Abstract
To understand the insect resistance mechanism of the larch, Larix olgensis, in a mixed forest, larch (Larix olgensis) seedlings and ashtree (Fraxinus mandshurica) seedlings were planted with mixed banding forests in the proportion of 1:1 (BMF1:1), 3:3 (BMF3:3) and 5:5 (BMF5:5), in pots and in the field. One year later, the content of secondary metabolites in the needles of each larch treatment were tested with an ultraviolet spectrophotometer. The results showed that the allelopathic effect of F. mandshuricas (ashtree) on L. olgensis (larch) could increase the content of secondary metabolites in larch needles. It was found that the flavonoid content in the needles of BMF5:5 was higher than that in the needles of BMF1:1 and BMF3:3 (p < 0.05). The tannin content in the needles of FBMF3:3 and FBMF5:5 was significantly higher than that of FBMF1:1, whereas the tannin content in the needles of PBMF3:3 reached 1.27 mg/g, which was the highest (p < 0.05). The lignin content in the needles of FBMF3:3 reached 2.27 mg/g, which was significantly more increased than that in the control group in a dose-dependent manner, while that in the needles of PBMF3:3 and PBMF5:5 was higher than that in the needles of PBMF1:1 (p < 0.05). The tannin and lignin content in the needles of FBMF was higher than that of PBMF. However, there was no difference in the content of flavonoids in the needles of FBMF and PBMF. These results suggest that banding mixed larches and ashtrees can significantly increase the content of secondary metabolites (phenolic compounds) in the needles of L. olgensis and improve its chemical defense, and the allelopathic effect of ashtrees on larches is related to the mixed proportion. Thus, the effect of mixed banding forests in the proportion of 3:3 and 5:5 is better.
1. Introduction
Plant interaction is one of the fundamental scientific problems in ecological research [1], in which the chemical interactions among and within plants have been widely and deeply studied. Plant allelopathy is a natural ecological phenomenon, in which a plant releases chemicals to affect another plant. It is a chemical response strategy of a plant to the same or different plants that coexist with it [2]. In the face of animal feeding, microbial infection and other plant competition, plants often respond by synthesizing and releasing secondary metabolites. They adjust their biomass distribution by identifying the information of adjacent species, thus deciding whether to adopt chemical defense strategies or not [3,4]. Secondary metabolites result from the interaction between plants and their living environment during long-term evolution. They are products of complex branching metabolic pathways, which determine the color, smell and taste of plants [5]. Although many secondary metabolites do not participate in the metabolism of plants, they can suppress the digestion and utilization of food by herbivorous insects and then interfere with their mating behavior, attracting natural enemies. As an important physiological indicator of performance, they play an essential role in the process of plants resisting insect invasion [6,7].
As the content of secondary metabolites in plant tissue cell walls increases, the number of nutrients, such as proteins and sugars, obtained by phytophagous insects decreases [8,9]. Phenolic compounds in plant secondary metabolites are essential chemical defense substances in plant insect resistance [10], and there is a close relationship between the composition and the content of such phenolic compounds, which are a class of substances with complex genes synthesized by the shikimate acid pathway and the malonate acid pathway, including flavonoids, tannins, lignin and other substances [11,12]. Tannin is a highly polymerized polyphenol compound in polyphenols that is usually divided into condensed tannins and hydrolyzable tannins. It can combine with proteins and digestive enzymes to form a complex compound insoluble in water that interferes with insect food utilization [13,14]. Liu XX et al. [15] added different mass fractions of tannins to an artificial diet to feed Hyphantria cunea larvae and found that tannins had a significant inhibitory effect on the food utilization of Hyphantria cunea. Flavonoids are also important phenolic compounds in the process of plant insect resistance. They exist in plants through the shikimic acid–phenylpropane metabolic pathway. They not only have antioxidant activity, but also increase the metabolic burden of insects and affect the normal life activities of insects, e.g., soybean, which can synthesize flavonoids to inhibit the feeding harm of lepidopteran larvae [16]. Lignin is a complex phenolic polymer filled in the cellulose framework and formed of three alcohol monomers. Its metabolic pathways intersect with those of other plant secondary metabolites. Its metabolism is closely related to plant disease resistance, insect resistance, waterlogging resistance and cold resistance, and other forms of stress resistance physiology have certain correlations [17]. After being ingested by insects, lignin can reduce the efficiency of insects’ food utilization [18].
At present, pure artificial forests with a single tree species in forestry production systems are considered to be typical representatives of simplified ecosystems, which are usually sensitive, easily disturbed, and even have outbreaks of insect pests. Therefore, increasing the diversity of tree species can improve the resistance of trees to pests [19]. In mixed forests, the interactions among the same or different trees affect the function of the forest ecosystem through competition, predation, parasitism and mutualism [20]. According to research, Ostryopsis davidiana can promote the growth of Pinus tabulaeformis [21]. The content of phenolic acid in the rhizosphere soil of a mixed forest of P. tabulaeformis and O. davidiana is significantly lower than that of pure forest P. tabulaeformis, which reduces the autotoxic effect caused by phenolic acid content that is too high in the soil of a pure forest of P. tabulaeformis. The author’s previous research found the 30-year or 20-year growth of L. olgensis (larch)-F. mandshurica (ashtree) banding mixed forests can significantly enhance the activity of the defense proteins in larch needles, thus improving the resistance of larch to phytophagous insects and their chemical defense [22]. In this study, the allelopathy of L. olgensis (larch)-F. mandshurica (ashtree) banding mixed forests on larch young trees was studied by measuring the content of the primary secondary metabolites (tannin, lignin and flavonoids) in larch needles, and the different allelopathy of banding mixed ashtree on larch young trees was compared by the pot experiment and the field experiment, which illuminated the allelopathic mechanism of the mixed banding forest in improving the insect resistance of larches. The results lay a theoretical foundation for applying forest management measures to control forest defoliators, such as Dendrolimus superans.
