimarães, B.R.; Paixão, R.K.; Lourdes, M.D.; Ruivo, P.; Costa, A.C.L.D.; Meir, P. Effects of an induced drought on the soil CO2 production and soil CO2 efflux in an eastern Amazonian rainforest, Brazil. Glob. Chang. Biol. 2007, 13, 2218–2229. [Google Scholar] [CrossRef]
- van Straaten, O.; Veldkamp, E.; Corre, M.D. Simulated drought reduces soil CO2 efflux and production in a tropical forest in Sulawesi, Indonesia. Ecosphere 2011, 2, 119. [Google Scholar] [CrossRef]
- Hinko-Najera, N.; Fest, B.; Livesley, S.J.; Arndt, S.K. Reduced throughfall decreases autotrophic respiration, but not heterotrophic respiration in a dry temperate broadleaved evergreen forest. Agric. For. Meteorol. 2015, 200, 66–77. [Google Scholar] [CrossRef]
- Xu, X.K.; Duan, C.T.; Wu, H.H.; Luo, X.B.; Han, L. Effects of changes in throughfall on soil GHG fluxes under a mature temperate forest, northeastern China. J. Environ. Manag. 2021, 294, 112950. [Google Scholar] [CrossRef]
- Davidson, E.A.; Nepstad, D.C.; Ishida, F.Y.; Brando, P.M. Effects of an experimental drought and recovery on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2008, 14, 2582–2590. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Wieder, W.R.; Reed, S.C.; Townsend, A.R. Experimental drought in a tropical rain forest increases soil carbon dioxide losses to the atmosphere. Ecology 2010, 91, 2313–2323. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.P.; Sha, L.Q.; Wu, C.S.; Tan, Z.H.; Song, Q.H.; Liu, Y.T.; Dong, L.Y. Effects of continuous drought stress on soil respiration in a tropical rainforest in southwest China. Plant Soil 2015, 394, 343–353. [Google Scholar] [CrossRef]
- Zhou, S.X.; Huang, C.D.; Xiang, Y.B.; Xiao, Y.X.; Tang, J.D.; Han, B.H. Effects of throughfall exclusion on soil respiration in natural evergreen broadleaved forest in rainy area of Western China. Chin. J. Appl. Environ. Biol. 2016, 22, 499–504. [Google Scholar]
- Xiang, Y.B.; Huang, C.D.; Hu, T.X.; Tu, L.H.; Zhou, S.X.; Xiao, Y.X.; Gao, B.D. Effects of simulated nitrogen deposition and precipitation changes on soil respiration in an evergreen broadleaved forest in the rainy area of western China. Acta Ecol. Sin. 2016, 36, 5227–5235. [Google Scholar]
- Liu, Y.C.; Liu, S.R.; Wan, S.Q.; Wang, J.X.; Luan, J.W.; Wang, H. Differential responses of soil respiration to soil warming and experimental reduction in a transitional oak forest in central China. Agric. For. Meteorol. 2016, 226–227, 186–198. [Google Scholar] [CrossRef]
- Chen, L.; Liu, S.R.; Zeng, J.; Wang, H.; Lu, L.H. Effect of precipitation reduction on soil greenhouse gas emission in Castanopsis hystrix and Pinus massoniana plantations. J. Cent. South Univ. For. Tech. 2019, 39, 86–92. [Google Scholar]
- Joffre, R.; Qurcival, J.M.; Rambal, S.; Rocheteau, A. The Key-role of topsoil moisture on CO2 efflux from a Mediterranean Quercus ilex forest. Annu. For. Sci. 2003, 60, 519–526. [Google Scholar] [CrossRef]
