Abstract
Pine wilt disease (PWD) is regarded as one of the most serious conifer diseases affecting pines worldwide. To date, an in-depth study of the driving mechanisms behind short-distance PWD spread is lacking. In this study, we collected PWD forest subcompartment data in Fushun, China, and analysed the effects of factors on the short-distance transmission of PWD; the analysed factors included the number of neighbouring PWD-infected forest subcompartments, the canopy density, the slope direction, and different traffic corridor types. The results suggested that the spatial spill-over effect of nearby PWD-infected subcompartments contributed the most to short-distance PWD transmission, with an impact of up to 78% on its propagation. The impact of the traffic corridor was 20%. With the help of a beetle vector, PWD can spread to nearby forest subcompartments, and this spatial PWD spill-over effect showed a linearly decaying trend as the distance to neighbouring subcompartments increased. Different traffic corridor types exhibited significant PWD transmission impact differences. County roadways and highways had great impacts, while others had relatively small impacts. For each additional 100 m of distance from a county roadway, highway, national, or provincial roadway, the PWD infection risks in forest subcompartments were reduced by 18%, 11%, 5%, and 3%, respectively. In this study, we quantified the influence of driving factors on the short-distance spread of PWD and provided a theoretical basis for the control of PWD transmission; the results obtained herein are critical for maintaining the ecological security of forests, promoting ecological forest management and stabilising forest carbon sinks.
1. Introduction
Pine wilt disease (PWD) is a devastating threat to forest ecosystems globally and is capable of destroying trees on a timescale of weeks to months [1]. Caused by invasive species, the PWD first appeared in mainland China in 1982 [2]. As of 31 December 2020, according to the latest PWD data released by the China Forestry and Grass Administration, PWD has been identified in a total of 5479 towns and villages in 726 county-level administrative regions in 18 provinces in China. In 2020 alone, 60 new county-level epidemic areas will be added, and there will be multiple outbreaks [3,4]. Faced with such a severe PWD situation, it is necessary to study the driving mechanism of pine wood nematode (PWN) transmission. In particular, the study of PWN propagation over short distances is important for controlling PWN diffusion. Therefore, the analysis of the main modes of short-distance PWD transmission has far-reaching importance for the regional control of PWD spread.
PWD causes vast economic and ecological losses to forest ecosystems and affects forest management in infected areas. Since the 1990s, more than 1.4 million trees have died from PWD in China, causing an average annual economic loss of more than seven billion yuan [5]. In recent years, PWD has spread to the Qinling and Liaoning Provinces, where it is expected to pose a fatal threat to more than 30 million hectares of pine forests [3]. Furthermore, forest carbon sinks are not permanent [6,7], and pine trees infected with PWD gradually weaken until they lose their carbon sequestration ability [8,9]. Moreover, the rapid release of carbon back into the atmosphere due to the death of pine trees poses a threat to the global forest carbon storage [10,11].
PWD is naturally transmitted with the help of the long-horned beetle (Coleoptera: Ceruginidae) vector, and Monochamus alternatus (M. alternatus), which is widely distributed in China, is the main vector of Bursaphelenchus xylophilus (B. xylophilus) in the nation [12]. M. alternatus adults carrying Bursaphelenchus xylophilus fly to nearby healthy pine trees to forage, at which time the PWNs leave the tracheas of the beetles and enter the healthy trees through the opening by which the beetle is feeding [13,14]. Once inside the new host tree, Bursaphelenchus xylophilus can proliferate over a long period of time [15]. In addition, authors who performed an M. alternatus flight distance test reported that the species can fly up to 800 m [13,16,17], while the propagation distance is generally approximately 2–3 km [18].
PWD exhibits a combination of short-distance and long-distance transmission and dispersal methods [19,20]. “Short-distance transmission” refers to the spread of PWD in a relatively small range. Three main modes of PWD transmission have been found: transmission through the Monochamus alternatus vector [21,22], movements of PWNs themselves, and physical contact between diseased roots and healthy trees in pure forests [23]. The long-distance transmission of PWD occurs in conjunction with human activities that span multiple regions [24,25,26]. In the main manifestation of long-distance PWD transmission, PWNs are transported to a new place after wood infected with PWD is felled, resulting in the spread of PWNs along roads [27]; alternatively, diseased wood can be used directly to build packaging boxes for trade circulation without being treated [28].
The process by which PWNs spread locally is affected by natural environmental factors such as climate, topography, host vegetation, and media factors [29]. PWD is likely to survive and spread only when tree species are susceptible and the environment and climate conditions are suitable [30,31]. Topographic factors such as slope direction also play important roles in mediating temperature, humidity, and wind conditions in forests [32]. High temperatures, dryness, and wind are also important factors affecting the spread of PWD [33,34,35,36,37]. Host vegetation also affects the occurrence of PWD [23], as volatiles from healthy host pine trees attract PWNs [38]. Trees in low-density stands are thought to be more susceptible to PWD infection than high-density-stand trees because volatiles are more likely to spread in lower-density forests [39,40]. However, past studies have also reported that PWNs can spread farther in high-density forests than in low-density forests [41,42].
Human factors also play major roles in the spread of PWD. As the population density increases, the risk of local trees contracting PWD increases [43] alongside the rate of spread [44,45]. When trees infected with PWD are processed into wood or wood products, the PWNs within the wood persist and survive [46]. PWN adults can thus be transported to roadsides far from their origin [47]. Studies have found that transportation hubs play an important role in human-mediated communication [48].
To date, there is no single study on the driving mechanism of PWN spread in the research; there is no single study on the short-distance transmission and dispersal of PWN. These existing studies mainly analysed the effects of the population density, the road density, the host vegetation, and terrain factors on PWD spread when researching the driving mechanisms of short-distance PWD spread [27,49,50,51]. However, the above driving factors are not suitable for studying short-distance transmission in small study areas. In past research on short-distance PWD transmission, there is also a lack of comparative analysis of the different effects of various driving factors. There is a lack of research that considers the spatial spill-over effect caused by the flight effect of the M. alternatus vector. In considering the impact of roads, the impact of deeper traffic corridors at different types on the spread of the PWN was not considered. In this study, we not only consider the different influences of multilevel traffic corridors, such as highways, national roadways, provincial roadways, and county roadways, on PWN transmission but also study the contribution of spatial spill-over effects of nearby PWD-infected subcompartments to PWN transmission. Corresponding strategies for the control of PWN are proposed according to different impacts, and the supervision of roads that easily spread PWN is strengthened to effectively control the spread of PWN. In this study, we collected fine-resolution PWD forest subcompartment data and took Fushun City as an example to address the following questions: (1) How much do the spatial spill-over effects and traffic corridors at different types contribute to the short-distance transmission of PWD? (2) What is the regularity of the spatial spill-over effects of nearby PWD-infected subcompartments on the spread of the PWD in short-distance transmission of PWN? and (3) Based on our in-depth analysis of roads in short-distance PWD transmission, are there significant differences in the impacts of different transportation corridor types on PWD transmission?