2. Materials and Methods
2.1. Mixed Mode Setting of L. olgensis and F. mandshurica
In mid-April 2015, two-year-old larch seedlings and one-year-old ashtree seedlings were planted in pots (23 × 23 × 25 cm) and in the field at the Maoershan experimental forestry farm of Northeast Forestry University, Shangzhi, Heilongjiang Province, P.R. China. Larch and ashtree seedlings were planted with mixed banding forests in the proportion of 1:1, 3:3 and 5:5, in pots and in the field, and then larch pure forest was used as the control (Table 1). The distance between one mixed mode and the other was 4 m, and the distance between the two lines of seedlings was 25 cm (Figure 1). The seedlings were covered with gauze to avoid the occurrence of diseases, insect pests and human-caused mechanical damage.

Table 1.
Two planting modes and different banding mixed proportions (four samples each, n = 4).

Figure 1.
Larch and ashtree young trees planted with mixed banding forests in the field and in pots.
2.2. Collection of Larch Needles
Larch needles were collected on 22 July, 1 August, 12 August, 22 August and 1 September. There were 4 repetitions for each treatment and about 30 g of needles for each repetition. Then, those needles were stored at −40 °C in a freezer for the sample testing.
2.3. Determination of Secondary Metabolites Content
2.3.1. Determination of Tannin Content
Tannin in needles was extracted and identified according to the method described by Yan S.C. et al. [23]. Then, 5 g of frozen needles of each treatment were frozen to a constant weight by a freeze dryer. Next, 1 g of dry needles were homogenized using a mortar and pestle, and then the powder was placed into a 20 mL screw-cap centrifuge tube with 10 mL of 95% ethanol and extracted for 24 h at −20 °C. The mixture was centrifuged for 10 min at 10,000 rpm and at 4 °C. Then, 1 mL of supernatant was added to a test tube containing 9 mL of 70% ethanol for the mixture solution, 0.5 mL mixture solution was added to an aluminum foil-covered test tube with 3 mL 4% vanillin of ethanol and 1.5 mL concentrated hydrochloric acid, and the tubes were heated at 20 °C for 20 min in the water bath. The absorbance was measured at 510 nm, and 70% ethanol was the blank control.
2.3.2. Determination of Flavonoid Content
The extraction and identification of flavonoid were performed according to the method described by Jiang et al. [24], with modifications. Needles were extracted with 50 mL of 95% aqueous methanol on a shaker for 24 h and then extracted by ultrasonic extraction for 2 h. The extracted liquid was centrifuged for 15 min at 14,000 rpm. Next, 1 mL of supernatant was added to a test tube containing 4 mL of water, and then 0.5 mL of 50% NaNO2 was added to the test tube. After setting for 6 min, 0.5 mL of 10% AlCl3 was added to the tube. The mixture in the tube was set for 6 min again, and 4 mL of 4% NaOH was added to the tube. The absorbance was measured at 510 nm, and 95% aqueous was the blank control. The flavonoid content was expressed with milligrams of rutin equivalents per gram of fresh leaf weight.
2.3.3. Determination of Lignin Content
Lignin in the needles was extracted and identified according to the method described by Ren et al. [25,26]. First, 0.5 g of needles in 2 mL of 95% ethanol were homogenized using a mortar and pestle and then were centrifuged for 10 min at 4500 rpm. The residues were washed by successive stirring and centrifugation—five times with 95% methanol and three times with 1:2 mixtures of ethanol and n-hexane (v/v)—and then were dried in an oven at 60 °C overnight. All dry residues were placed into a 10 mL screw-cap centrifuge tube with 1 mL of glacial acetic acid containing 25% acetyl bromide. While the tubes were heated at 70 °C for 30 min in the water bath, the reaction was immediately stopped by adding 0.9 mL of 2 M NaOH solution, and then 5 mL of glacial acetic acid and 0.1 mL 7.5 M hydroxylammonium chloride were added. The mixture was centrifuged for 5 min at 4500 rpm, and the absorption of the supernatant after dilution with glacial acetic acid was determined at 280 nm, and 95% ethanol was the blank control. The lignin content was expressed as OD/g (optical density, OD) at the weight of the fresh needles.
2.4. Statistical Analysis
All the data were analyzed with SPSS 19.0 for Windows. The contents data of secondary metabolites in the pot and field treatments were analyzed by one-way analysis of variance (ANOVA), followed by least significant difference (Bonferroni), multiple comparisons at α = 0.05. Two-tailed t-tests of independent samples were used for comparing the contents data of tannin, flavonoid and lignin in the same mixed proportion treatments in planting pots and the field, whose data were log-transformed to achieve variance homogeneity and normal distribution.
