Comparative Study of the Phenology of Seven Native Deciduous Tree Species in Two Different Mesoclimatic Areas in the Carpathian Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Methods
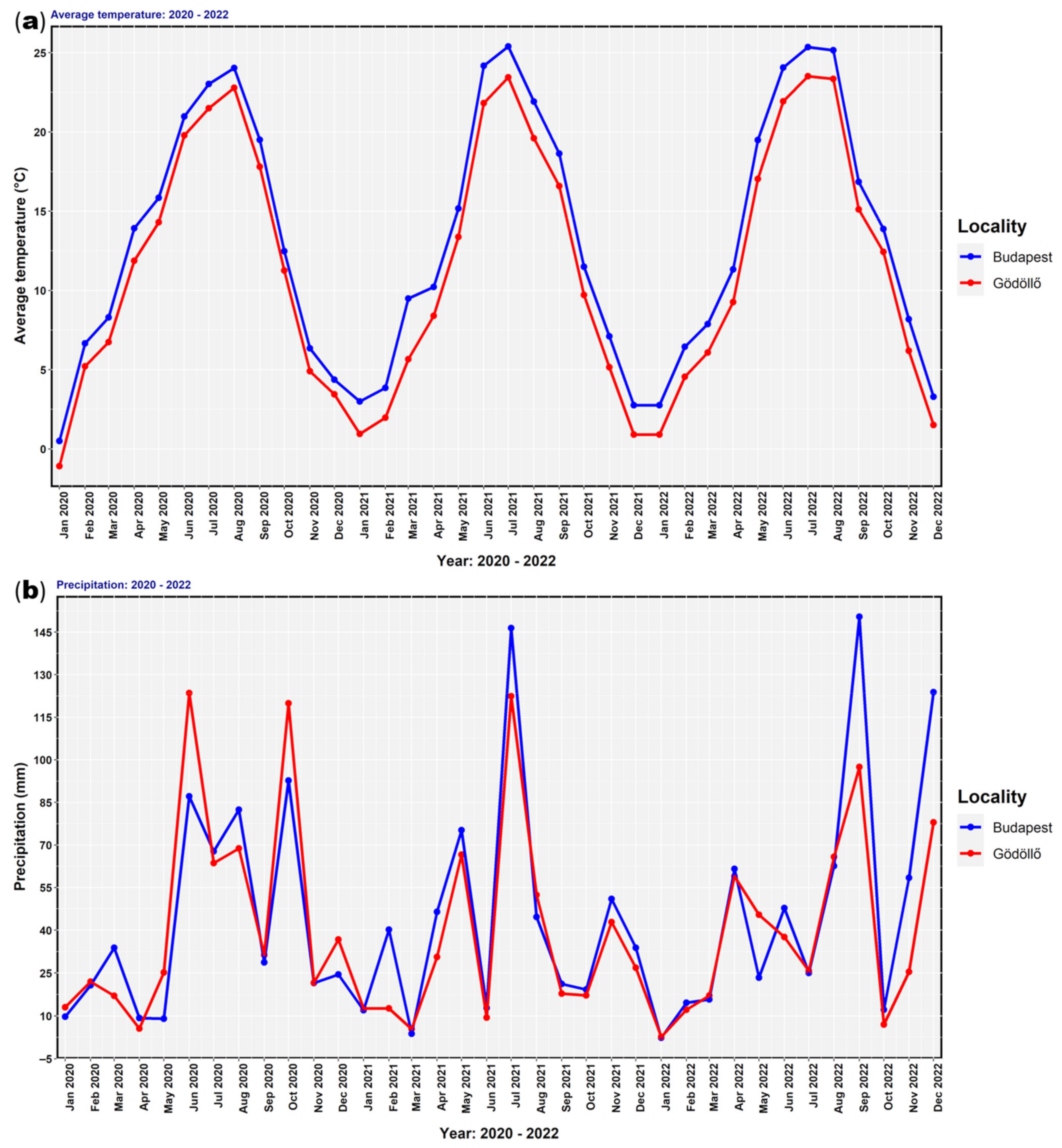
2.3. Climate Data and Phenophase Observation Measurements of Biotic and Abiotic Data (Environmental Parameters)
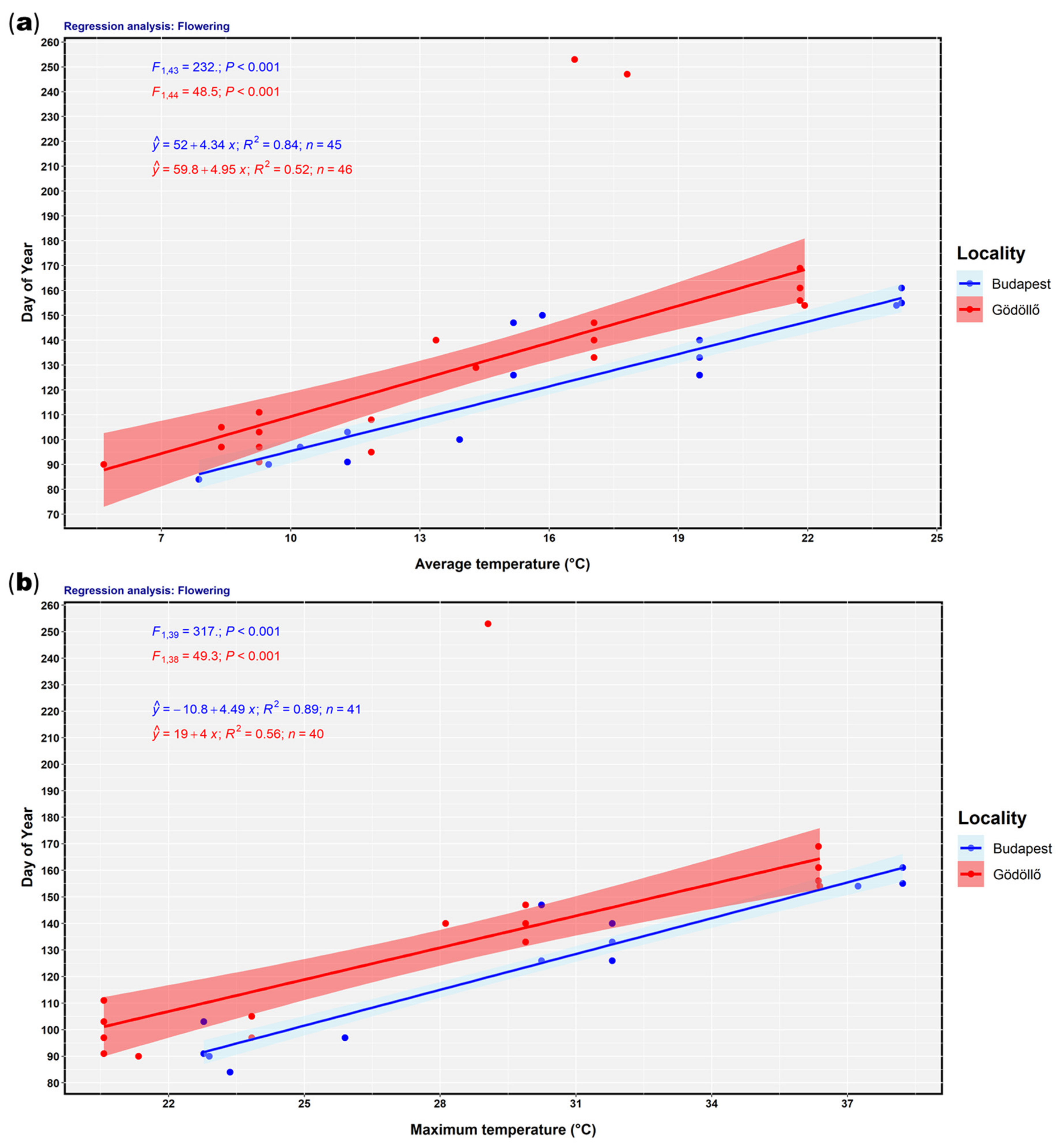
2.4. Statistical Analysis
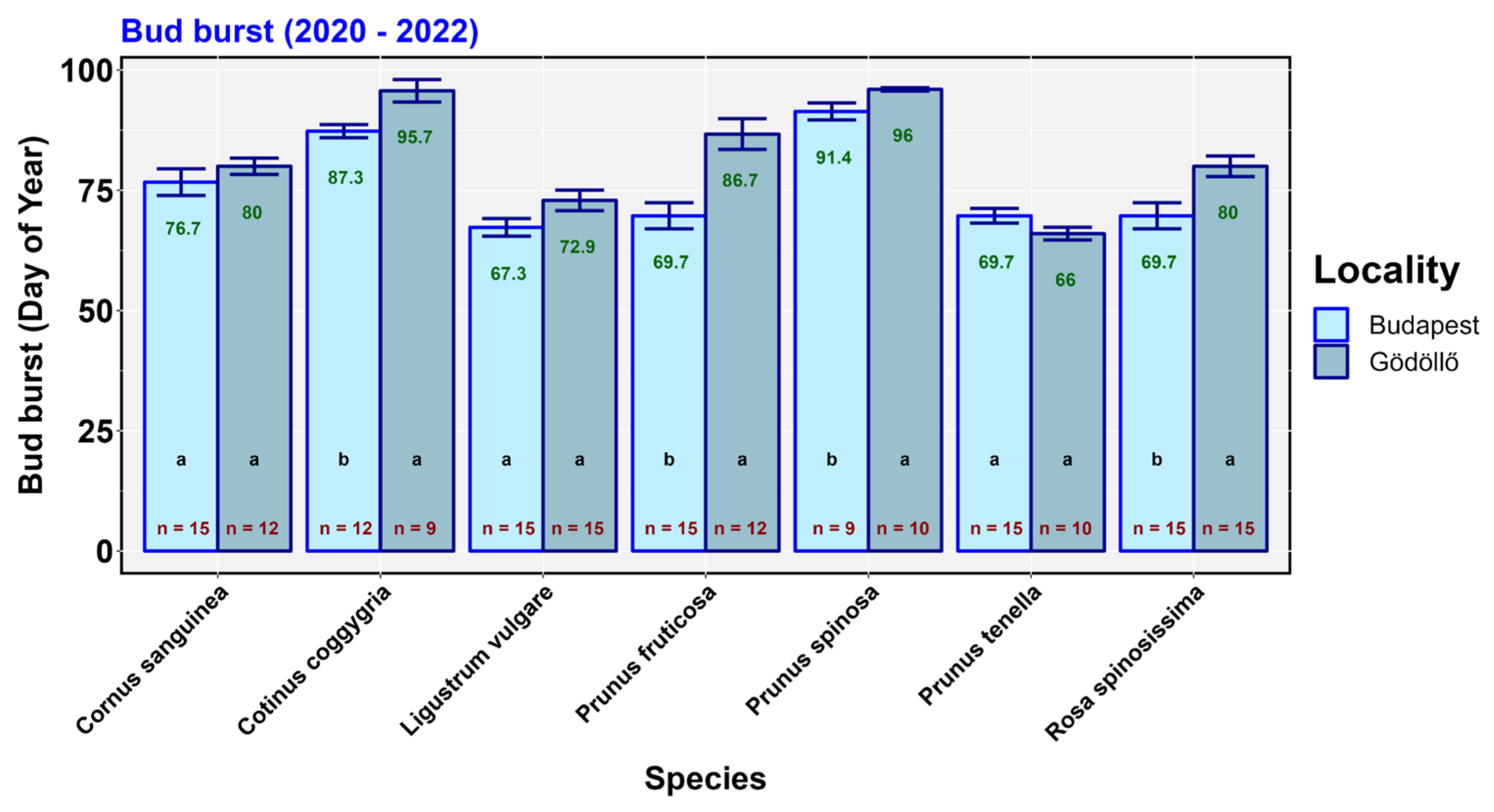
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Phenophase/Driving Factors | r2 | F-Statistic | n | a | b | Significance Level |
|---|---|---|---|---|---|---|
| Bud burst | ||||||
| Mean temperature (March–April) | 0.060 | 12.050 | 179 | 61.180 | 2.060 | <0.001 *** |
| Precipitation (March–April) | 0.120 | 25.220 | 179 | 72.170 | 0.260 | <0.001 *** |
| Min. temperature (March–April) | 0.030 | 3.370 | 114 | 85.610 | 1.090 | 0.07 + |
| Max. temperature (March–April) | 0.010 | 1.000 | 114 | 61.860 | 0.880 | 0.32 |
| Number of frost days | 0.220 | 31.430 | 114 | 91.390 | −1.140 | <0.001 *** |
| Budding | ||||||
| Mean temperature (March–April–May) | 0.550 | 118.300 | 99 | 67.570 | 3.600 | <0.001 *** |
| Precipitation (March–April–May) | 0.140 | 15.220 | 99 | 95.430 | 0.260 | <0.001 *** |
| Min. temperature (March–April–May) | 0.520 | 86.860 | 83 | 108.590 | 2.910 | <0.001 *** |
| Max. temperature (March–April–May) | 0.430 | 60.730 | 83 | 34.200 | 3.010 | <0.001 *** |
| Number of frost days | 0.430 | 60.070 | 83 | 114.190 | −2.460 | <0.001 *** |
| Flowering | ||||||
| Mean temperature (March–June) | 0.580 | 120.800 | 91 | 59.150 | 4.430 | <0.001 *** |
| Precipitation (March–June) | 0.010 | 0.610 | 91 | 131.300 | −0.120 | 0.44 |
| Min. temperature (March–June) | 0.560 | 98.740 | 81 | 111.730 | 3.870 | <0.001 *** |
| Max. temperature (March–June) | 0.640 | 142.40 | 81 | 8.750 | 4.090 | <0.001 *** |
| Number of frost days (March–June) | 0.360 | 45.210 | 81 | 135.990 | −5.930 | <0.001 *** |
| Number of tropical nights (March–June) | 0.240 | 25.520 | 81 | 119.410 | 5.490 | <0.001 *** |
| Fruiting | ||||||
| Mean temperature (May–June) | 0.370 | 44.750 | 78 | 86.000 | 3.380 | <0.001 *** |