2. Materials and Methods
2.1. Study Area
The study area is located in the northeastern part of Liaoning Province, China, which is Fushun City. The study area is located between the longitude lines of 123°39′42″ and 125°28′58″ E and the latitude lines of 41°14′10″ and 42°28′32″ N, with a total area of 11,271.5 square km (Figure 1). The study area is bordered by Jilin Province to the east, the provincial capital Shenyang 45 km to the west, Tieling in the north, and Benxi in the south. The average elevation of Fushun City is 80 m. The city is located in the middle-temperate zone and belongs to the continental monsoon climate. The annual average temperature is 7.0 °C, and the annual average precipitation total is 800 mm. Fushun City is located in the remnants of the Changbai Mountains and is surrounded by mountains on three sides. The forest coverage rate of Fushun City is 68.44%, and the forest volume reaches 80.3829 million square m. The local forests are composed of coniferous forests (comprising Korean pine, Pinus tabuliformis, and Pinus sylvestris) and broad-leaved forests [52].
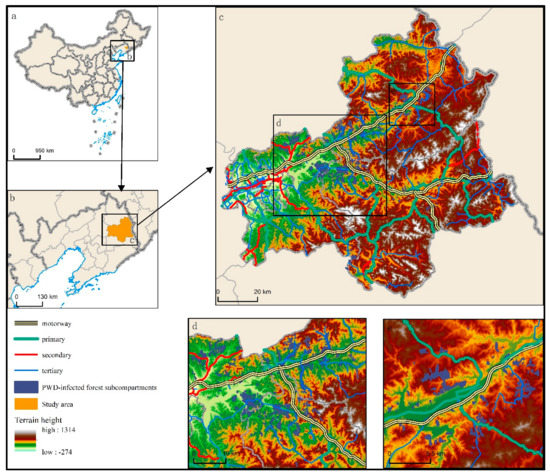
Figure 1.
Study area of Fushun City, Liaodong Province, China (the indigo areas indicate PWD forest subcompartments; (a): the location of Liaoning Province in China; (b): the location of Fushun City in Liaoning Province; (c): distribution of forest subcompartments in Fushun City in the study area; (d): the concentrated distribution area of forest subcompartments in the southwest part of Fushun City; (e): the concentrated distribution area of of forest subcompartments in northeast Fushun City).
2.2. Data
In this study, we conducted analyses on the forest subcompartment scale. Subcompartmental PWD data in Fushun City were obtained from the Biological Disaster Prevention and Control Center of the State Forestry and Grassland Administration (www.forestpest.cn). The data provided by the Biological Disaster Prevention and Control Center of the State Forestry and Grassland Administration are based on the second category of forest resources survey in 2017, and the ground mobile phone is used to conduct a field survey of all forest subcompartments in Fushun City. Among the 285,646 forest subcompartments in the second-category survey data of forest resources in Fushun City, 164,521 forest subcompartments were pine subcompartments, 805 forest subcompartments were found to have PWD, and their spatial locations were recorded (Figure 1c). The second category of forest resources survey is a forest resource survey conducted by forest management units such as state-owned forest farms, nature reserves, forest parks, or county-level administrative regions to meet the needs of forest management plans, overall design, forestry zoning, and planning and design. The attribute information, including the slope direction and canopy density, was considered in the PWD forest subcompartment data. The slope directions were divided into eight categories, including the north, northeast, east, southeast, south, southwest, west, and northwest slope directions. The canopy density ranged from 0 to 1 and referred to the ratio of the total projected area (crown width) of tree crowns on the ground under direct total-area (stand) sunlight conditions in each forest subcompartment.
Road data collected in Fushun City were obtained from the 2020 vector road network data of OpenStreetMap (https://www.openstreetmap.org/, accessed on 3 May 2021). The road categories were verified using the 2020 China real-time road network navigation data categories, and four different traffic corridor types were established: highways, national roadways, provincial roadways, and county roadways. The expressways in Fushun City include the Shenji Highway, Futong Highway, and Yongyong Highway. Shenji Highway runs through Fushun City, with a total length of 428.8 km, of which the length of Fushun City accounts for 137 km. The Futong Highway runs through Xinbin Manchu Autonomous County and Qingyuan. At the junction of Manchu Autonomous County, the Futong Highway intersects with the Shenji Highway. The national roadways and highways in Fushun City complement each other and connect the whole city. The county roadways intersect with the national and provincial roadways and run through various mountain areas, thus connecting the county with the outside area.
The spread of PWD is affected by the natural environment and human activities and can be divided into two types: long-distance and short-distance transmission [22]. Because the scope of this study is small, the propagation mode analysed herein is mainly short-distance transmission. In the study area, the spatial variations in meteorological factors such as temperature, humidity, and the regional population density are very small, so the influence of climate conditions and the population density is not considered in this study. Fushun has a complex topography, and there are large differences in the aspect of the forest subcompartments. At the same time, stand density differences also exist among different forest subcompartments; thus, the slope direction, canopy density, and spatial spill-over effect of the PWD forest subcompartments themselves were selected as natural influencing factors in this work. To evaluate the impacts of human activities on the spread of PWD, the distances from traffic corridors of different types (highways, national roadways, provincial roadways, and county roadways) to the forest subcompartments were used as a proxy variable (Figure 2).
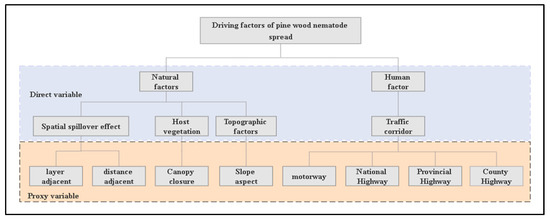
Figure 2.
Graph showing the proxy variables used to analyse the spread of PWD.
2.3. Methods
2.3.1. Spatial Spill-Over Effect of PWD
In this study, because PWD can spread among host vegetation in different forest subcompartments, an obvious physical–geographic spatial PWD spill-over effect exists [53]. After PWNs infect a host tree and reproduce within the tree to reach a certain population density, the PWNs must find a new host to survive and reproduce. In addition, if host pine trees that are suitable for PWN survival are located near epidemic forest subcompartments, the volatiles of the uninfected host pine trees can attract M. alternatus adults. The PWNs then gathered on the M. alternatus adults and spread to the healthy forest subcompartments. If no suitable trees are located in any adjacent forest subcompartment, M. alternatus spreads to appropriate forest subcompartments within their flight distance abilities. In rare cases, PWNs can also spread through infected sawdust to the injured roots of adjacent trees, thereby infecting the adjacent trees [54,55]. In this paper, the infection probabilities of forest subcompartments at different distances to a PWD-infected forest subcompartment were studied, and the spatial spill-over effect of PWD was subsequently analysed.