3. Results
3.1. Effect of the Banding Mixed Forest in the Field on Content of Secondary Metabolites in Needles of L. olgensis
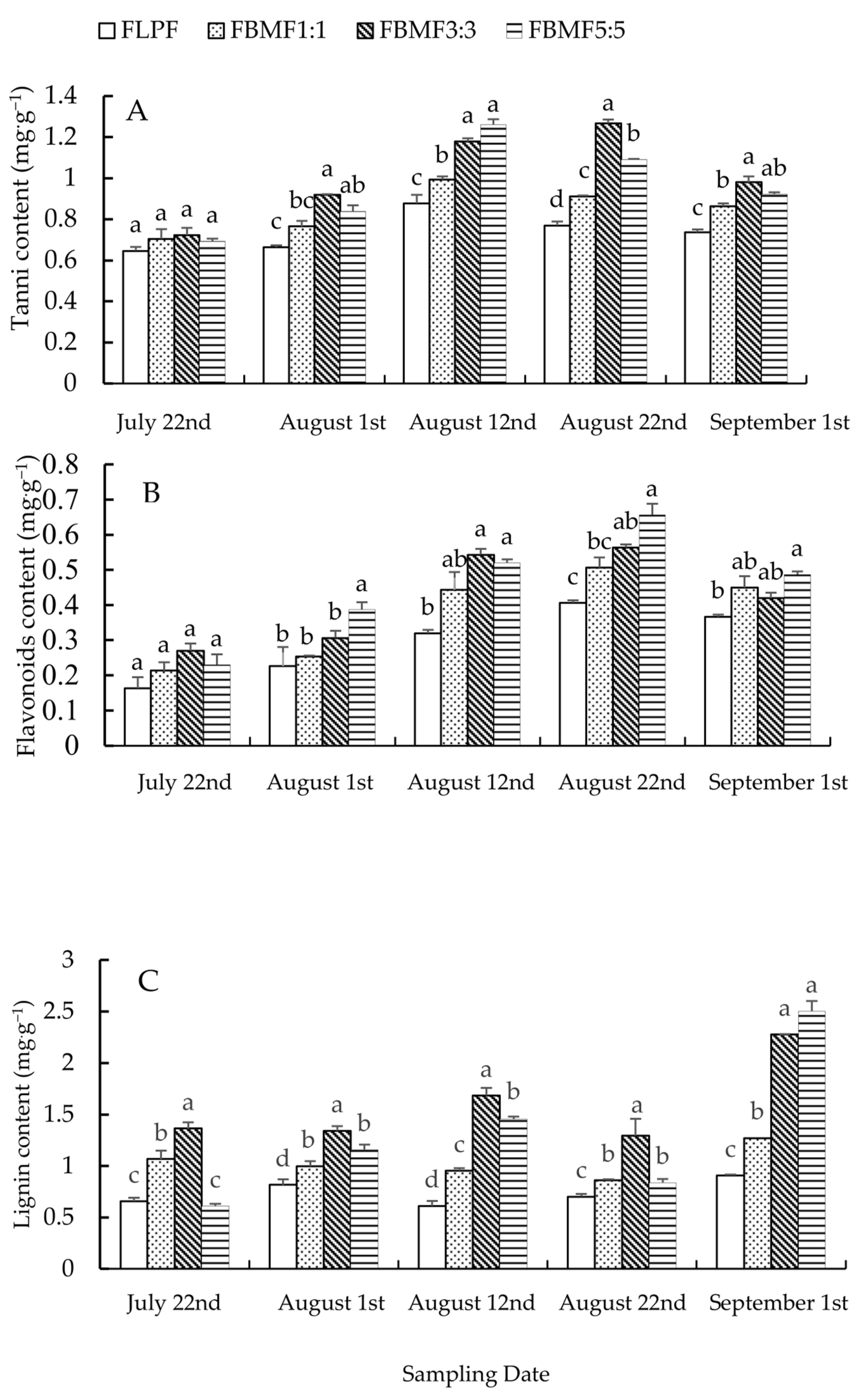
Except for 22 July (F = 0.97, df1 = 3, df2 = 8, p > 0.05), the tannin content of needles in FBMF was significantly higher than that in FLPF. On 1 August and 12 August the tannin content in needles of FBMF3:3 was significantly higher than that of FBMF1:1 (F = 27.10, df1 = 3, df2 = 8, p < 0.05 on 1 August; F = 39.73, df1 = 3, df2 = 8, p < 0.05 on 12 August), but there was no difference between FBMF3:3 and FBMF5:5. On 22 August, the tannin content in needles of FBMF3:3 was significantly higher than that of FBMF1:1 and FBMF5:5, while that of FBMF5:5 was significantly higher than that of FBMF1:1 (F = 243.26, df1 = 3, df2 = 8, p < 0.05). On 1 September, the tannin content in needles of FBMF3:3 was significantly higher than that of FBMF1:1 (F = 33.06, df1 = 3, df2 = 8, p < 0.05), but that of FBMF5:5 was not different from FBMF3:3 and FBMF1:1 (Figure 2A). The results indicated that the allelopathic effect of ashtrees on larches in FBMF could increase the tannin content in needles, and then the tannin content in needles of FBMF3:3 and FBMF5:5 significantly increased.
Figure 2.
Changes of contents of tannin, flavonoids and lignin in needles of FBMF. (A) Tannin content, (B) Flavonoids content, (C) Lignin content. The values presented in the figure are means ± standard deviation (n = 3). Different lowercase letters in the same age histogram mean there is a significant difference between different treatments at the same time in the same mixed forest (ANOVA followed by Bonferroni multiple comparisons, p < 0.05). The same holds for Figure 2 and Figure 3 below.