| Precipitation (May–June) | 0.030 | 2.590 | 78 | 142.990 | 0.140 | 0.11 |
| Min. temperature (May–June) | 0.310 | 32.990 | 76 | 126.610 | 2.950 | <0.001 *** |
| Max. temperature (May–June) | 0.360 | 41.050 | 76 | 49.990 | 3.090 | <0.001 *** |
| Number of tropical nights (May–June) | 0.300 | 31.130 | 76 | 142.100 | 3.150 | <0.001 *** |
| Beginning of leaf coloring | ||||||
| Mean temperature (July–October) | 0.780 | 664.800 | 195 | 362.110 | −6.230 | <0.001 *** |
| Precipitation (July–October) | 0.170 | 39.190 | 195 | 227.510 | 0.350 | <0.001 *** |
| Min. temperature (July–October) | 0.570 | 172.800 | 130 | 279.580 | −5.100 | <0.001 *** |
| Max. temperature (July–October) | 0.860 | 777.700 | 130 | 443.340 | −6.300 | <0.001 *** |
| Number of tropical nights (July–October) | 0.320 | 61.240 | 130 | 243.770 | −2.850 | <0.001 *** |
| End of leaf fall | ||||||
| Mean temperature (November–December) | 0.500 | 187.700 | 191 | 361.800 | −6.040 | <0.001 *** |
| Precipitation (November–December) | 0.030 | 6.140 | 191 | 326.570 | 0.100 | 0.01 * |
| Min. temperature (November–December) | 0.400 | 82.370 | 125 | 314.160 | −4.610 | <0.001 *** |
| Max. temperature (November–December) | 0.500 | 124.100 | 125 | 373.220 | −3.150 | <0.001 *** |
| Number of frost days (November–December) | 0.350 | 65.860 | 125 | 315.130 | 1.550 | <0.001 *** |
Appendix B
| Phenophase/Driving Factors | r2 | F-Statistic | n | a | b | Significance Level |
|---|---|---|---|---|---|---|
| Bud burst | ||||||
| Mean temperature (March–April) | 0.020 | 2.200 | 96 | 57.600 | 1.950 | 0.14 |
| Precipitation (March–April) | 0.050 | 4.480 | 96 | 71.250 | 0.160 | 0.04 * |
| Min. temperature (March–April) | 0.160 | 11.580 | 62 | 88.330 | 4.080 | 0.001 ** |
| Max. temperature (March–April) | 0.270 | 21.930 | 62 | −109.360 | 8.040 | <0.001 *** |
| Number of frost days | 0.210 | 16.120 | 62 | 92.020 | −1.920 | <0.001 *** |
| Budding | ||||||
| Mean temperature (March–April–May) | 0.490 | 4.490 | 47 | 63.700 | 3.770 | <0.001 *** |
| Precipitation (March–April–May) | 0.250 | 15.000 | 47 | 91.070 | 0.360 | <0.001 *** |