- Mission, L.; Rocheteau, A.; Rambal, S.; Ourcival, J.M.; Limousin, J.M.; Rodriguez, R. Functional changes in the control of carbon fluxes after 3 years of increased drought in a Mediterranean evergreen forest? Glob. Chang. Biol. 2010, 16, 2461–2475. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Alberti, G.; Inglima, I.; Marjanovic, H.; LeCain, D.; Zaldei, A.; Peressotti, A.; Miglietta, F. Decreased summer drought affects plant productivity and soil carbon dynamics in a Mediterranean woodland. Biogeosciences 2011, 8, 2729. [Google Scholar] [CrossRef]
- Shvaleva, A.; Lobo-do-Vale, R.; Cruz, C.; Castaldi, S.; Rosa, A.P.; Chaves, M.M.; Pereira, J.S. Soil-atmosphere greenhouse gases (CO2, CH4 and N2O) exchange in evergreen oak woodland in southern Portugal. Plant Soil Environ. 2011, 57, 471–477. [Google Scholar] [CrossRef]
- Wu, Z.; Dijkstra, P.; Koch, G.W.; Penuelas, J.; Hungate, B.A. Responses of terrestrial ecosystems to temperature and precipitation change: A meta-analysis of experimental manipulation. Glob. Chang. Biol. 2011, 17, 927–942. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Zhang, D.; Zhou, G.; Liu, J.; Liu, S.; Chu, G.; Li, J. Effects of precipitation increase on soil respiration: A three-year field experiment in subtropical forests in China. PLoS ONE 2012, 7, e41493. [Google Scholar] [CrossRef]
- Billings, S.A.; Richter, D.D.; Yarie, J. Soil carbon dioxide fluxes and profile concentrations in two boreal forests. Can. J. For. Res. 1998, 28, 1773–1783. [Google Scholar] [CrossRef]
- Xu, X.K.; Han, L.; Wang, Y.S.; Inubushi, K. Influence of vegetation types and soil properties on microbial biomass carbon and metabolic quotients in temperate volcanic and tropical forest soils. Soil Sci. Plant Nutr. 2007, 53, 430–440. [Google Scholar] [CrossRef]
- Wood, T.E.; Silver, W.L. Strong spatial variability in trace gas dynamics following experimental drought in a humid tropical forest. Glob. Biogeochem. Cycles 2012, 26, GB3005. [Google Scholar] [CrossRef]
- Xu, X.K.; Luo, X.B. Effect of wetting intensity on soil GHG fluxes and microbial biomass under a temperate forest floor during dry season. Geoderma 2012, 170, 118–126. [Google Scholar] [CrossRef]
- Fender, A.C.; Gansert, D.; Jungkunst, H.F.; Fiedler, S.; Beyer, F.; Schützenmeister, K.; Thiele, B.; Valtanen, K.; Polle, A.; Leuschner, C. Root-induced tree species effects on the source/sink strength for greenhouse gases (CH4, N2O and CO2) of a temperate deciduous forest soil. Soil Biol. Biochem. 2013, 57, 587–597. [Google Scholar] [CrossRef]
- van Straaten, O.; Veldkamp, E.; Kohler, M.; Anas, I. Spatial and temporal effects of drought on soil CO2 efflux in a cacao agroforestry system in Sulawesi, Indonesia. Biogeosciences 2010, 7, 1223–1235. [Google Scholar] [CrossRef]
- Suseela, V.; Dukes, J.S. The responses of soil and rhizosphere respiration to simulated climatic changes vary by season. Ecology 2013, 94, 403–413. [Google Scholar] [CrossRef]
- Bréchet, L.M.; Lopez-Sangil, L.; George, C.; Birkett, A.J.; Baxendale, C.; Trujillo, B.C.; Sayer, E.J. Distinct responses of soil respiration to experimental litter manipulation in temperate woodland and tropical forest. Ecol. Evol. 2018, 8, 3787–3796. [Google Scholar] [CrossRef]