The flavonoids content of needles in FBMF was higher than that in FLPF. On 1 August, the flavonoids content of needles in FBMF5:5 was significantly higher than that in FBMF1:1, FBMF3:3 and FLPF (F = 4.76, df1 = 3, df2 = 8, p < 0.05), but there was no difference in FBMF1:1, FBMF3:3 and FLPF. On 12 August and 22 August, the flavonoids content of needles in FBMF5:5 and FBMF3:3 was significantly higher than that in FLPF (F = 13.40, df1 = 3, df2 = 8, p < 0.05 on 12 August; F = 22.16, df1 = 3, df2 = 8, p < 0.05 on 22 August), while that in FBMF5:5 was significantly higher than that in FBMF1:1 on 22 August, but there was no difference between that in FBMF3:3 and FBMF1:1. On 1 September, the flavonoids content of needles in FBMF5:5 was significantly higher than that in and FLPF (F = 7.41, df1 = 3, df2 = 8, p < 0.05), but there was no difference between that in FBMF5:5 and FBMF1:1 or FBMF3:3 (Figure 2B). The results showed that ashtree promoted the flavonoids compound in needles, and the flavonoids content of needles in FBMF5:5 was higher than that in FBMF1:1 and FBMF3:3.
Except for 22 July and 1 August, the lignin content in needles treated with FBMF was significantly higher than that of needles treated with FLPF (p < 0.05). On 22 July, the lignin content in needles of FBMF1:1 and FBMF3:3 was significantly higher than that of FLPF (F = 32.63, df1 = 3, df2 = 8, p < 0.05), but there was no difference between them. On 1 August and 12 August, the lignin content in needles of larch–ashtree FBMF was, from high to low, FBMF3:3 > FBMF5:5 > FBMF1:1 (F = 6.84, df1 = 3, df2 = 8, p < 0.05 on 1 August; F = 11.63, df1 = 3, df2 = 8, p < 0.05 on 12 August). On 22 August, the lignin content of needles in FBMF3:3 was significantly higher than that in FBMF1:1 and FBMF5:5 (F = 31.60, df1 = 3, df2 = 8, p < 0.05), but there was no difference between that in FBMF1:1 and FBMF5:5. On 1 September, the lignin content in needles of FBMF3:3 and FBMF5:5 was significantly higher than that of FBMF1:1 (F = 87.68, df1 = 3, df2 = 8, p < 0.05), but there was no significant difference between FBMF3:3 and FBMF5:5 (Figure 2C). These results indicated that ashtree in FBMF had a significant effect on the lignin content of needles, and then the lignin content in the needles of FBMF3:3 was higher than that of other treatments.
3.2. Effects of the Banding Mixed Forest in Pots on Content of Secondary Metabolites in Needles of L. olgensis
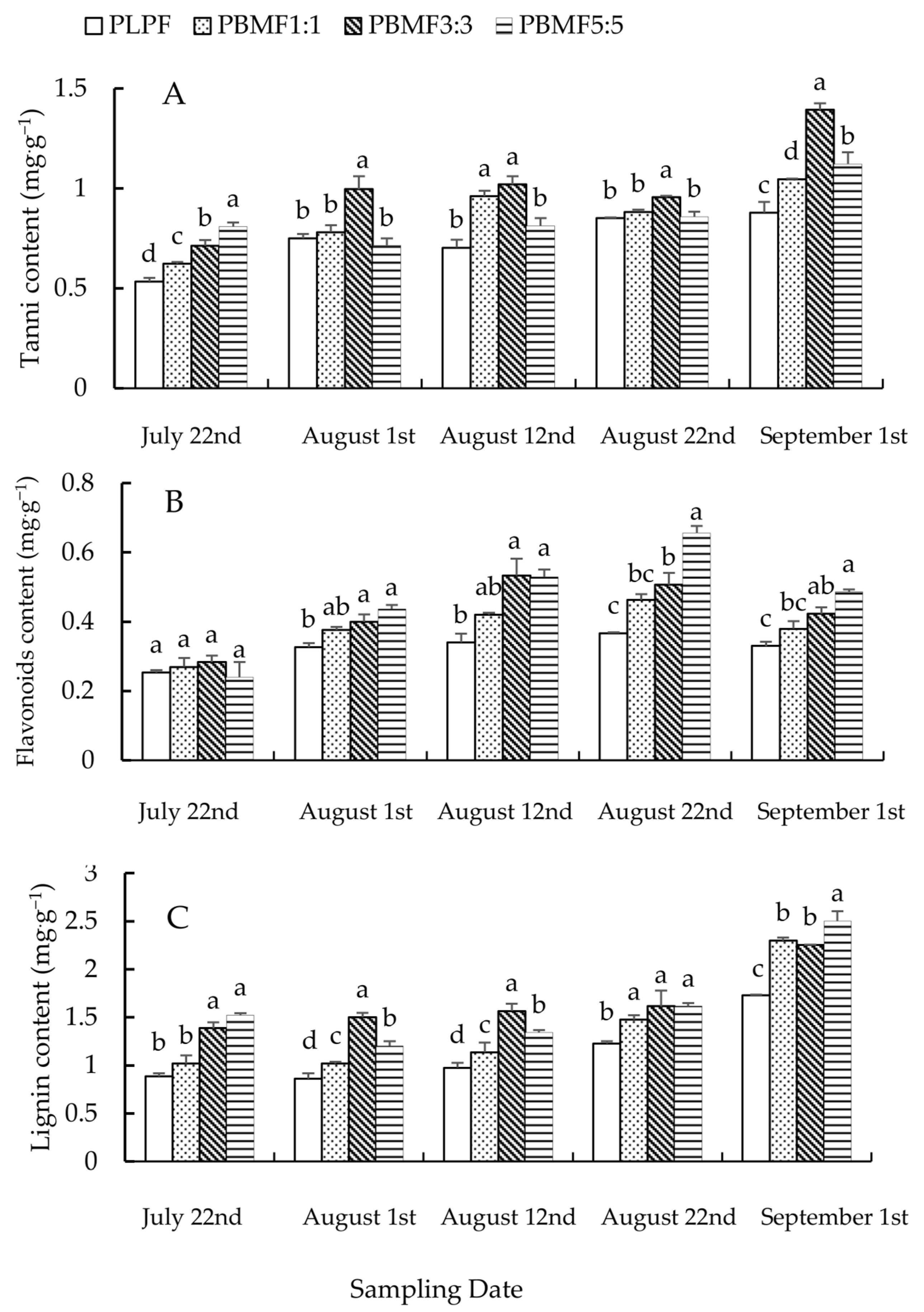
The tannin content of needles in PBMF3:3 was always significantly higher than that in PLPF, and the tannin content of needles in PBMF5:5 was significantly higher than that in PLPF on 22 July and 1 September. On 22 July, the tannin content in needles of PBMF was, from high to low, PBMF5:5 > PBMF3:3 > PBMF1:1 (F = 32.38, df1 = 3, df2 = 8, p < 0.05). On 1 August, 22 August and 1 September, the tannin content of needles in PBMF3:3 was significantly higher than in PBMF1:1 and PBMF5:5 (F = 9.13, df1 = 3, df2 = 8, p < 0.05 on 1 August; F = 11.52, df1 = 3, df2 = 8, p < 0.05 on 22 August; F = 25.50, df1 = 3, df2 = 8, p < 0.05 on 1 September), but there was no difference in PBMF1:1 and PBMF5:5 on 1 August and 22 August. On 12 August, the tannin content in needles of PBMF1:1 and PBMF3:3 was significantly higher than that of PBMF5:5 (F = 15.11, df1 = 3, df2 = 8, p < 0.05), but there was no difference between them (Figure 3A). These results showed that the allelopathy of ashtree in PBMF could increase the content of tannin in larch needles, and the tannin content in the needles of PBMF3:3 was higher than that of PBMF1:1 and PBMF5:5.