| Min. temperature (March–April–May) | 0.490 | 36.040 | 40 | 105.440 | 3.150 | <0.001 *** |
| Max. temperature (March–April–May) | 0.320 | 17.560 | 40 | 31.510 | 3.050 | <0.001 *** |
| Number of frost days | 0.560 | 48.410 | 40 | 114.850 | −3.330 | <0.001 *** |
| Flowering | ||||||
| Mean temperature (March–June) | 0.840 | 232.300 | 45 | 51.970 | 4.340 | <0.001 *** |
| Precipitation (March–June) | 0.002 | 0.090 | 45 | 119.560 | 0.050 | 0.76 |
| Min. temperature (March–June) | 0.820 | 180.900 | 41 | 100.230 | 4.120 | <0.001 *** |
| Max. temperature (March–June) | 0.890 | 317.300 | 41 | −10.770 | 4.490 | <0.001 *** |
| Number of frost days (March–June) | 0.410 | 27.500 | 41 | 130.550 | −5.350 | <0.001 *** |
| Number of tropical nights (March–June) | 0.460 | 33.480 | 41 | 111.410 | 5.460 | <0.001 *** |
| Fruiting | ||||||
| Mean temperature (May–June) | 0.380 | 21.930 | 38 | 74.140 | 3.910 | <0.001 *** |
| Precipitation (May–June) | 0.100 | 3.880 | 38 | 137.910 | 0.240 | 0.06 + |
| Min. temperature (May–June) | 0.360 | 20.450 | 38 | 115.380 | 3.690 | <0.001 *** |
| Max. temperature (May–June) | 0.340 | 18.520 | 38 | 35.900 | 3.530 | <0.001 *** |
| Number of tropical nights (May–June) | 0.370 | 20.940 | 38 | 138.040 | 3.300 | <0.001 *** |
| Beginning of leaf coloring | ||||||
| Mean temperature (July–October) | 0.820 | 454.800 | 102 | 356.660 | −5.670 | <0.001 *** |
| Precipitation (July–October) | 0.210 | 27.290 | 102 | 226.390 | 0.360 | <0.001 *** |
| Min. temperature (July–October) | 0.710 | 161.600 | 68 | 298.000 | −5.790 | <0.001 *** |
| Max. temperature (July–October) | 0.900 | 596.800 | 68 | 446.770 | −6.250 | <0.001 *** |
| Number of tropical nights (July–October) | 0.540 | 76.740 | 68 | 251.280 | −2.860 | <0.001 *** |
| End of leaf fall | ||||||
| Mean temperature (November–December) | 0.650 | 191.400 | 103 | 371.720 | −7.050 | <0.001 *** |
| Precipitation (November–December) | 0.001 | 0.200 | 103 | 333.560 | 0.020 | 0.66 |
| Min. temperature (November–December) | 0.720 | 168.200 | 68 | 313.620 | −8.060 | <0.001 *** |
| Max. temperature (November–December) | 0.540 | 76.180 | 68 | 375.050 | −3.210 | <0.001 *** |