- Davidson, E.A.; Ishida, F.Y.; Nepstad, D.C. Effects of an experimental drought on soil emissions of carbon dioxide, methane, nitrous oxide, and nitric oxide in a moist tropical forest. Glob. Chang. Biol. 2004, 10, 718–730. [Google Scholar] [CrossRef]
- Yu, S.Q.; Mo, Q.F.; Chen, Y.Q.; Li, Y.W.; Li, Y.X.; Zou, B.; Xia, H.P.; Jun, W.; Li, Z.A.; Wang, F.M. Effects of seasonal precipitation change on soil respiration processes in a seasonally dry tropical forest. Ecol. Evol. 2020, 10, 467–479. [Google Scholar] [CrossRef]
- Wood, T.E.; Detto, M.; Silver, W.L. Sensitivity of soil respiration to variability in soil moisture and temperature in a humid tropical forest. PLoS ONE 2013, 8, e80965. [Google Scholar] [CrossRef]
- Post, W.M.; Kwon, K.C. Soil carbon sequestration and land-use change: Processes and potential. Glob. Chang. Biol. 2000, 6, 317–327. [Google Scholar] [CrossRef]
- Rosenberg, M.S.; Adams, D.C.; Gurevitch, J. MetaWin: Statistical Software for Meta-Analysis, Version 2; Sinauer Associates: Sunderland, MA, USA, 1999. [Google Scholar]
- Liu, L.; Estiarte, M.; Peñuelas, J. Soil moisture as the key factor of atmospheric CH4 uptake in forest soils under environmental change. Geoderma 2019, 355, 113920. [Google Scholar] [CrossRef]
- Li, L.F.; Zheng, Z.Z.; Wang, W.J.; Biederman, J.A.; Xu, X.L.; Ran, Q.W.; Qian, R.Y.; Xu, C.; Zhang, B.; Wang, F.; et al. Terrestrial N2O emissions and related functional genes under climate changes: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 931–943. [Google Scholar] [CrossRef]
- Nepstad, D.C.; Veríssimo, A.; Alencar, A.; Nobre, C.; Lima, E.; Lefebvre, P.; Schlesinger, P.; Potter, C.; Moutinho, P.; Mendoza, E.; et al. Large-scale impoverishment of Amazonian forests by logging and fire. Nature 1999, 398, 505–508. [Google Scholar] [CrossRef]
- Costa, M.H.; Foley, J.A. Combined effects of deforestation and doubled atmospheric CO2 concentrations on the climate of Amazonia. J. Clim. 2000, 13, 18–34. [Google Scholar] [CrossRef]
- Waring, B.G.; Hawkes, C.V. Short-term precipitation exclusion alters microbial responses to soil moisture in a wet tropical forest. Microb. Ecol. 2015, 69, 843–854. [Google Scholar] [CrossRef]
- Bhattacharyya, A.; Campbell, A.N.; Tfaily, M.M.; Lin, Y.; Kukkadapu, R.K.; Silver, W.L.; Nico, P.S.; Pett-Ridge, J. Redox fluctuations control the coupled cycling of iron and carbon in tropical forest soils. Environ. Sci. Technol. 2018, 52, 14129–14139. [Google Scholar] [CrossRef] [PubMed]
- Nepstad, D.C.; Decarvalho, C.R.; Davidson, E.A.; Jipp, P.H.; Lefebvre, P.A.; Negreiros, G.H.; Dasilva, E.D.; Stone, T.A.; Trumbore, S.E.; Vieira, S. The role of deep roots in the hydrological and carbon cycles of Amazonian forests and pastures. Nature 1994, 372, 666–669. [Google Scholar] [CrossRef]
- Schenk, H.J.; Jackson, R.B. The global biogeography of roots. Ecol. Monogr. 2002, 72, 311–328. [Google Scholar] [CrossRef]
- Hertel, D.; Moser, G.; Culmsee, H.; Erasmi, S.; Horna, V.; Schuldt, B.; Leuschner, C. Below- and above-ground biomass and net primary production in a paleotropical natural forest (Sulawesi, Indonesia) as compared to neotropical forests. For. Ecol. Manag. 2009, 258, 1904–1912. [Google Scholar] [CrossRef]