Figure 3.
Changes of contents of tannin, flavonoids and lignin in needles of PBMF. (A) Tannin content, (B) Flavonoids content, (C) Lignin content.
Except for 22 July, the flavonoids content in needles treated with PBMF3:3 and PBMF5:5 was significantly higher than that of needles treated with PLPF (p < 0.05). On 1 August and 12 August, the content of flavonoids in needles of PBMF5:5 was significantly higher than that of PBMF1:1 (F = 10.61, df1 = 3, df2 = 8, p < 0.05 on 1 August; F = 9.95, df1 = 3, df2 = 8, p < 0.05 on 12 August), and there was no difference between that of PBMF3:3 and PBMF1:1. On 22 August, the content of flavonoids in needles of PBMF5:5 was significantly higher than that of PBMF1:1 and PBMF3:3, but there was no difference between that of PBMF1:1 and PBMF3:3 (F = 30.49, df1 = 3, df2 = 8, p > 0.05). On 1 September, the content of flavonoids in needles of PBMF5:5 was significantly higher than that of PBMF1:1 (F = 11.79, df1 = 3, df2 = 8, p < 0.05), but there was no difference between that of PBMF3:3 and PBMF1:1 or PBMF5:5. (Figure 3B). These results showed that the allelopathy of ashtree in PBMF could increase the content of flavonoids in needles, and the content of flavonoids in the needles of PBMF5:5 was higher than that of PBMF1:1 and PBMF3:3, which is consistent with the results of the field experiment.
Except for 22 July, the lignin content in needles treated with PBMF was significantly higher than that of needles treated with PLPF (p < 0.05). On 22 July, the lignin content of needles in PBMF3:3 and PBMF5:5 were significantly higher than that of PLPF and PBMF1:1(F = 33.87, df1 = 3, df2 = 8, p < 0.05), but there was no difference between them. On 1 August and 12 August, the lignin content in needles treated with PBMF was, from high to low, PBMF3:3 > PBMF5:5 > PBMF1:1. On 1 September, the lignin content in needles of PBMF5:5 was significantly higher than that of PBMF1:1 and PBMF3:3. However, there was no difference between that of PBMF1:1 and PBMF3:3 (F = 4.69, df1 = 3, df2 = 8, p > 0.05) (Figure 3C). These results showed that the allelopathy of ashtree in needles of PBMF could increase the lignin content, and the lignin content in needles of PBMF3:3 and PBMF5:5 was significantly higher than that of PBMF1:1 (p < 0.05).
3.3. Effects of Two Planting Methods on Content of Secondary Metabolites in Needles of L. olgensis
The difference of the tannin content in needles was irregular in FBMF and PBMF. On 12 August, the tannin content in needles of FBMF1:1 was significantly lower than that of PBMF1:1 (t = −5.89, df = 4, p < 0.01), and there was no difference between them at other times. On 22 July and 1 September, the tannin content in needles of FBMF3:3 was significantly lower than that of PBMF3:3 (t = −3.07, df = 4, p < 0.05 on 22 July; t = −4.90, df = 4, p < 0.05 on 1 September), and on 22 August, the tannin content in needles of FBMF3:3 was significantly higher than that of PBMF3:3(t = 4.95, df = 4, p < 0.05). On 1 August, 12 and 22, the tannin content in needles of FBMF5:5 was significantly higher than that of PBMF5:5 (t = 3.55, df = 4, p < 0.05 on 1 August; t = 4.5, df = 4, p < 0.05 on 12 August; t = 6.33, df = 2.02, p < 0.05 on 22 August), while the tannin content in needles of FBMF5:5 was significantly lower than that of PBMF5:5 on 22 July (t = −10.78, df=4, p < 0.01). These results showed that the allelopathy of ashtree in needles of FBMF5:5 was more beneficial to improving the tannin content than that of PBMF5:5 (Table 2).