| Number of frost days (November–December) | 0.500 | 64.830 | 68 | 315.950 | 2.260 | <0.001 *** |
Appendix C
| Phenophase/Driving Factors | r2 | F-Statistic | n | a | b | Significance Level |
|---|---|---|---|---|---|---|
| Bud burst | ||||||
| Mean temperature (March–April) | 0.440 | 64.190 | 83 | 46.920 | 4.730 | <0.001 *** |
| Precipitation (March–April) | 0.280 | 31.000 | 83 | 73.660 | 0.370 | <0.001 *** |
| Min. temperature (March–April) | 0.620 | 82.930 | 52 | 109.880 | 4.820 | <0.001 *** |
| Max. temperature (March–April) | 0.170 | 10.340 | 52 | 12.490 | 3.390 | 0.002 ** |
| Number of frost days | 0.710 | 122.000 | 52 | 99.700 | −1.370 | <0.001 *** |
| Budding | ||||||
| Mean temperature (March–April–May) | 0.630 | 86.640 | 52 | 69.000 | 3.640 | <0.001 *** |
| Precipitation (March–April–May) | 0.060 | 3.250 | 52 | 99.180 | 0.170 | 0.08 + |
| Min. temperature (March–April–May) | 0.620 | 67.840 | 43 | 111.800 | 3.170 | <0.001 *** |
| Max. temperature (March–April–May) | 0.560 | 52.700 | 43 | 32.290 | 3.160 | <0.001 *** |
| Number of frost days | 0.360 | 22.840 | 43 | 113.860 | −2.040 | <0.001 *** |
| Flowering | ||||||
| Mean temperature (March–June) | 0.520 | 48.510 | 46 | 59.770 | 4.950 | <0.001 *** |
| Precipitation (March–June) | 0.060 | 2.630 | 46 | 148.290 | −0.420 | 0.11 |
| Min. temperature (March–June) | 0.580 | 51.580 | 40 | 120.110 | 4.700 | <0.001 *** |
| Max. temperature (March–June) | 0.560 | 49.340 | 40 | 18.950 | 4.000 | <0.001 *** |
| Number of frost days (March–June) | 0.350 | 20.130 | 40 | 141.700 | −6.660 | <0.001 *** |
| Number of tropical nights (March–June) | 0.230 | 11.290 | 40 | 123.950 | 10.120 | 0.002 ** |
| Fruiting | ||||||
| Mean temperature (May–June) | 0.400 | 24.920 | 40 | 98.250 | 2.780 | <0.001 *** |
| Precipitation (May–June) | 0.090 | 3.870 | 40 | 161.280 | −0.300 | 0.06 + |
| Min. temperature (May–June) | 0.310 | 16.460 | 38 | 132.530 | 2.670 | <0.001 *** |
| Max. temperature (May–June) | 0.440 | 28.100 | 38 | 66.620 | 2.600 | <0.001 *** |
| Number of tropical nights (May–June) | 0.210 | 9.410 | 38 | 142.910 | 5.280 | 0.005 ** |
| Beginning of leaf coloring | ||||||