2.3.2. Binary Logistic Regression
In this study, binary logistic regression was used to analyse the connections among the spread of PWD, traffic corridors, and nearby PWD-infected forest subcompartments [56]. Binary logistic regression is a nonlinear multivariate statistical method [57] in which maximum-likelihood estimation is used to ensure that each point is fitted optimally. This method has been widely used in natural disaster assessments and prediction research [58]. The dependent variable in the binary logistic regression model is represented by a binary categorical variable, where 1 indicates true (infection) and 0 indicates false (noninfection). The independent model variables are described by a series of exposure factors. If we let the probability of an event occurring be P and the probability of the event not occurring be 1-P, the regression relationship between the probability P of the forest subcompartment being infected and the independent variable (influencing factor) can be expressed as follows:
where represents the risk probability of a PWD infection in forest subcompartment ; represents the logarithm of the ratio of the probability of the forest subcompartment being infected with PWD and being disease-free; is the influencing factor representing the distance from forest subcompartment to the nearest traffic corridors of different types, the canopy density, the slope direction, or the influence of the number of adjacent PWD-infected forest subcompartments; and is the logistic regression coefficient corresponding to the impact factor represented by the ratio of the probability of infection in the subcompartment to the probability of noninfection in the subcompartment. Due to the large number of independent variables analysed in this study, the significance level of the variable regression coefficient in the model was set to 0.1.
The two influencing factors in the model, the number of PWD-infected forest subcompartments in the vicinity of the analysed subcompartment and traffic corridors of different types, were obtained as follows. The number of PWD-infected forest subcompartments among the nearest neighbours was divided into two cases: layer neighbours and distance neighbours. The statistics for the number of neighbouring infected forest subcompartments include the statistics of the number of first-level neighbouring infected forest subcompartments and the second-level neighbouring infected forest subcompartments of the training forest subcompartments. The number of first-level neighbouring infected forest subcompartments refers to the number of infected forest subcompartments in the forest subcompartments that are connected by common vertices or shared edges with the training forest subcompartments based on the principle of proximity matrix (Queen adjacency) in spatial correlation analysis. The number of second-level neighbouring infected forest subcompartments is the number of infected forest subcompartments in the forest subcompartments that are connected with the first-level neighbouring infected forest subcompartments in common vertex connection or common edge connection. The distance neighbours were used with the “Generate Neighbor Table” tool in ArcMap to determine all the infected forest subcompartments within a certain distance of each training forest subcompartment. Then, the number of infected forest subcompartments within a certain distance of each training forest subcompartment was determined. When considering the influence of different traffic corridor types, we assumed that forest subcompartments were affected only by the nearest road and used nearest-neighbour analysis on each forest subcompartment to calculate the distance to the graded road and the adjacent value.
When studying the factors influencing PWD infection in forest subcompartments, the dependent variable took one of only two states: infected or uninfected. All sample data were selected from the pine forest subcompartment data. Using the statsmodels library in python, a binary logistic regression model was thus used to assess the relationships between different factors and the infection or noninfection status of forest subcompartments. The utilised model can explore the comprehensive effect of the influencing factors on the occurrence of PWD. To avoid the randomness of the results of the noninfected forest subcompartments selected in the study of the spread of PWD, the data were prepared according to the ratio of 1:10 between the infected and noninfected forest subcompartments. To ensure the balance of training samples for the study on the spread of PWD, a number of noninfected forest subcompartment samples equal to the number of infected forest subcompartments were randomly selected for training. During training, 80% of the sample data were selected as training data and 20% as test data. We randomly selected 100 different unaffected subcompartments to form 100 models with PWD-infected subcompartments. Finally, the independent variable coefficients were synthesised from these 100 models to enhance the robustness of the training model. At the same time, the ratio of infected to noninfected subcompartments in the training data was 1:1.
According to the particularities of logistic regression, the absolute value of the logistic regression coefficient () cannot be used to compare the relative effect of each independent variable; thus, standardised logistic regression coefficients were introduced for this purpose. These logistic regression coefficients were standardised to eliminate the influence of dimension and order-of-magnitude differences. The use of standardised regression coefficients can improve the comparability among different variables and facilitate the comparison of different independent variable contributions to the model. The formula used to obtain the standardised logistic regression coefficients is as follows:
where represents the standardised regression coefficient, represents the standard deviation of the independent variable , and represents the standard deviation of the dependent variable in the binary logistic regression model; the standard deviation of the random distribution function in the binary logistic regression model is . The standardised regression coefficient calculated using the above formula was then converted to an absolute value after exponential (e) conversion. The relative effects of different independent variables on the dependent variable were expressed by comparing the corresponding absolute values.
In this study, the statsmodels library in python language was used to perform logistic regression and t tests (p value). The sklearn.metrics under the sklearn module was used to calculate the coefficient of determination (R2) and the area under the curve (AUC) for the evaluation metrics (Table A3). The significance test (p value) was used to verify whether the regression coefficients of the model variables were significant, that is, whether each independent variable could explain the corresponding dependent variable. The coefficient of determination (R2) was used to test the fitting effect of the binary logistic regression model. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the performance of the logistic regression model.
3. Results
3.1. Differences in the Transmission and Spread of the PWN by Individual Drivers
Short-distance PWD spread refers to the process by which PWD is transmitted by the M. alternatus vector to new nearby host trees in stands with suitable natural-environmental conditions. We found that the spatial spill-over effect of PWD was the main factor affecting short-distance PWD spread and dispersal. Among the driving factors of different types of traffic corridors, spatial spill-over effects, and canopy density currently studied, 78% of the occurrence of PWD-infected forest subcompartments is affected by the spatial spill-over effect of nearby PWD-infected subcompartments, and 20% is caused by different types of traffic corridors. In addition, the contribution of canopy density is only 2% (Table A2).
3.2. Spatial Spill-Over Effect in PWD Spread
We found that the spread of PWD exhibited significant spatial spill-over effects. PWD-infected forest subcompartments had very significant transmission effects on their first-order and second-order adjacent subcompartments. When a forest subcompartment becomes infected with PWD, the risk of an outbreak in the first-order neighbouring subcompartments of the same class increased 11.4-fold, while the risk of an outbreak in the second-order neighbouring subcompartments increased 8.5-fold (Table A1).
As the distance from an uninfected forest subcompartment to a PWD outbreak gradually increased, the risk of PWD infection in the forest subcompartment showed an approximately linear downwards trend (Figure 3a). In this study, we found that the risk of a PWD infection within 0.5 km of a PWD-infected forest subcompartment was reduced by approximately four times when the distance from the epidemic subcompartment increased by 0.1 km. At 0.5 km, the impact of a PWD-infected forest subcompartment on the neighbouring forest subcompartments dropped to a certain level. In addition, the impact of PWD-infected forest subcompartments on the disease infection risk of forest subcompartments dropped to below 75% when a distance of 1.5 km was reached. The impact of epidemic subcompartments on forest subcompartments located 1.5 km away was basically stable, suggesting that in forest subcompartments located farther than 1.5 km away from a PWD-infected forest subcompartment, the risk of contracting PWD is basically reduced to the minimum impact.