Table 2.
Comparative analysis of content of secondary metabolites in larch needles by two planting modes.
The content of flavonoids in needles of FBMF3:3 was significantly higher than that of PBMF3:3 on 22 July (t = 3.71, df = 4, p < 0.05); the content of flavonoids in needles of FBMF5:5 was significantly higher than that of PBMF5:5 on 1 August (t = 3.61, df = 4, p < 0.05); and the flavonoids content of needles in PBMF5:5 was significantly higher than that of FBMF5:5 on 1 September (t = −4.85, df = 4, p < 0.05). Except for those results, there was no difference between FBMF and PBMF at other times or treatments. (Table 2). These results showed that the effects of the allelopathy of ashtree on needles in FBMF and PBMF were similar for improving the content of flavonoids.
The lignin content of needles in FBMF was higher than that in PBMF. Except for that result, the lignin content in needles of FBMF5:5 was significantly lower than that of PBMF5:5 on 22 July (t = −9.675, df = 4, p < 0.01), while the lignin content in needles of FBMF1:1 (t = 5.76, df = 4, p < 0.01) and FBMF3:3(t=6.25, df=4, p < 0.01) on 22 July; that of FBMF1:1 (t = 6.93, df = 4, p < 0.01), FBMF3:3 (t=13.96, df = 4, p < 0.01) and FBMF5:5 (t = 17.49, df = 4, p < 0.01) on 12 August; and that of FBMF3:3 (t = 20.96, df = 4, p < 0.01) and FBMF5:5 (t = 16.04, df = 4, p < 0.01) on 1 September were all significantly higher than that of PBMF (Table 2). These results showed that compared with PBMF, FBMF was more beneficial in increasing the lignin content in larch needles.
4. Discussion
Plant allelopathy is a way of transmitting information between plants. It is also a natural chemical regulation phenomenon in the ecosystem and an ecological mechanism for plants to adapt to the environment [27]. Plants can sense and recognize the information substances that coexist within the same species or alien plants. Different plants regulate plant growth and development through different types of allelochemicals; change plants’ physiological, biochemical and compositional levels; and then improve their adaptability [28]. When Mallotus japonicus grows with its related species, the nectar secretion will be reduced, and their own chemical defense capabilities will be changed [29]. Among secondary metabolites, flavonoids and lignin are important phenylpropanoid pathway metabolites that can increase the metabolic burden of herbivores and inhibit their food consumption, digestibility and assimilation rates [16] (Matthias and Danie, 2020). Our study results showed that the allelopathy of ashtree can increase the content of tannin, flavonoids and lignin in needles, whether planting occurred in pots or the field. The reason may be due to the induction of some chemical substances released by the ashtree volatilization, rain and fog leaching, plant residue decomposition, root exudation and other substances infiltrating the soil to reach the larch [30]. The results are also consistent with the research results, in which the growth of larch–ashtree banded mixed forest for twenty and thirty years significantly enhanced larch’s resistance to insect pests [22]. This shows that a mixed forest of two-year-old larch–ashtree can promote a level of secondary metabolism in larches and the synthesis of phenols to enhance larch’s resistance to phytophagous insects and improve its chemical defenses.
The stable stand structure of mixed plantations gives full play to forest ecological function and benefit, compared with monocultural L. olgensis plantations with large-scale and successive planting [31]. It has many obvious advantages in enhancing system stability, resisting diseases and pests and increasing biological diversity. At the same time, increasing the richness of stand species can improve the trees’ resistance to pests [32]. In a broad-leaved forest, the overall damage of forest pests to broad-leaved tree species is significantly reduced with an increased number of tree species [33]. Cao B [34] used 3-year-old Ailanthus altissima and Populus bolleana to establish a mixed forest for controlling Anoplophora glabripennis. The results showed that the insect resistance of P. bolleana was better when P. bolleana and A. altissima were banding mixed in a ratio of 3:2 and 2:3. Our study showed that BMF3:3 and BMF5:5 of larch–ashtree had the most obvious effect on improving the content of secondary metabolites in needles, which was consistent with the previous study, which demonstrated that the content of secondary metabolites in the needles of 20-year-old larch–ashtree mixed by strips of 4:4 was significantly stronger than that in needles of 20-year-old larch–ashtree mixed by strips of 2:10 [22]. This shows that the allelopathy intensity of F. mandshurica on larch is different in different mixed modes. There are differences in stand density, population structure, interspecific competition, nutrient absorption, the content of allelochemicals released by ashtrees and the allelochemicals secreted by its roots among the three banding mixed modes of larch–ashtree—BMF1:1, BMF3:3 and BMF5:5—which all affect the synthesis of chemical defense substances in the needles. Therefore, when constructing larch–ashtree banding mixed forest, three or five rows of banded mixed forest are more conducive to improving the chemical defense ability of the trees.