| Mean temperature (July–October) | 0.850 | 502.900 | 93 | 388.460 | −7.970 | <0.001 *** |
| Precipitation (July–October) | 0.090 | 8.940 | 93 | 228.580 | 0.330 | 0.003 ** |
| Min. temperature (July–October) | 0.880 | 446.400 | 62 | 290.010 | −8.180 | <0.001 *** |
| Max. temperature (July–October) | 0.900 | 544.800 | 62 | 456.070 | −6.870 | <0.001 *** |
| Number of tropical nights (July–October) | 0.790 | 228.200 | 62 | 252.400 | −14.060 | <0.001 *** |
| End of leaf fall | ||||||
| Mean temperature (November–December) | 0.450 | 69.010 | 88 | 352.510 | −5.250 | <0.001 *** |
| Precipitation (November–December) | 0.140 | 13.630 | 88 | 313.340 | 0.390 | <0.001 *** |
| Min. temperature (November–December) | 0.550 | 66.920 | 57 | 308.920 | −4.120 | <0.001 *** |
| Max. temperature (November–December) | 0.360 | 31.390 | 57 | 365.810 | −2.740 | <0.001 *** |
| Number of frost days (November–December) | 0.480 | 50.190 | 57 | 311.050 | 1.370 | <0.001 *** |
References
- Peñuelas, J.; Ciais, P.; Canadell, J.G.; Janssens, I.A.; Fernández-Martínez, M.; Carnicer, J.; Obersteiner, M.; Piao, S.; Vautard, R.; Sardans, J. Shifting from a fertilization-dominated to a warming-dominated period. Nat. Ecol. Evol. 2017, 1, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Begum, R.A.; Lempert, R.; Ali, E.; Benjaminsen, T.A.; Bernauer, T.; Cramer, W.; Cui, X.; Mach, K.; Nagy, G.; Stenseth, N.C.; et al. Chapter 1: Point of Departure and Key Concepts In IPCC. Climate Change 2022: Impacts, Adaptation, and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Eds.; Cambridge University Press: Cambridge, UK, 2022; Volume 1, pp. 123–181. [Google Scholar]
- Schwartz, M.D. Introduction. In Phenology: An Integrative Environmental Science, 2nd ed.; Schwartz, M.D., Ed.; Springer Dordrecht: Berlin/Heidelberg, Germany, 2013; Volume 1, pp. 1–5. [Google Scholar]
- Sparks, T.H.; Menzel, A. Observed changes in seasons: An overview. Int. J. Climatol. 2002, 22, 1715–1725. [Google Scholar] [CrossRef]