- Martins, C.S.C.; Nazaries, L.; Delgado-Baquerizo, M.; Macdonald, C.A.; Anderson, I.C.; Hobbie, S.E.; Venterea, R.T.; Reich, P.B.; Singh, B.K. Identifying environmental drivers of greenhouse gas emissions under warming and reduced rainfall in boreal-temperate forests. Funct. Ecol. 2017, 31, 2356–2368. [Google Scholar] [CrossRef]
- Post, W.M.; Emanuel, W.R.; Zinke, P.J.; Stangenberger, A.G. Soil carbon pools and world life zones. Nature 1982, 298, 156–159. [Google Scholar] [CrossRef]
- Thom, D.; Golivets, M.; Edling, L.; Meigs, G.W.; Gourevitvh, D.J.; Sonter, L.J.; Galford, G.L.; Keeton, W.S. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal-temperate North America. Glob. Chang. Biol. 2019, 25, 2446–2458. [Google Scholar] [CrossRef]
- Stuenzi, S.; Boike, J.; Gädeke, A.; Herzschuh, U.; Kruse, S.; Pestryakova, L.A.; Westermann, S.; Langer, M. Sensitivity of ecosystem-protected permafrost under changing boreal forest structures. Environ. Res. Lett. 2021, 16, 084045. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Lajeunesse, M.J.; Miao, G.; Piao, S.; Wan, S.; Wu, Y.; Wang, Z.; Yang, S.; Li, P. A cross-biome synthesis of soil respiration and its determinants under simulated precipitation changes. Glob. Chang. Biol. 2016, 22, 1394–1405. [Google Scholar] [CrossRef]
- Darenova, E.; Čater, M. Different structure of sessile oak stands affects soil moisture and soil CO2 efflux. For. Sci. 2018, 64, 340–348. [Google Scholar]
- Amundson, R. The carbon budget in soils. Annu. Rev. Earth Planet. Sci. 2001, 29, 535–562. [Google Scholar] [CrossRef]
- Hayakawa, C.; Funakawa, S.; Fujii, K.; Kadono, A.; Kosaki, T. Effects of climatic and soil properties on cellulose decomposition rates in temperate and tropical forests. Biol. Fertil. Soils 2014, 50, 633–643. [Google Scholar] [CrossRef]
- Xu, X.K. Effect of freeze-thaw disturbance on soil C and N dynamics and GHG fluxes of East Asia forests: Review and future perspectives. Soil Sci. Plant Nutr. 2022, 68, 15–26. [Google Scholar] [CrossRef]
- Xu, X.K.; Duan, C.T.; Wu, H.H.; Li, T.S.; Cheng, W.G. Effect of intensity and duration of freezing on soil microbial biomass, extactable C and N pools, and N2O and CO2 emissions from forest soils in cold temperate region. Sci. China Earth Sci. 2016, 59, 156–169. [Google Scholar] [CrossRef]
- Wu, H.H.; Xu, X.K.; Cheng, W.G.; Han, L. Dissolved organic matter and inorganic N jointly regulate greenhouse gases fluxes from forest soils with different moistures during a freeze-thaw period. Soil Sci. Plant Nutr. 2020, 66, 163–176. [Google Scholar] [CrossRef]
- Wu, H.H.; Xu, X.K.; Fu, P.Q.; Cheng, W.G.; Fu, C.S. Responses of soil WEOM quantity and quality to freeze-thaw and litter manipulation with contrasting soil water content: A laboratory experiment. Catena 2021, 198, 105058. [Google Scholar] [CrossRef]
- Martínez-Vilalta, J.; Sala, A.; Asensio, D.; Galiano, L.; Hoch, G.; Palacio, S.; Piper, F.I.; Lloret, F. Dynamics of non-structural carbohydrates in terrestrial plants: A global synthesis. Ecol. Monogr. 2016, 86, 495–516. [Google Scholar] [CrossRef]