Allelopathy among plants is mainly concentrated in the aboveground and underground parts [35]. Plants release specific secondary metabolites (allelochemicals) to the environment through aboveground parts’ (stems, leaves, flowers, fruits or seeds) volatilization, leaching and root exudation, which directly or indirectly affect the growth and development of neighboring plants, allowing neighboring plants to adjust their biomass allocation in order to determine whether to employ strategies such as chemical defense [36,37]. The present study showed that compared with the pot experiment, larches in the field experiment were more conducive to increasing their content of tannin and lignin. This shows that the synergistic chemical action of the aboveground volatiles and underground compounds of F. mandshurica is more conducive to enhancing the synthesis of insect-resistant secondary metabolites in needles. However, there is no significant difference between the effects of the two planting methods on the content of flavonoids in needles. It may be that some allelochemicals secreted by the roots of F. mandshurica in the ground-planting method have an antagonistic effect on the synthesis of secondary metabolites of flavonoids [38,39], which leads to the fact that the allelopathic effect of F. mandshurica in the field experiment on flavonoids is not stronger than that of F. mandshurica in the pot experiment. The mechanism of this phenomenon and the allelochemicals involved in enhancing the chemical defense of larch should be further studied.
With the improvement of people’s awareness of ecological and environmental protection, pest control has developed from comprehensive control measures based on chemical control to natural control based on forestry measures. The establishment of a mixed forest is an essential means of forest pest control and a measure of ecological pest control, too. Furthermore, allelopathy is an ecological factor that cannot be ignored in diverse forest ecosystems. It not only exists universally, but also greatly impacts the structural layout, function, benefit and development of forest communities [34,40]. Therefore, we should fully use the current healthy forest ecosystem and combine the underground and aboveground chemical links and their ecological synergy to explore the corresponding mechanisms and further construct natural chemical regulation in the future [21,32].
5. Conclusions
In summary, banding mixed larch and ashtree can significantly increase the content of secondary metabolites (phenolic compounds) in L. olgensis and improve its chemical defense. FBMF treatments showed more significant effects on the tannin and lignin content in needles than PBMF treatments. However, the allelopathic intensity of F. mandshurica on larches is different in different mixed modes, and then the effect of banding mixed forests by the proportion of 3:3 and 5:5 is better. These findings lay a theoretical foundation for applying forest management measures to control forest defoliators, such as Dendrolimus superans. Yet, the underground and aboveground chemical links and their ecological synergy mechanism for larch–ashtree banding mixed forests are still not clear, which needs further research.
Author Contributions
H.J. and Z.M. designed the study, performed the experiments and participated in developing, drafting and finalizing the manuscript. S.Y. formulated the overarching research goals, commentary and revision of the manuscript. S.Z. and D.J. helped analyze the data. P.L. helped analyze the data, finalized the manuscript and provided helpful advice on the revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Natural Science Foundation of Heilongjiang Province of China (LH2021C086) and the Special Fund Project for Basic Scientific Research Business Expenses of Central Universities (DL13BAX31).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data supporting the conclusions of this study are included in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kong, C. Inter-specific and intra-specific chemical interactions among plants. Chin. J. Appl. Ecol. 2020, 31, 2141–2150. [Google Scholar]
- Li, Y.; Xia, Z.; Kong, C. Allelobiosis in the interference of allelopathic wheat with weeds. Pest Manag. Sci. 2016, 72, 2146–2153. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Lou, Y.G. Research progresses in chemical interactions between plants and phytophagous Insects. Chin. J. Appl. Ecol. 2020, 31, 2151–2160. [Google Scholar]
- Wu, J.S. The “chemical defense”of plants against pathogenic microbes: Phytoa-lexins biosynthesis and molecular regulations. Chin. J. Appl. Ecol. 2020, 31, 2161–2167. [Google Scholar]
- Zhang, W.H.; Liu, G.J. A review on plant secondary substances in plant resistance to insect pests. Chin. Bull. Bot. 2003, 20, 522–530. [Google Scholar]
- Jiang, D.; Yan, S. Effects of Cd, Zn or Pb stress in Populus alba berolinensis on the development and reproduction of Lymantria dispar. Ecotoxicology 2017, 26, 1305–1313. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Q.; He, D. Temporal changes of phenolicacids in Phellodendron amurense Rupr. Leaves and its resistance to insects. J. Northeast. For. Univ. 2014, 42, 126–130. [Google Scholar]
- Jiang, D.; Wang, Y.Y.; Yan, S.C. Effects of Zn stress on growth development and chemical defense of Populus alba’berolinensis’ seedlings. J. Beijing For. Univ. 2018, 40, 42–48. [Google Scholar]