- Cleland, E.E.; Chuine, I.; Menzel, A.; Mooney, H.A.; Schwartz, M.D. Shifting plant phenology in response to global change. Trends Ecol. Evol. 2007, 22, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Sherry, R.A.; Zhou, X.; Gu, S.; Arnone, J.A., III; Schimel, D.S.; Verburg, P.S.; Wallace, L.L.; Luo, Y. Divergence of reproductive phenology under climate warming. Proc. Natl. Acad. Sci. USA 2007, 104, 198–202. [Google Scholar] [CrossRef]
- Wolkovich, E.; Donahue, M.J. How phenological tracking shapes species and communities in nonstationary environments. Biol. Rev. 2021, 96, 2810–2827. [Google Scholar] [CrossRef] [PubMed]
- Walkovszky, A. Changes in phenology of the locust tree (Robinia pseudoacacia L.) in Hungary. Int. J. Biometeorol. 1998, 41, 155–160. [Google Scholar] [CrossRef]
- Menzel, A.; Fabian, P. Growing season extended in Europe. Nature 1999, 397, 659. [Google Scholar] [CrossRef]
- Chmielewski, F.-M.; Rötzer, T. Response of tree phenology to climate change across Europe. Agric. Meteorol. 2001, 108, 101–112. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Ahas, R.; Aasa, A. Onset of spring starting earlier across the Northern Hemisphere. Glob. Chang. Biol. 2006, 12, 343–351. [Google Scholar] [CrossRef]
- Vitasse, Y.; François, C.; Delpierre, N.; Dufrêne, E.; Kremer, A.; Chuine, I.; Delzon, S. Assessing the effects of climate change on the phenology of European temperate trees. Agric. Meteorol. 2011, 151, 969–980. [Google Scholar] [CrossRef]
- Chitu, E.; Paltineanu, C. Timing of phenological stages for apple and pear trees under climate change in a temperate-continental climate. Int. J. Biometeorol. 2020, 64, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Vander Mijnsbrugge, K.; Malanguis, J.M.; Moreels, S.; Turcsán, A.; Paino, E.N. Stimulation, reduction and compensation growth, and variable phenological responses to spring and/or summer–autumn warming in Corylus taxa and Cornus sanguinea L. Forests 2022, 13, 654. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Gong, Y.; Wang, S.; Zhang, W.; Zhang, S.; De Boeck, H.J.; Fu, Y.H. Climate warming shifts the time interval between flowering and leaf unfolding depending on the warming period. Sci. China Life Sci. 2022, 65, 2316–2324. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, W.; Liu, S.; Dong, W. Divergent responses of leaf phenology to changing temperature among plant species and geographical regions. Ecosphere 2015, 6, 250. [Google Scholar] [CrossRef]