- Harteveld, M.; Hertel, D.; Wiens, M.; Leuschner, C. Spatial and temporal variability of fine root abundance and growth in tropical moist forests and agroforestry systems (Sulawesi, Indonesia). Ecotropica 2007, 13, 111–120. [Google Scholar]
- Jiang, H.; Deng, Q.; Zhou, G.; Hui, D.; Zhang, D.; Liu, S.; Chu, G.; Li, J. Responses of soil respiration and its temperature/moisture sensitivity to precipitation in three subtropical forests in southern China. Biogeosciences 2013, 10, 3963–3982. [Google Scholar] [CrossRef]
- Huang, S.D.; Ye, G.F.; Lin, J.; Chen, K.T.; Xu, X.; Ruan, H.H.; Tan, F.L.; Chen, H.Y.H. Autotrophic and heterotrophic soil respiration responds asymmetrically to drought in a subtropical forest in the Southeast China. Soil Biol. Biochem. 2018, 123, 242–249. [Google Scholar] [CrossRef]
- Zhang, J.L.; Liu, S.R.; Liu, C.J.; Wang, H.; Luan, J.W.; Liu, X.J.; Guo, X.W.; Niu, B.L. Different mechanisms underlying divergent responses of autotrophic and heterotrophic respiration to long-term throughfall reduction in a warm-temperate oak forest. For. Ecosyst. 2021, 8, 41. [Google Scholar] [CrossRef]
- Deng, Q.; Zhang, D.; Han, X.; Chu, G.; Zhang, Q.; Hui, D. Changing rainfall frequency rather than drought rapidly alters annual soil respiration in a tropical forest. Soil Biol. Biochem. 2018, 121, 8–15. [Google Scholar] [CrossRef]
- Kiikkilä, O.; Kitunen, V.; Spetz, P.; Smolander, A. Characterization of dissolved organic matter in decomposing Norway spruce and silver birch litter. Eur. J. Soil Sci. 2012, 63, 476–486. [Google Scholar] [CrossRef]
- Hensgens, G.; Lechtenfeld, O.J.; Guillemette, F.; Laudon, H.; Berggren, M. Impacts of litter decay on organic leachate composition and reactivity. Biogeochemistry 2020, 154, 99–117. [Google Scholar] [CrossRef]
- Xu, J.W.; Ji, J.H.; Hu, D.N.; Zheng, Z.; Mao, R. Tree fresh leaf- and twig-leached dissolved organic matter quantity and biodegradability in subtropical plantations in China. Forests 2022, 13, 833. [Google Scholar] [CrossRef]
- Wu, P.P.; Ding, Y.D.; Li, S.L.; Sun, X.X.; Zhang, Y.; Mao, R. Carbon, nitrogen and phosphorus stoichiometry controls interspecific patterns of leaf litter-derived dissolved organic matter biodegradation in subtropical plantations of China. iForest 2021, 14, 80–85. [Google Scholar] [CrossRef]
- Xu, X.K.; Yin, L.; Duan, C.T.; Jing, Y.S. Effect of N addition, moisture, and temperature on soil microbial respiration and microbial biomass in forest soil at different stages of litter decomposition. J. Soils Sediments 2016, 16, 1421–1439. [Google Scholar] [CrossRef]
- Oberhuber, W.; Gruber, A.; Lethaus, G.; Winkler, A.; Wieser, G. Stem gridling indicates prioritized carbon allocation to the root system at the expense of radial stem growth in Norway spruce under drought conditions. Environ. Exp. Bot. 2017, 138, 109–118. [Google Scholar] [CrossRef]
- Smith, M.G.; Miller, R.E.; Arndt, S.K.; Kasel, S.; Bennett, L.T. Whole-tree distribution and temporal variation of non-structural carbohydrates in broadleaf evergreen trees. Tree Physiol. 2018, 38, 570–581. [Google Scholar] [CrossRef]