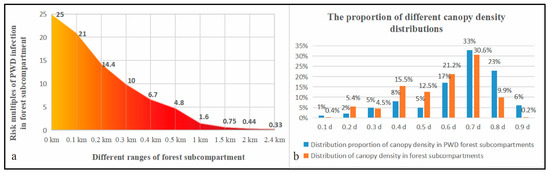
Figure 3.
Risk statistics of the number of PWD-infected forest subcompartments at different distances from an infected forest subcompartment (a); canopy density distribution of the PWD-infected forest subcompartments (b).
3.3. Effects of the Canopy Density and Slope Directions on PWD
The canopy density degree of the PWD-infected forest subcompartments in Fushun City peaked at 0.7; the canopy density degrees were mainly distributed in the 0.6–0.8 range (Figure 3b). According to the canopy density, 61.6% of the forest subcompartments were distributed in the 0.6–0.8 range in the study area, of which 21.2% had a canopy density of 0.6; 73% of the PWD-infected forest subcompartments were distributed in the 0.6–0.8 range, of which only 17% had a canopy density of 0.6. When the canopy density of PWD-infected forest subcompartments was considered alone, the results showed that whether the subcompartments were infected was significantly positively correlated with the canopy density range. When the forest canopy density was within the 0.6–0.8 range, the PWD infection risk increased by 22% (Table A1).
The slope directions of the PWD-infected forest subcompartments in Fushun City exhibited a total of eight possible directions. The proportion of PWD-infected forest subcompartments with these directions (clockwise from the north slope direction) basically first increased and then decreased. This change trend was similar to the overall slope direction trend of the Fushun forest subcompartments. We also found that the p-value coefficient of the slope directions obtained in the logistic regression analysis was much greater than 0.1, indicating that the effect of the slope directions on the PWD status was not significant.
3.4. Spatial Proximity Characteristics of the Distribution of PWD-Infected Forest Subcompartments
The PWD-infected forest subcompartments showed spatially proximal characteristics. As the distance between the target forest subcompartment and other recently PWD-infected forest subcompartments continued to increase, the number of PWD-infected forest subcompartments with other neighbouring epidemic forest subcompartments showed an increasing trend (Figure 4). Within the 0–0.5 km range surrounding PWD-infected forest subcompartments, the number of epidemic forest subcompartments recently increased rapidly to 87%, indicating that most epidemic forest subcompartments are located very close to each other. Then, the increasing trend of the number of PWD-infected forest subcompartments flattened out, exhibiting an average increase of only approximately 3% per kilometre. Epidemic forest subcompartments exist within 2.4 km of basically all (99%) PWD-infected subcompartments.
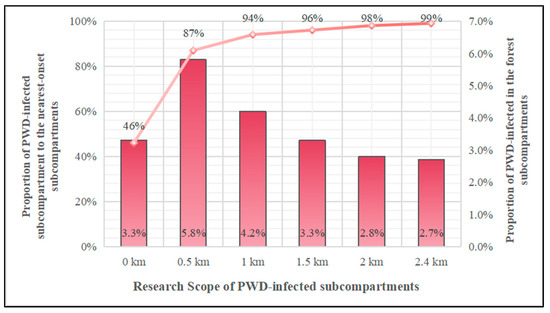
Figure 4.
Relationships between neighbouring PWD-infected forest subcompartments in Fushun City.
As the impacts of PWD-infected forest subcompartments continuously increased, the proportion of PWD infections among the nearby forest subcompartments showed a trend of first increasing and then decreasing (Figure 4). Among the subcompartments directly adjacent to the analysed PWD-infected subcompartment, epidemic subcompartments accounted for 3.3% of all adjacent forest subcompartments. The proportion of the epidemic subcompartments within the range of 0–0.5 km rose to the maximum value (5.8%). In addition, a high clustering distribution was observed in the forest subcompartments within 0.5 km of the analysed PWD-infected subcompartment. With the expansion of the scope of PWD-infected forest subcompartments, the proportion of epidemic subcompartments among adjacent forest subcompartments dropped significantly within the range of 0.5–2.4 km of the PWD-infected forest subcompartments. The proportion of PWD-infected forest subcompartments decreased by an average of approximately 1.6% for each 1 km expansion.
3.5. Different Traffic Corridor Types Differentially Affected the Spread of PWD
Different traffic corridor types are a type of human factor in the study, and the influence of these various types on PWD transmission differs significantly. Our research shows that county roadways and highways very significantly impact the spread of PWD, while the impacts of national and provincial roadways were relatively small. With the other factors remaining constant, the risk of PWD infection decreased by 18% and 11% for forest subcompartments in each 100 m increment area from county roadways and highways, respectively. In contrast, the risk of PWD infection was reduced by only 5% and 3% for the forest subcompartments located at each additional 100 m distance from national and provincial roadways, respectively (Table A1).
3.5.1. Spatial Correlation Characteristics concerning PWD-Infected Forest Subcompartments with Traffic Corridors
The PWD-infected forest subcompartments showed significant spatial correlation characteristics with the traffic corridors in Fushun City. Most of the PWD-infected forest subcompartments were located at intersections between Fushun traffic roads or spread along the road traffic network (Figure 1c). Most PWD-infected forest subcompartments were concentrated in two locations: the junction of the Shenji Expressway and Futong Expressway, which represents a gathering centre from which roads spread in all four directions (Figure 1d), and the junction of two multilevel roads on the northeast side of the Shenji Expressway (Figure 1e). The first PWD-infected forest subcompartment was concentrated within 20 km of the gathering centre, where roads of different types intersect. The PWD-infected forest subcompartments in this area were scattered on both sides of the highways, national roadways and county roadways, accounting for approximately 61% of all PWD-infected subcompartments identified in Fushun City. The PWD forest subcompartments distributed on the sides of the county roads were the densest. In the second gathering centre, the PWD-infected forest subcompartments accounted for 18% of the total number of identified PWD-infected subcompartments. The national roadways at this location are relatively dense, and PWD-infected subcompartments concentrate in the areas between national roadways. Some PWD-infected subcompartments are also located near county roadways.
3.5.2. Characteristics of the PWD-Infected Forest Subcompartments within 3 km Traffic Corridors
The PWD-infected forest subcompartments identified in Fushun City were concentrated within 3 kilometres of traffic corridors and showed a monotonic increasing trend (Figure 5). When considering the distribution distance between the PWD-infected forest subcompartments and the traffic corridors, 7% of the infected subcompartments were distributed near roads, and half of the infected subcompartments were distributed within 1 km of traffic corridors. When the distance between a PWD-infected forest subcompartment and a traffic corridor was greater than 1 km, the growth rate of PWD-infected forest subcompartments slowed significantly. When the infected forest subcompartments were located 3 km away from roads, the proportion of PWD-infected forest subcompartments reached 93%. Between 1 km and 3 km from roads, the proportions of infected subcompartments per kilometre increased by 20% on average. Briefly, the PWD-infected forest subcompartments were located within 1 km, 1–2 km, and 2–3 km ranges of traffic corridors at proportions of 52%, 25%, and 16%, respectively.