- Scheifinger, H.; Menzel, A.; Koch, E.; Peter, C.H. Trends of spring time frost events and phenological dates in Central Europe. Theor. Appl. Climatol. 2003, 74, 41–51. [Google Scholar] [CrossRef]
- Vitasse, Y.; Basler, D. Is the use of cuttings a good proxy to explore phenological responses of temperate forests in warming and photoperiod experiments? Tree Physiol. 2014, 34, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Fitter, A.H.; Fitter, R.S. Rapid changes in flowering time in British plants. Science 2002, 296, 1689–1691. [Google Scholar] [CrossRef] [PubMed]
- Buonaiuto, D.M.; Wolkovich, E.M. Differences between flower and leaf phenological responses to environmental variation drive shifts in spring phenological sequences of temperate woody plants. J. Ecol. 2021, 109, 2922–2933. [Google Scholar] [CrossRef]
- Gallinat, A.S.; Primack, R.B.; Wagner, D.L. Autumn, the neglected season in climate change research. Trends Ecol. Evol. 2015, 30, 169–176. [Google Scholar] [CrossRef]
- Chen, L.; Hänninen, H.; Rossi, S.; Smith, N.G.; Pau, S.; Liu, Z.; Feng, G.; Gao, J.; Liu, J. Leaf senescence exhibits stronger climatic responses during warm than during cold autumns. Nat. Clim. Chang. 2020, 10, 777–780. [Google Scholar] [CrossRef]
- Gill, A.L.; Gallinat, A.S.; Sanders-DeMott, R.; Rigden, A.J.; Short Gianotti, D.J.; Mantooth, J.A.; Templer, P.H. Changes in autumn senescence in northern hemisphere deciduous trees: A meta-analysis of autumn phenology studies. Ann. Bot. 2015, 116, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Menzel, A.; Sparks, T.; Estrella, N.; Koch, E.; Aasa, A.; Ahas, R.; Alm-Kübler, K.; Bissolli, P.; Braslavska, O.; Briede, A.; et al. European phenological response to climate change matches the warming pattern. Glob. Chang. Biol. 2006, 12, 1969–1976. [Google Scholar] [CrossRef]