- Guo, Y.; Zhang, P.; Guo, M. Secondary metabolites and plant defence against pathogenic disease. Plant Physiol. Jounal. 2012, 48, 429–434. [Google Scholar]
- Weston, L. Mechanisms for cellular transport and release of allelochemicals from plant roots into the rhizosphere. J. Exp. Bot. 2012, 63, 3445–3454. [Google Scholar] [CrossRef]
- Li, M.; Zeng, R.; Luo, S. Secondary metabolites related with plant resistance against pathogenic microorganisms and insect pests. Chin. J. Biol. Control. 2007, 23, 269–273. [Google Scholar]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Shi, X.P.; Chen, Y.P.; Yan, Z.Q. Research progress on plant allelopathy. Biotechnol. Bull. 2020, 36, 215–222. [Google Scholar]
- Yuan, H.; Yan, S.; Tong, L. Content differences of condensed tannin in needles of Larix gmelinii by cutting needles and insect feeding. Acta Ecol. Sin. 2009, 29, 1415–1420. [Google Scholar]
- Liu, X.X.; Jiang, D.; Meng, Z.J. Effects of secondary substances on food Utilization by Hyphantria cunea larvae. J. Northeast. For. Univ. 2020, 48, 99–103. [Google Scholar]
- Dučaiová, Z.; Sajko, M.; Mihaličová, S.; Repčák, M. Dynamics of accumulation of coumarin-related compounds in leaves of Matricaria chamomilla after methyl jasmonate elicitation. Plant Growth Regul. 2016, 79, 81–94. [Google Scholar] [CrossRef]
- Shahabinejad, M.; Shojaaddini, M.; Maserti, B. Exogenous application of methyl jasmonate and salicylic acid increases antioxidant activity in the leaves of pistachio (Pistacia vera L. cv. Fandoughi) trees and reduces the performance of the phloem-feeding psyllid Agonoscena pistaciae. Arthropod Plant Interact. 2014, 8, 525–530. [Google Scholar] [CrossRef]
- Mitsuda, N.; Seki, M.; Shinozaki, K.; Ohmetakagi, M. The NAC Transcription Factors NST1 and NST2 of Arabidopsis Regulate Secondary Wall Thickenings and Are Required for Anther Dehiscence. Plant Cell 2005, 17, 2993–3006. [Google Scholar] [CrossRef]
- Klapwijk, M.J.; Björkman, C. Mixed forests to mitigate risk of insect outbreaks. Scand. J. For. Res. 2018, 33, 772–780. [Google Scholar] [CrossRef]
- Jactel, H.; Bauhus, J.; Boberg, J. Tree diversity drives forest stand resistance to natural disturbances. Curr. For. Rep. 2017, 3, 223–243. [Google Scholar] [CrossRef]
- Zhang, Y.J. Allelopathic Effects of Pinus tabulaeformis Carr.Littles Extract on Castanea mollissima Bl. and Quercus variabilis Bl. Seedling Growth. Diss. Beijing For. Univ. 2009.
- Jiang, H.; Yan, S.; Xue, Y. Effects of forest type on activity of several defense proteins and contents of secondary metabolites in Larch needles. For. Res. 2018, 31, 24–28. [Google Scholar]
- Wu, Y.Q.; Guo, Y.Y. Determination of tannin in cotton plant. J. Appl. Ecol. 2000, 11, 243–245. [Google Scholar]
- Jiang, D.; Wang, Y.Y.; Dong, X.W.; Yan, S.C. Inducible defense responses in populus alba berolinensis to pb stress. S. Afr. J. Bot. 2018, 119, 295–300. [Google Scholar] [CrossRef]
- Ren, Q.; Hu, Y.J.; Li, Z.Y. Content variation of lignin and peroxidase activities from damaged Pinus massioniana. ActaEcologicaSinica 2007, 27, 4895–4899. [Google Scholar]
- Fukushima, R.; Hatfield, R. Extraction and isolation of lignin for utilization as a standard to determine lignin concentration using the acetyl bromide spectrophotometric method. J. Agric. Food Chem. 2001, 49, 3133–3139. [Google Scholar] [CrossRef]
- Hisashi, K.; Fukiko, K.; Osamu, O. Involvement of allelopathy in inhibition of understory growth in red pine forests. J. Plant Physiol. 2017, 218, 66–73. [Google Scholar]
- Kong, C. Chemical interactions between plant and othe rorganisms: A potential strategy for pest mana-gement. Sci. Agric. Sin. 2007, 40, 712–720. [Google Scholar]
- Yamawo, A. Relatedness of Neighboring Plants Alters the Expression of Indirect Defense Traits in an Extrafloral Nectary-Bearing Plant. Evol. Biol. 2015, 42, 12–19. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Jiang, Y. Allelochemicals from root exudates and their effects on soil biota. Adv. Earth Sci. 2005, 20, 330–337. [Google Scholar]
- Pretzsch, P.H. Tree species mixing can increase maximum stand density. Can. J. For. Res. 2016, 46, 45–52. [Google Scholar] [CrossRef]
- Chen, B.; During, H.; Anten, N. Detect thy neighbor: Identity recognition at the root level in plants. Plant Sci. 2012, 195, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Guyot, V.; Castagneyrol, B.; Vialatte, A. Tree diversity reduces pest damage in mature forests across Europe. Biol. Lett. 2016, 4, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, X. Effects of mixed forest of Ailanthus altissima and Populus bolleana on host choice of Anoplophora glabripennis. Sci. Silvae Sin. 2006, 42, 56–60. [Google Scholar]
- Zhang, Y.; Chang, S.; Song, Y. Application of plant allelopathy in agro-ecosystems. Chin. Agric. Sci. Bull. 2018, 34, 61–68. [Google Scholar] [CrossRef]
- Semchenko, M.; Saar, S.; Lepik, A. Plant root exudates mediate neighbour recognition and trigger complex behavioural changes. New Phytol. 2014, 204, 631–637. [Google Scholar] [CrossRef]
- Semchenko, M.; John, E.A.; Hutchings, M.J. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytol. 2007, 176, 644–654. [Google Scholar] [CrossRef]
- Santonja, M.; Bousquet-Mélou, A.; Greff, S. Allelopathic effects of volatile organic compounds released from Pinus halepensis needles and roots. Ecol. Evol. 2019, 9, 8201–8213. [Google Scholar] [CrossRef]
- Qian, C.Y.; Tang, F.H.; Li, C.C. Review on allelopathic effect of forest tree. J. Northwest For. Univ. 2019, 34, 79–85. [Google Scholar]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).