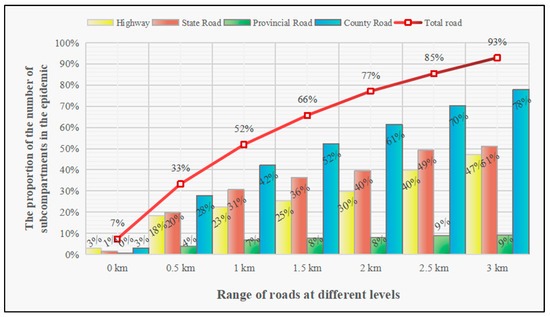
Figure 5.
Relationships characterising the proportions of PWD-infected forest subcompartments at various distances from different traffic corridor types in Fushun City.
We found that, as the areas affected by roads of different levels continuously increased, the number of PWD-infected forest subcompartments showed an upwards trend (Figure 5). The numbers of PWD-infected forest subcompartments falling directly on highways and county roadways were the same. However, as the areas affected by these road types expanded, the number of PWD-infected forest subcompartments within 3 km of the county roadways showed a linear upwards trend. Within the corresponding scope of influence, the change trend of the number of PWD-infected forest subcompartments near highways steadily increased and then rapidly increased. The proportion of PWD-infected forest subcompartments near national roadways showed a fluctuating increase.
3.5.3. The Main Distribution Locations of the PWD-Infected Forest Subcompartments on Low-Level County Roads
PWD was mainly distributed near low-level county roads, while relatively few PWD-infected forest subcompartments were identified near other high-level traffic corridors (Figure 5). PWD-infected forest subcompartments located within 0.5 km of county roads accounted for 28% of the total number of PWD-infected forest subcompartments. Within 0.5–3 km around county roads, the area affected by the county roadways increased by 20% for each 1 km expansion, while the proportion of PWD-infected subcompartments correspondingly increased by 20%. The proportion of PWD-infected subcompartments located within 0.5 km of expressways accounted for 18% of the total number of PWD-infected forest subcompartments, and the proportion of PWD-infected subcompartments between 0.5 and 2 km of expressways showed a steady increase; the proportion of infected subcompartments identified beyond 2 km from expressways increased rapidly by 17%. The proportion of PWD-infected forest subcompartments along national roadways accounted for only 1% of the total number of infected subcompartments. When the area affected by national roadways expanded to 3 km, the PWD-infected forest subcompartments grew to account for 51% of the total infected subcompartments. The number of PWD-infected subcompartments within 1 km of provincial roadways accounted for 7% of the total. As the influence of provincial roadways expanded, the number of PWD-infected forest subcompartments remained basically unchanged.
4. Discussion
In this study, we assessed the influence of various natural and human factors on the spatial patterns of short-distance PWD transmission among forest subcompartments. The following results were obtained: (1) In the short-distance transmission of PWD, the spatial spill-over effects of nearby PWD-infected subcompartments play a dominant role; their contribution to PWD transmission reached 78%, while different types of traffic corridors contributed 20%. (2) The spread of PWD exhibited a significant spatial spill-over effect, and the PWD infection risk in the forest subcompartments showed an approximately linear downwards trend. (3) Different traffic corridor types exhibited significant differences in their influence on PWD transmission; county roadways and highways had larger impacts, while other traffic corridor types had relatively smaller impacts.
These results show that the influence of the spatial spill-over effects of nearby PWD-infected subcompartments was far greater than that of different types of traffic corridors in the short-distance transmission of PWD. The study area was Fushun City, and the distribution of PWD-infected forest subcompartments in this region was found to be relatively concentrated. A large number of suitable host pine trees are located in adjacent forest subcompartments. As a PWD vector, M. alternatus plays an important role in the spread of PWD, and the spatial spill-over effects of nearby PWD-infected subcompartments play a large role in the transmission process overall [13,44]. Previous studies have reported that factors such as population density and traffic density can greatly increase the speed of beetle-transmitted PWD spread [59]. However, when the study scale was small and roads between forest subcompartments were sparse, road transport played a lesser role in the short-distance transmission of PWD. As we studied short-distance PWD transmission in this paper with a limited research scope, we found that the spread of PWD is mainly affected by the spatial spill-over effects of nearby PWD-infected subcompartments.
The flight distance of M. alternatus is limited, so the spatial spill-over effect of PWD-infected forest subcompartments showed linear attenuation with increasing distance. Spatial PWD spill-over can be achieved through the M. alternatus vector and is thus limited by the flying ability of the beetle [60,61]. In flight mill experiments, M. alternatus can fly more than 5 km during their entire life cycle [17]. In a mark–release–recapture experiment performed on mature M. alternatus, most of the beetles were recaptured at distances of 800 m from the release point [13]. Adult M. alternatus can reach flight distances of 1 km, and the recorded maximum transmission distance is 1442 m [62]. The spatial spill-over effect of PWD obtained in this study was basically similar to the flight-transmission ability of M. alternatus. However, we analysed the specific effects of different PWD spatial spill-over distances in more detail in our study compared to past work and provided more meaningful guidance for inhibiting the spread of PWD.
In this study, we also found that the canopy density of forest subcompartments affects the transmission of PWD. When the canopy density of host pine trees was within the 0.6–0.8 range, the forest subcompartments were more likely to be infected with PWD. From the perspective of airflow, low-forest-density stands are more conducive to the volatilisation of pheromones than places with high forest densities [63]. The results of some other studies were consistent with our finding that beetles travel longer in contiguous high-density forests than in low-density forest landscapes [42].
In this study, the slope direction was selected among topographic factors for analysis, but we found no relationship between the slope direction and PWD spread. Complex topographic changes induce climatic conditions and other variables and thus affect the ecological environment [41,64]. Park (2013) and others reported that geographical features such as elevation, slope angle, slope direction, and soil moisture status are very important for the occurrence of PWNs. Previous studies have shown that different slope directions affect the natural environment and thus affect the PWD status. However, this was inconsistent with our research results. Due to the limited scope of the study area analysed herein and the concentrated distribution of PWD-infected subcompartments, the influence of terrain factors on short-distance transmission was limited.
When considering different traffic corridor types, we found that county roadways had the greatest impact on PWD spread, followed by highways, national roadways, and provincial roadways. Previous studies have reported that many alien organisms are often distributed near roads [65]. Thus, the greater the road density is, the better PWNs can spread [44,50]. Road density also plays an important role in the spread of other beetle species [66,67]. In this work, we studied the contribution of traffic corridors by analysing the contributions of different traffic corridor types separately and determining the distances at which different traffic corridor types impact the spread of PWNs through roadways. To limit and prevent the spread of PWD, it is critical that the vehicle management measures applied in wood transportation activities are strengthened along county roads.