- Peltier, D.M.P.; Ogle, K. Legacies of more frequent drought in ponderosa pine across the western United States. Glob. Chang. Biol. 2019, 25, 3803–3816. [Google Scholar] [CrossRef]
- Li, S.L.; Xu, J.W.; Ding, Y.D.; Mao, R. Litter water-holding and water-loss characteristics of trees and ferns in the water conservation forests at the middle reaches of the Gan River. J. Soil Water Conserv. 2021, 35, 170–176. [Google Scholar]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Rousk, J.; Brookes, P.C.; Bääth, E. Investigating the mechanisms for the opposing pH relationships of fungal and bacterial growth in soil. Soil Biol. Biochem. 2010, 42, 926–934. [Google Scholar] [CrossRef]
- Smolander, A.; Kitunen, V. Comparison of tree species effects on microbial C and N transformations and dissolved organic matter properties in boreal forest floors. Appl. Soil Ecol. 2011, 49, 224–233. [Google Scholar] [CrossRef]
- Bouma, T.J.; Bryla, D.R. On the assessment of root and soil respiration for soils of different textures: Interactions with soil moisture contents and soil CO2 concentrations. Plant Soil 2000, 227, 215–221. [Google Scholar] [CrossRef]
- Dilustro, J.J.; Collins, B.; Duncan, L.; Crawford, C. Moisture and soil texture effects on soil CO2 efflux components in southeastern mixed pine forests. For. Ecol. Manag. 2005, 204, 85–95. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.R.; Luan, J.W.; Chen, C.; Cai, C.J.; Zhou, F.; Di, Y.P.; Gao, X.M. Nitrogen addition exacerbates the negative effect of throughfall reduction on soil respiration in a Bamboo Forest. Forests 2021, 12, 724. [Google Scholar] [CrossRef]
- Lu, H.B.; Liu, S.R.; Wang, H.; Luan, J.W.; Schindlbacher, A.; Liu, Y.C.; Wang, Y. Experimental throughfall reduction barely affects soil carbon dynamics in a warm-temperate oak forest, central China. Sci. Rep. 2017, 7, 15099. [Google Scholar] [CrossRef]
- Abramoff, R.; Xu, X.F.; Hartman, M.; O’Brien, S.; Feng, W.T.; Davidson, E.; Finzi, A.C.; Moorhead, D.; Schimel, J.; Torn, M. The Millennial model: In search of measurable pools and transformations for modeling soil carbon in the new century. Biogeochemistry 2018, 137, 51–71. [Google Scholar] [CrossRef]
- Zhang, H.C.; Goll, D.S.; Wang, Y.P.; Ciais, P.; Wieder, W.R.; Abramoff, R.; Haung, Y.Y.; Guenet, B.; Preschner, A.K.; Rossel, R.A.V.; et al. Microbial dynamics and soil physicochemical properties explain large-scale variations in soil organic carbon. Glob. Chang. Biol. 2020, 26, 2668–2685. [Google Scholar] [CrossRef]
- Epron, D.; Bahn, M.; Derrien, D.; Lattanzi, F.A.; Pumpanen, J.; Gessler, A.; Högberg, P.; Maillard, P.; Dannoura, M.; Gérant, D.; et al. Pulse-labelling trees to study carbon allocation dynamics: A review of methods, current knowledge and future prospects. Tree Physiol. 2012, 32, 776–798. [Google Scholar] [CrossRef]
- McCarroll, D.; Whitney, M.; Young, G.H.F.; Loader, N.J.; Gagen, M.H. A simple stable carbon isotope method for investigating changes in the use of recent versus old carbon in oak. Tree Physiol. 2017, 37, 1021–1027. [Google Scholar] [CrossRef]