This study also had certain shortcomings. Due to the complex distribution of traffic corridors with different types, it was difficult to consider the degree to which the PWD-infected forest subcompartments were affected by all nearby traffic corridors of different types simultaneously. In this paper, by assuming that the PWD-infected forest subcompartments were affected only by the nearest roads of different types, we created an ideal model. The scope of our research comprised only short-distance transmission, and we emphasise only the PWD-transmission differences between the effects of traffic corridors and spatial spill-over. In the future, the influence of climatic factors and other anthropogenic factors on long-distance PWD transmission should also be considered.
5. Conclusions
In this study, the spatial spill-over effects were found to be the most important driver affecting the short-distance transmission of PWD, and the risk of such transmission was negatively correlated with the distance from the PWD-infected forest subcompartments. Traffic corridors at different types are considered to be the main routes for the spread of the PWN. County roads and highways were most conducive to the spread of the PWN. These findings collectively improve the understanding of PWD spread in nature and provide important empirical observational evidence and a theoretical basis that ultimately contribute to the management and conservation practices of minimising PWD spread in northern China and potentially can be extended to other regions in China and the world.
Author Contributions
Conceptualisation, J.H.; data curation, Y.L.; methodology, Y.L. and J.H.; project administration, J.H. and T.Y.; resources, J.H.; writing—original draft, Y.L.; writing—review and editing, Y.L., T.Y. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Key Research and Development Program of China [grant number 2019YFA0606600].
Data Availability Statement
Restrictions apply to the availability of these data. Data was obtained from the Biological Disaster Prevention and Control Center of the State Forestry and Grass-land Administration and are available www.forestpest.cn with the permission of the Biological Disaster Prevention and Control Center of the State Forestry and Grass-land Administration.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A

Table A1.
(a) Separate consideration of the coefficients of different types of traffic corridors (coefficients of highways, national roadway, provincial roadway, and county roadway) in the logistic regression model and t-test statistical results (p < 0.1). (b) Separate consideration of the coefficients of the spatial spill-over effect of the PWD forest subcompartments in the logistic regression model and t-test statistical results (p < 0.1). (c) Separate consideration of the coefficient of canopy density in the logistic regression model and t-test statistical results (p < 0.1).
Table A1.
(a) Separate consideration of the coefficients of different types of traffic corridors (coefficients of highways, national roadway, provincial roadway, and county roadway) in the logistic regression model and t-test statistical results (p < 0.1). (b) Separate consideration of the coefficients of the spatial spill-over effect of the PWD forest subcompartments in the logistic regression model and t-test statistical results (p < 0.1). (c) Separate consideration of the coefficient of canopy density in the logistic regression model and t-test statistical results (p < 0.1).
| (a) | ||
| Variable | B | p |
| highways | −0.011 | <0.001 |
| national roadway | −0.005 | 0.006 |
| provincial roadway | −0.003 | <0.001 |
| county roadway | −0.018 | <0.001 |
| const | 2.257 | <0.001 |
| (b) | ||
| Variable | B | p |
| first-order adjacent PWD subcompartments | 2.516 | <0.001 |
| second-order adjacent PWD subcompartments | 2.247 | <0.001 |
| const | −1.162 | <0.001 |
| (c) | ||
| Variable | B | p |
| canopy density | 0.201 | 0.049 |
| const | −0.074 | 0.241 |
Appendix B

Table A2.
Coefficients of overall driving factors in logistic regression and t-test statistical results (p < 0.1).
Table A2.
Coefficients of overall driving factors in logistic regression and t-test statistical results (p < 0.1).
| Variable | B | p |
|---|---|---|
| highways | −0.012 | <0.001 |
| national roadway | 0 | 0.966 |
| provincial roadway | −0.002 | 0.016 |
| county roadway | −0.01 | 0.001 |
| first-order adjacent PWD subcompartments | 2.285 | <0.001 |
| second-order adjacent PWD subcompartments | 1.843 | <0.001 |
| canopy density | 0.307 | 0.046 |
| const | 0.388 | 0.093 |
Appendix C

Table A3.
Overall accuracy, R-square, and AUC on training and test data in logistic regression.
Table A3.
Overall accuracy, R-square, and AUC on training and test data in logistic regression.
| OA | R2 | AUC | |
|---|---|---|---|
| Training data | 0.86 | 0.62 | 0.94 |
| Test data | 0.84 | 0.56 | 0.93 |
OA: Overall accuracy, the total number of correct classifications in the sample divided by the total number of samples. R2: Coefficient of determination—an important statistic that reflects the goodness of fit of the model is the ratio of the regression sum of squares to the total sum of squares. AUC: Area Under Curve.
References
- Yusuf, A.; Acay, B.; Mustapha, U.T.; Inc, M.; Baleanu, D. Mathematical modeling of pine wilt disease with Caputo fractional operator, Chaos. Solitons Fractals 2021, 143, 110569. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Wang, L.; Piao, C.; Li, C. Current status of pine wilt disease and its control status. J. Environ. Entomol. 2018, 40, 256–267. (In Chinese) [Google Scholar]
- Hao, Z.; Huang, J.; Li, X.; Sun, H.; Fang, G. A multi-point aggregation trend of the outbreak of pine wilt disease in China over the past 20 years. For. Ecol. Manag. 2022, 505, 119890. [Google Scholar] [CrossRef]
- Li, J.; Pan, J.; Liu, C.; Cheng, T.; Peng, Z.; Yan, H. Analysis of the epidemic situation of pine wilt disease in China in 2020. For. Pest Dis. 2021, 40, 45–48. [Google Scholar]
- Zhao, J.; Huang, J.; Yan, J.; Fang, G. Economic loss of pine wood nematode disease in mainland China from 1998 to 2017. Forests 2020, 11, 1042. [Google Scholar] [CrossRef]
- Körner, C. A matter of tree longevity. Science 2017, 355, 130–131. [Google Scholar] [CrossRef]
- Seidl, R.; Klonner, G.; Rammer, W.; Essl, F.; Moreno, A.; Neumann, M.; Dullinger, S. Invasive alien pests threaten the carbon stored in Europe’s forests. Nat. Commun. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Peltzer, D.; Allen, R.; Lovett, G.; Whitehead, D.; Wardle, D. Effects of biological invasions on forest carbon sequestration. Glob. Change Biol. 2010, 16, 732–746. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, C.; Lee, K.-S.; Bolan, N.S.; Naidu, R. Carbon storage and soil CO2 efflux rates at varying degrees of damage from pine wilt disease in red pine stands. Sci. Total Environ. 2013, 465, 273–278. [Google Scholar] [CrossRef]
- Running, S.W. Ecosystem disturbance, carbon, and climate. Science 2008, 321, 652–653. [Google Scholar] [CrossRef]
- Seidl, R.; Schelhaas, M.-J.; Rammer, W.; Verkerk, P.J. Increasing forest disturbances in Europe and their impact on carbon storage. Nat. Clim. Change 2014, 4, 806–810. [Google Scholar] [CrossRef]
- Wu, Y.; Wickham, J.D.; Zhao, L.; Sun, J. CO2 drives the pine wood nematode off its insect vector. Curr. Biol. 2019, 29, R619–R620. [Google Scholar] [CrossRef]
- Kobayashi, F.; Yamane, A.; Ikeda, T. The Japanese pine sawyer beetle as the vector of pine wilt disease. Annu. Rev. Entomol. 1984, 29, 115–135. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, S.; Wei, W.; Hao, H.; Zhang, B.; Butcher, R.A.; Sun, J. Chemical signals synchronize the life cycles of a plant-parasitic nematode and its vector beetle. Curr. Biol. 2013, 23, 2038–2043. [Google Scholar] [CrossRef]
- Fielding, N.; Evans, H. The pine wood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickle (= B. lignicolus Mamiya and Kiyohara): An assessment of the current position. For. Int. J. For. Res. 1996, 69, 35–46. [Google Scholar] [CrossRef]
- Togashi, K. A field experiment on dispersal of newly emerged adults of Monochamus alternatus (Coleoptera: Cerambycidae). Popul. Ecol. 1990, 32, 1–13. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jung, J.K.; Jung, C.; Han, H.; Koh, S.H. Dispersal capacity of Monochamus saltuarius on flight mills. Entomol. Exp. Appl. 2018, 166, 420–427. [Google Scholar] [CrossRef]
- Takasu, F.; Yamamoto, N.; Kawasaki, K.; Togashi, K.; Kishi, Y.; Shigesada, N. Modeling the expansion of an introduced tree disease. Biol. Invasions 2000, 2, 141–150. [Google Scholar] [CrossRef]
- Liebhold, A.M.; Tobin, P.C. Population ecology of insect invasions and their management. Annu. Rev. Entomol. 2008, 53, 387–408. [Google Scholar] [CrossRef]
- Robinet, C.; Van Opstal, N.; Baker, R.; Roques, A. Applying a spread model to identify the entry points from which the pine wood nematode, the vector of pine wilt disease, would spread most rapidly across Europe. Biol. Invasions 2011, 13, 2981–2995. [Google Scholar] [CrossRef]
- Steiner, G.; Buhrer, E.M. Aphelenchoides xylophilus n. sp., a nematode associated with blue-stain and other fungi in timber. J. Agric. Res. 1934, 48, 949–951. [Google Scholar]
- Zhao, L.; Mota, M.; Vieira, P.; Butcher, R.A.; Sun, J. Interspecific communication between pinewood nematode, its insect vector, and associated microbes. Trends Parasitol. 2014, 30, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, J.; Li, X.; Fang, G.; Liu, D. The interaction of environmental factors increases the risk of spatiotemporal transmission of pine wilt disease. Ecol. Indic. 2021, 133, 108394. [Google Scholar] [CrossRef]
- Roques, A.; Zhao, L.; Jianghua, S.; Robinet, C. Pine Wood Nematode, Pine wilt Disease, Vector Beetle and Pine Tree: How a Multiplayer System Could Reply to Climate Change. In Climate Change and Insect Pests; CABI: Wallingford, UK, 2015; pp. 220–234. [Google Scholar]
- Shin, S.-C. Pine wilt disease in Korea. In Pine wilt Disease; Springer: Tokyo, Japan, 2008; pp. 26–32. [Google Scholar]
- An, H.; Lee, S.; Cho, S.J. The effects of climate change on pine wilt disease in South Korea: Challenges and Prospects. Forests 2019, 10, 486. [Google Scholar] [CrossRef]
- Robinet, C.; Roques, A.; Pan, H.; Fang, G.; Ye, J.; Zhang, Y.; Sun, J. Role of human-mediated dispersal in the spread of the pinewood nematode in China. PLoS ONE 2009, 4, e4646. [Google Scholar] [CrossRef] [PubMed]
- Meurisse, N.; Rassati, D.; Hurley, B.P.; Brockerhoff, E.G.; Haack, R.A. Common pathways by which non-native forest insects move internationally and domestically. J. Pest Sci. 2019, 92, 13–27. [Google Scholar] [CrossRef]
- Haynes, K.J.; Allstadt, A.J.; Klimetzek, D. Forest defoliator outbreaks under climate change: Effects on the frequency and severity of outbreaks of five pine insect pests. Glob. Change Biol. 2014, 20, 2004–2018. [Google Scholar] [CrossRef]
- De la Fuente, B.; Saura, S. Long-term projections of the natural expansion of the pine wood nematode in the Iberian Peninsula. Forests 2021, 12, 849. [Google Scholar] [CrossRef]
- Rutherford, T.; Mamiya, Y.; Webster, J. Nematode-induced pine wilt disease: Factors influencing its occurrence and distribution. For. Sci. 1990, 36, 145–155. [Google Scholar]
- Eckerstorfer, M.; Christiansen, H.H. Topographical and meteorological control on snow avalanching in the Longyearbyen area, central Svalbard 2006–2009. Geomorphology 2011, 134, 186–196. [Google Scholar] [CrossRef]
- Firmino, P.N.; Calvão, T.; Ayres, M.P.; Pimentel, C.S. Monochamus galloprovincialis and Bursaphelenchus xylophilus life history in an area severely affected by pine wilt disease: Implications for forest management. For. Ecol. Manag. 2017, 389, 105–115. [Google Scholar] [CrossRef]
- Ikegami, M.; Jenkins, T.A. Estimate global risks of a forest disease under current and future climates using species distribution model and simple thermal model–Pine Wilt disease as a model case. For. Ecol. Manag. 2018, 409, 343–352. [Google Scholar] [CrossRef]
- Koralewski, T.E.; Wang, H.-H.; Grant, W.E.; Brewer, M.J.; Elliott, N.C.; Westbrook, J.K. Modeling the dispersal of wind-borne pests: Sensitivity of infestation forecasts to uncertainty in parameterization of long-distance airborne dispersal. Agric. For. Meteorol. 2021, 301, 108357. [Google Scholar] [CrossRef]
- Mundt, C.C.; Sackett, K.E.; Wallace, L.D. Landscape heterogeneity and disease spread: Experimental approaches with a plant pathogen. Ecol. Appl. 2011, 21, 321–328. [Google Scholar] [CrossRef]