- Diao, H.Y.; Wang, A.Z.; Yuan, F.H.; Guan, D.X.; Dai, G.H.; Wu, J.B. Environmental effects on carbon isotope discrimination from assimilation to respiration in a coniferous and broadleaf-leaved mixed forest of Northeast China. Forests 2020, 11, 1156. [Google Scholar] [CrossRef]
- Wen, T.; Qu, Y.X.; Lu, K.L.; Guan, C.; Zhao, C.M. Combining tree-ring width and carbon isotope data to investigate stem carbon allocation in an evergreen coniferous species. Agric. For. Meteorol. 2022, 316, 108845. [Google Scholar] [CrossRef]
- Trumbore, S. Age of soil organic matter and soil respiration: Radiocarbon constraints on belowground C dynamics. Ecol. Appl. 2000, 10, 399–411. [Google Scholar] [CrossRef]
- Moyes, A.B.; Gaines, S.J.; Siegwolf, R.T.W.; Bowling, D.R. Diffusive fractionation complicates isotopic partitioning of autotrphic and heterotrophic sources of soil respiration. Plant Cell Environ. 2010, 33, 1804–1819. [Google Scholar] [CrossRef]
- Dařenová, E.; Crabbe, R.A.; Knott, R.; Uherková, B.; Kadavý, J. Effect of coppicing, thinning and throughfall reduction on soil water content and soil CO2 efflux in a sessile oak forest. Silva Fennica 2018, 52, 9927. [Google Scholar] [CrossRef]
- Muhr, J.; Borken, W. Delayed recovery of soil respiration after wetting of dry soil further reduced C losses from a Norway spruce forest soil. J. Geophys. Res. 2009, 114, G04023. [Google Scholar] [CrossRef]
- Borken, W.; Davidson, E.A.; Savage, K.; Sundquist, E.T.; Steudler, P. Effect of summer throughfall exclusion, summer drought, and winter snow cover on methane fluxes in a temperate forest soil. Soil Biol. Biochem. 2006, 38, 1388–1395. [Google Scholar] [CrossRef]
- Liu, Y.C.; Shang, Q.; Wang, L.; Tian, Y.; Ju, Y.X.; Gan, J.B. Responses of soil respiration to changing precipitation regimes in an oak forest at a climate transitional zone. Acta Ecol. Sin. 2016, 36, 8054–8061. [Google Scholar]
- Peng, X.H.; Xu, X.F.; Han, H.R.; Cheng, X.Q.; Cai, M.K.; Bai, Y.C. Effects of throughfall exclusion on soil respiration in Larix principis-rupprechtii plantation. Ecol. Environ. Sci. 2017, 26, 1310–1316. [Google Scholar]
- Vasconcelos, S.S.; Zarin, D.J.; Capanu, M.; Littell, R.; Davidson, E.A.; Ishida, F.Y.; Santos, E.B.; Araújo, M.M.; Aragão, D.V.; Rangel-Vasconcelos, L.G.T.; et al. Moisture and substrate availability constrain soil trace gas fluxes in an eastern Amazonian regrowth forest. Global Biogeochem. Cycle 2004, 18, GB2009. [Google Scholar] [CrossRef]
- Bréchet, L.; Courtois, E.A.; Saint-Germain, T.; Janssens, I.A.; Asensio, D.; Ramirez-Rojas, I.; Soong, J.L.; Langenhove, L.V.; Verbruggen, E.; Stahl, C. Disentangling drought and nutrient effects on soil carbon dioxide and methane fluxes in a tropical forest. Front. Environ. Sci. 2019, 7, 180. [Google Scholar] [CrossRef]
- Xu, J. Response of Soil Carbon Processes to Throughfall Exclusion in Two Subtropical Plantations in Southern China. Ph.D. Thesis, Chinese Academy of Forestry, Beijing, China, 2014. [Google Scholar]