- Osada, Y.; Yamakita, T.; Shoda-Kagaya, E.; Liebhold, A.M.; Yamanaka, T. Disentangling the drivers of invasion spread in a vector-borne tree disease. J. Anim. Ecol. 2018, 87, 1512–1524. [Google Scholar] [CrossRef]
- Futai, K. Pine wood nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef]
- Kaiser, K.E.; McGlynn, B.L.; Emanuel, R.E. Ecohydrology of an outbreak: Mountain pine beetle impacts trees in drier landscape positions first. Ecohydrology 2013, 6, 444–454. [Google Scholar] [CrossRef]
- Togashi, K.; Shigesada, N. Spread of the pinewood nematode vectored by the Japanese pine sawyer: Modeling and analytical approaches. Popul. Ecol. 2006, 48, 271–283. [Google Scholar] [CrossRef]
- Calvao, T.; Duarte, C.M.; Pimentel, C.S. Climate and landscape patterns of pine forest decline after invasion by the pinewood nematode. For. Ecol. Manag. 2019, 433, 43–51. [Google Scholar] [CrossRef]
- Etxebeste, I.; Sanchez-Husillos, E.; Álvarez, G.; Gisbert, H.M.I.; Pajares, J. Dispersal of Monochamus galloprovincialis (Col. : Cerambycidae) as recorded by mark–release–recapture using pheromone traps. J. Appl. Entomol. 2016, 140, 485–499. [Google Scholar]
- Gilbert, M.; Guichard, S.; Freise, J.; Grégoire, J.-C.; Heitland, W.; Straw, N.; Tilbury, C.; Augustin, S. Forecasting Cameraria ohridella invasion dynamics in recently invaded countries: From validation to prediction. J. Appl. Ecol. 2005, 42, 805–813. [Google Scholar] [CrossRef]
- Choi, W.I.; Song, H.J.; Kim, D.S.; Lee, D.-S.; Lee, C.-Y.; Nam, Y.; Kim, J.-B.; Park, Y.-S. Dispersal patterns of pine wilt disease in the early stage of its invasion in South Korea. Forests 2017, 8, 411. [Google Scholar] [CrossRef]
- Cushman, J.H.; Meentemeyer, R.K. Multi-scale patterns of human activity and the incidence of an exotic forest pathogen. J. Ecol. 2008, 96, 766–776. [Google Scholar] [CrossRef]
- Sousa, E.; Naves, P.; Bonifácio, L.; Inácio, L.; Henriques, J.; Evans, H. Survival of Bursaphelenchus xylophilus and Monochamus galloprovincialis in pine branches and wood packaging material. EPPO Bull. 2011, 41, 203–207. [Google Scholar] [CrossRef]
- Kwon, T.-S.; Shin, J.H.; Lim, J.-H.; Kim, Y.-K.; Lee, E.J. Management of pine wilt disease in Korea through preventative silvicultural control. For. Ecol. Manag. 2011, 261, 562–569. [Google Scholar] [CrossRef]
- Floerl, O.; Inglis, G.; Dey, K.; Smith, A. The importance of transport hubs in stepping-stone invasions. J. Appl. Ecol. 2009, 46, 37–45. [Google Scholar] [CrossRef]
- Torres-Vila, L.M.; Zugasti, C.; De-Juan, J.M.; Oliva, M.J.; Montero, C.; Mendiola, F.J.; Conejo, Y.; Sánchez, Á.; Fernández, F.; Ponce, F. Mark-recapture of Monochamus galloprovincialis with semiochemical-baited traps: Population density, attraction distance, flight behaviour and mass trapping efficiency. For. Int. J. For. Res. 2015, 88, 224–236. [Google Scholar] [CrossRef]
- Zhang, Y.; Dian, Y.; Zhou, J.; Peng, S.; Hu, Y.; Hu, L.; Han, Z.; Fang, X.; Cui, H. Characterizing Spatial Patterns of Pine Wood Nematode Outbreaks in Subtropical Zone in China. Remote Sens. 2021, 13, 4682. [Google Scholar] [CrossRef]
- Park, Y.-S.; Chung, Y.-J.; Moon, Y.-S. Hazard ratings of pine forests to a pine wilt disease at two spatial scales (individual trees and stands) using self-organizing map and random forest. Ecol. Inform. 2013, 13, 40–46. [Google Scholar] [CrossRef]
- Rong, G. Status and development suggestions of forest resources in Fushun area. Seed Sci. Technol. 2021, 39, 118–120. (In Chinese) [Google Scholar]
- Du, Y.; Wan, Q.; Liu, H.; Liu, H.; Kapsar, K.; Peng, J. How does urbanization influence PM2.5 concentrations? Perspective of spillover effect of multi-dimensional urbanization impact. J. Clean. Prod. 2019, 220, 974–983. [Google Scholar] [CrossRef]
- Ryss, A.Y.; Kulinich, O.A.; Sutherland, J.R. Pine wilt disease: A short review of worldwide research. For. Sci. Pract. 2011, 13, 132. [Google Scholar] [CrossRef]
- Hopf-Biziks, A.; Schröder, T.; Schütz, S. Long-term survival and non-vector spread of the pinewood nematode, Bursaphelenchus xylophilus, via wood chips. For. Pathol. 2017, 47, e12340. [Google Scholar] [CrossRef]
- Cunningham, M.A.; Johnson, D.H. Proximate and landscape factors influence grassland bird distributions. Ecol. Appl. 2006, 16, 1062–1075. [Google Scholar] [CrossRef]
- Schein, A.I.; Ungar, L.H. Active learning for logistic regression: An evaluation. Mach. Learn. 2007, 68, 235–265. [Google Scholar] [CrossRef]
- Nefeslioglu, H.A.; Gokceoglu, C.; Sonmez, H. An assessment on the use of logistic regression and artificial neural networks with different sampling strategies for the preparation of landslide susceptibility maps. Eng. Geol. 2008, 97, 171–191. [Google Scholar] [CrossRef]
- Bigsby, K.M.; Tobin, P.C.; Sills, E.O. Anthropogenic drivers of gypsy moth spread. Biol. Invasions 2011, 13, 2077–2090. [Google Scholar] [CrossRef]
- Chen, X.; Shao, S.; Tian, Z.; Xie, Z.; Yin, P. Impacts of air pollution and its spatial spillover effect on public health based on China’s big data sample. J. Clean. Prod. 2017, 142, 915–925. [Google Scholar] [CrossRef]
- Miller, T.E.; Tenhumberg, B. Contributions of demography and dispersal parameters to the spatial spread of a stage-structured insect invasion. Ecol. Appl. 2010, 20, 620–633. [Google Scholar] [CrossRef]
- Smith, M.T.; Bancroft, J.; Li, G.; Gao, R.; Teale, S. Dispersal of Anoplophora glabripennis (Cerambycidae). Environ. Entomol. 2001, 30, 1036–1040. [Google Scholar] [CrossRef]
- Choi, W.I.; Park, Y.S. Dispersal patterns of exotic forest pests in South Korea. Insect Sci. 2012, 19, 535–548. [Google Scholar] [CrossRef]
- Mulder, O.; Sleith, R.; Mulder, K.; Coe, N.R. A Bayesian analysis of topographic influences on the presence and severity of beech bark disease. For. Ecol. Manag. 2020, 472, 118198. [Google Scholar] [CrossRef]
- Christen, D.C.; Matlack, G.R. The habitat and conduit functions of roads in the spread of three invasive plant species. Biol. Invasions 2009, 11, 453–465. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Edge type defines alien plant species invasions along Pinus contorta burned, highway and clearcut forest edges. For. Ecol. Manag. 2006, 223, 327–335. [Google Scholar] [CrossRef]
- Maheu-Giroux, M.; de Blois, S. Landscape ecology of Phragmites australis invasion in networks of linear wetlands. Landsc. Ecol. 2007, 22, 285–301. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).