- Liu, W.; Wang, L.H.; Fu, R.; Zhou, S.X.; Liu, L.; Wu, X.H.; Huang, C.D. Effects of precipitation increase on soil respiration of a Pinus yunnanensis plantation in a dry river valley area. Acta Ecol. Sin. 2017, 37, 1391–1400. [Google Scholar]
- Li, S.J. Effects of Precipitation Exclusion on Soil Microbial Community Structure and Greenhouse Gas Fluxes in a Natural Castanopsis carlesii Forest. Master’s Thesis, Fujian Normal University, Fuzhou, China, 2018. [Google Scholar]
- Ju, H. The Characteristic of Soil Activated Carbon and Nitrogen and Greenhouse Gas Fluxes of the Different Types of Forests in Shennongjia. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2016. [Google Scholar]
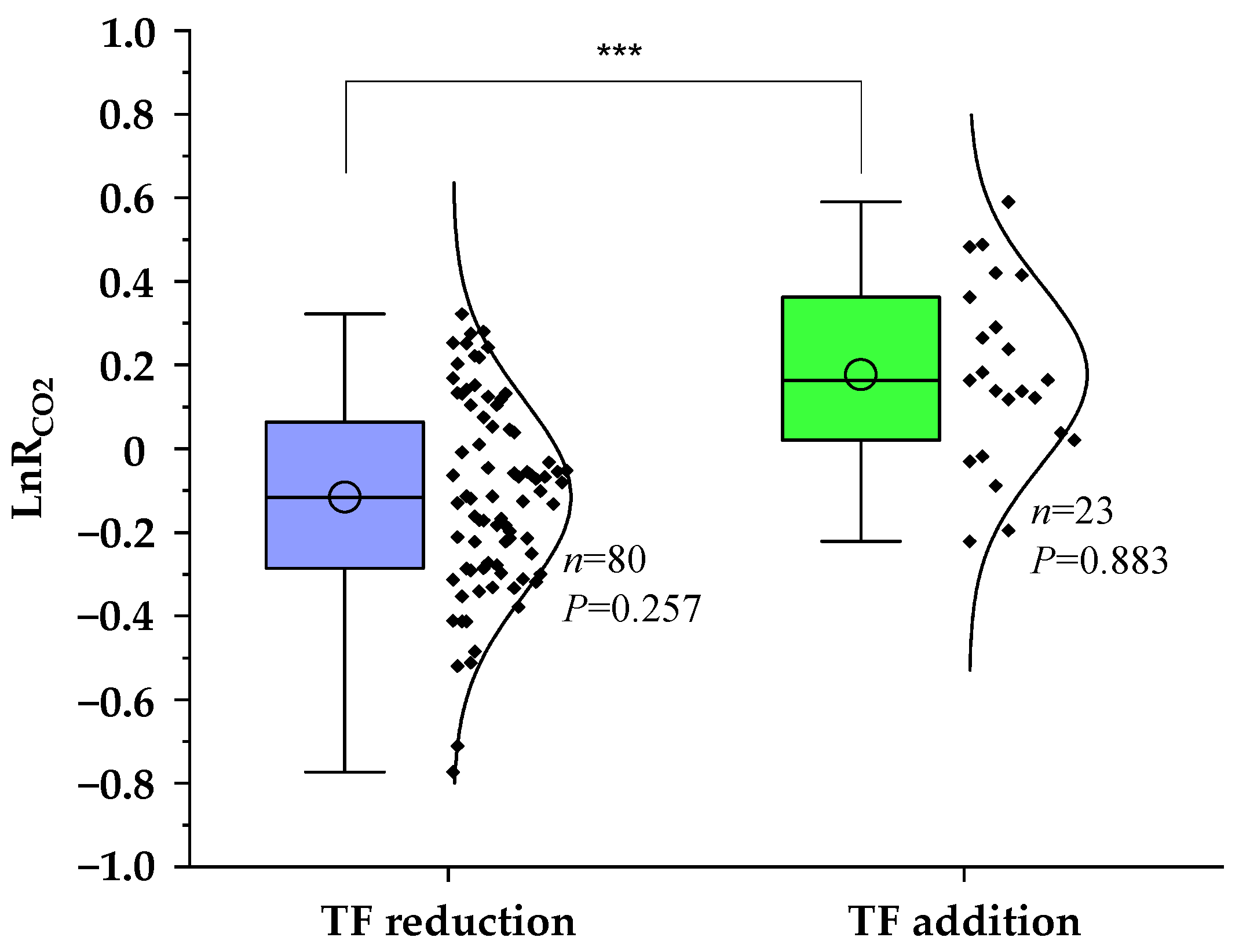
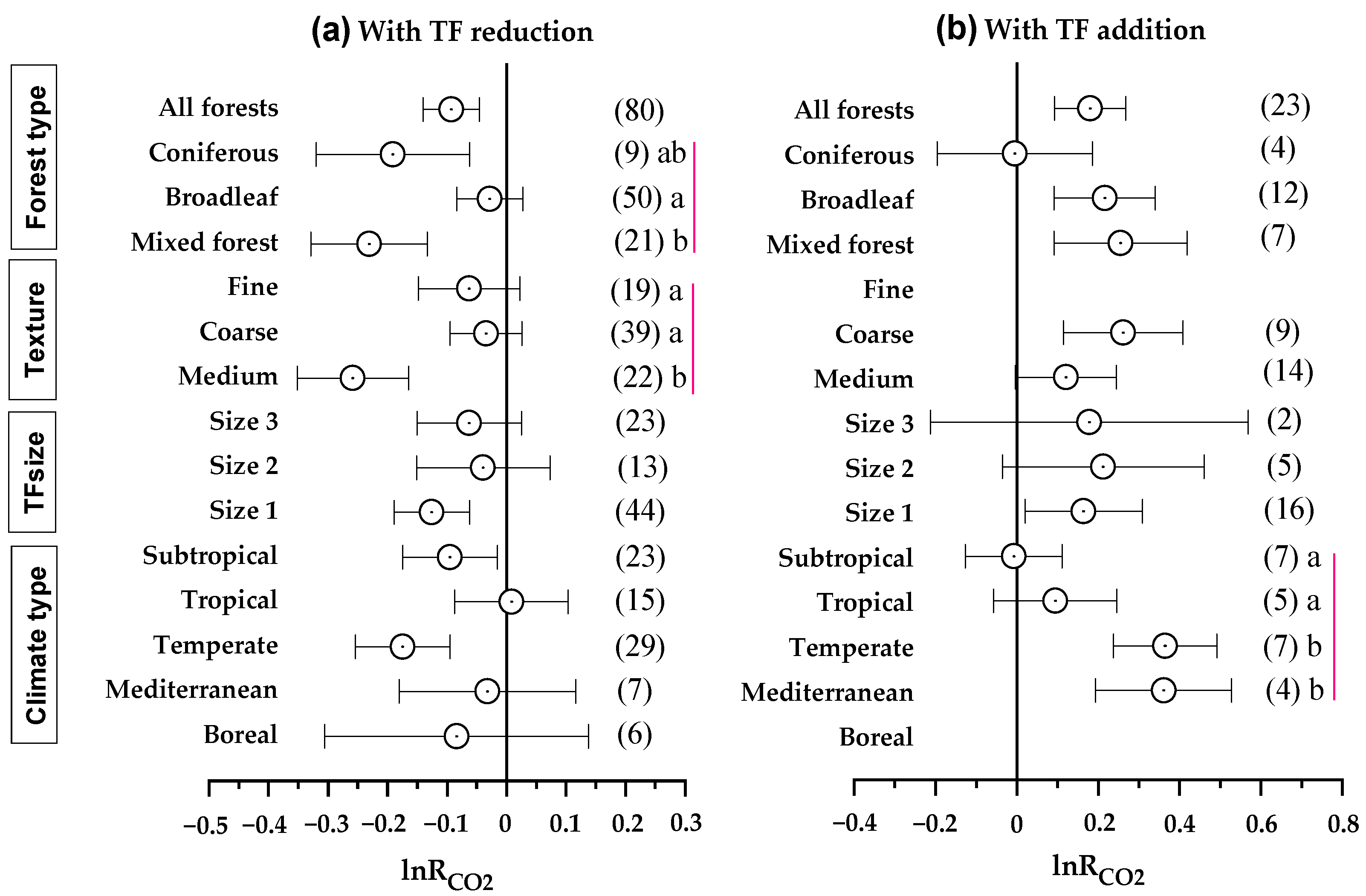


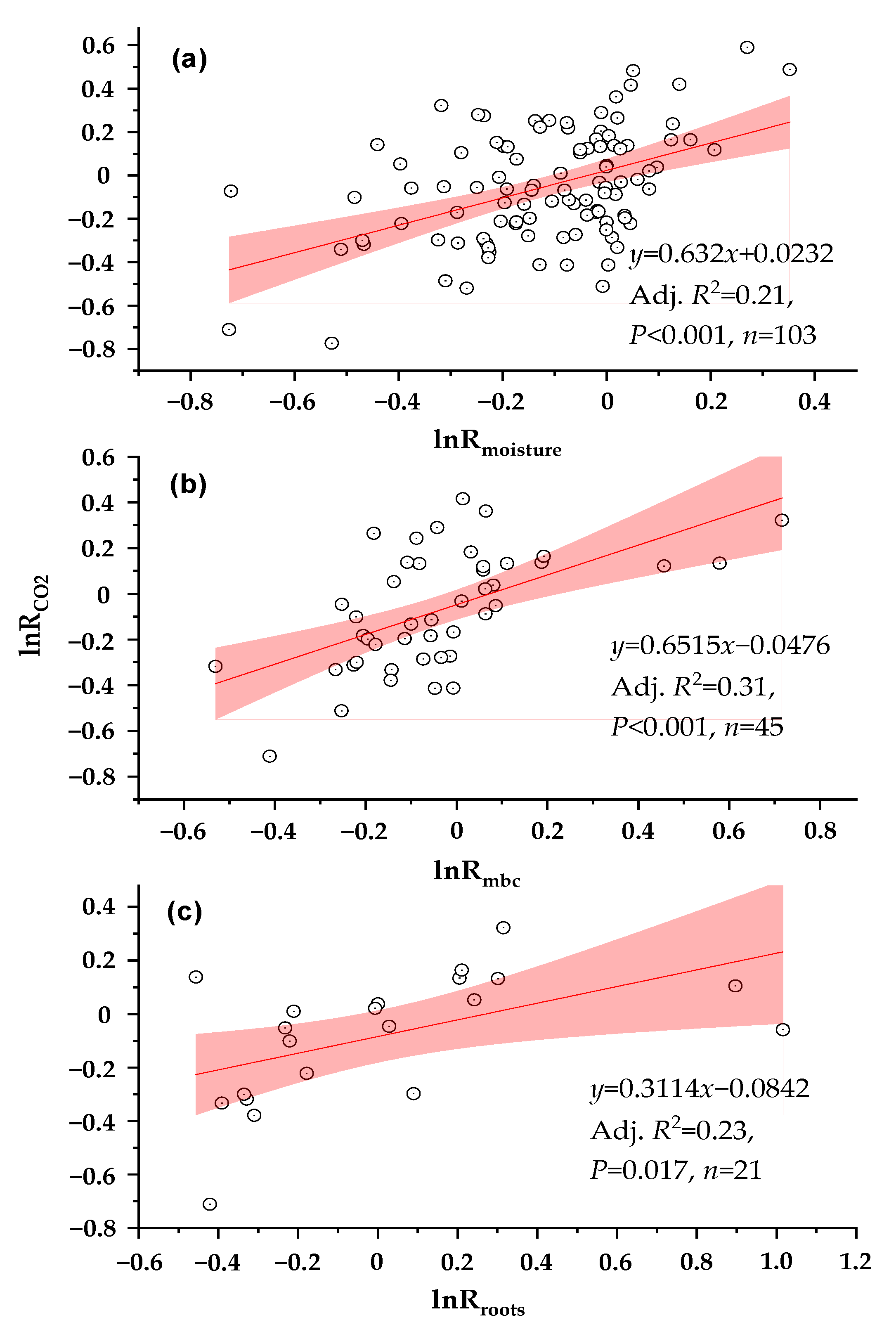

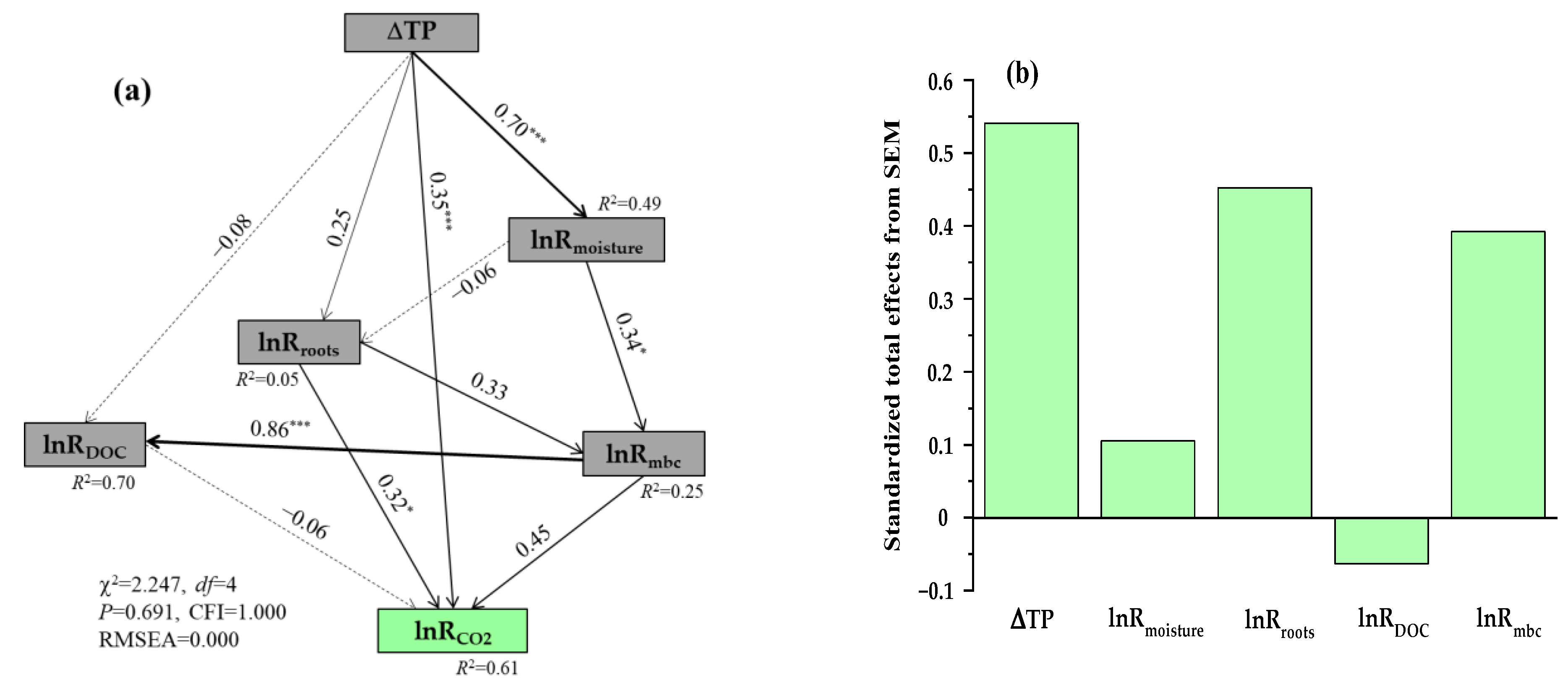

| Experimental Categories | Specific Conditions (Number of Paired Observation Data Points) a | Methods of Statistical Analysis |
|---|---|---|
| Climate conditions | Climate types (103): Boreal (6), Mediterranean (11), Temperate (36), Tropical (20), and Subtropical (30) | Analysis of variance (ANOVA) |
| Annual rainfall (103): 493 to 3528 mm | Structural equation modeling (SEM) | |
| Annual mean air temperature (103): −3.5 to 28.0 °C | SEM | |
| Forest types | Coniferous (13), Broadleaf (62), and Mixed forest (28) | ANOVA |
| Soil properties | Texture (103) b: Fine (19), Medium (36), and Coarse (48) | ANOVA |
| pH (103): 3.2 to 7.5 | SEM | |
| SOC (103): 1.4 to 83.0 g C kg−1 | SEM | |
| TN (103): 0.1 to 11.3 g N kg−1 | SEM | |
| Ratio of SOC to TN (103): 5.8 to 44.7 | SEM | |
| Bulk density (103): 0.5 to 1.6 g cm−3 | SEM | |
| TFsize c | Sizes (103): Size 1 (≤5 m × 5 m) (60), Size 2 (>5 m × 5 m and <20 m × 20 m) (18), and Size 3 (≥20 m × 20 m) (25) | ANOVA |
| Experimental Categories | Effects | lnRCO2 Upon TF Reduction | lnRCO2 Upon TF Addition | ||
|---|---|---|---|---|---|
| F Values | p Values | F Values | p Values | ||
| Climate conditions | Climate types | 1.439 | 0.230 | 10.261 | 0.000 |
| Annual rainfall | NA a | NA | |||
| Annual mean air temperature | NA | NA | |||
| Forest types | Forest types | 7.955 | 0.001 | 1.819 | 0.188 |
| Soil properties | Texture | 13.616 | 0.000 | 0.607 | 0.444 |
| pH | NA | NA | |||
| SOC | NA | NA | |||
| TN | NA | NA | |||
| Ratio of SOC to TN | NA | NA | |||
| Bulk density | NA | NA | |||
| TFsize | Sizes | 0.775 | 0.464 | 0.031 | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X. Effect of Changes in Throughfall on Soil Respiration in Global Forest Ecosystems: A Meta-Analysis. Forests 2023, 14, 1037. https://doi.org/10.3390/f14051037
Xu X. Effect of Changes in Throughfall on Soil Respiration in Global Forest Ecosystems: A Meta-Analysis. Forests. 2023; 14(5):1037. https://doi.org/10.3390/f14051037
Chicago/Turabian StyleXu, Xingkai. 2023. "Effect of Changes in Throughfall on Soil Respiration in Global Forest Ecosystems: A Meta-Analysis" Forests 14, no. 5: 1037. https://doi.org/10.3390/f14051037
APA StyleXu, X. (2023). Effect of Changes in Throughfall on Soil Respiration in Global Forest Ecosystems: A Meta-Analysis. Forests, 14(5), 1037. https://doi.org/10.3390/f14051037