- Delpierre, N.; Dufrêne, E.; Soudani, K.; Ulrich, E.; Cecchini, S.; Boé, J.; François, C. Modelling interannual and spatial variability of leaf senescence for three deciduous tree species in France. Agric. Meteorol. 2009, 149, 938–948. [Google Scholar] [CrossRef]
- Tanino, K.K.; Kalcsits, L.; Silim, S.; Kendall, E.; Gray, G.R. Temperature-driven plasticity in growth cessation and dormancy development in deciduous woody plants: A working hypothesis suggesting how molecular and cellular function is affected by temperature during dormancy induction. Plant Mol. Biol. 2010, 73, 49–65. [Google Scholar] [CrossRef]
- Mijnsbrugge, K.V.; Malanguis, J.M.; Moreels, S.; Turcsán, A.; Van der Schueren, N.; Notivol Paino, E. Direct phenological responses but later growth stimulation upon spring and summer/autumn warming of Prunus spinosa L. in a common garden environment. Forests 2022, 13, 23. [Google Scholar] [CrossRef]
- Zani, D.; Crowther, T.W.; Mo, L.; Renner, S.S.; Zohner, C.M. Increased growing-season productivity drives earlier autumn leaf senescence in temperate trees. Science 2020, 370, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Hänninen, H.; Tanino, K. Tree seasonality in a warming climate. Trends Plant Sci. 2011, 16, 412–416. [Google Scholar] [CrossRef]
- Morin, X.; Roy, J.; Sonié, L.; Chuine, I. Changes in leaf phenology of three European oak species in response to experimental climate change. New Phytol. 2010, 186, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; O’keefe, J. Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef]
- Wang, H.; Ge, Q.; Rutishauser, T.; Dai, Y.; Dai, J. Parameterization of temperature sensitivity of spring phenology and its application in explaining diverse phenological responses to temperature change. Sci. Rep. 2015, 5, 8833. [Google Scholar] [CrossRef]
- Wolkovich, E.M.; Cook, B.I.; Allen, J.M.; Crimmins, T.M.; Betancourt, J.L.; Travers, S.E.; Pau, S.; Regetz, J.; Davies, T.J.; Kraft, N.J.; et al. Warming experiments underpredict plant phenological responses to climate change. Nature 2012, 485, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Pongracz, R.; Bartholy, J.; Pieczka, I.; Hunyady, A. Estimation of regional climate change in the Carpathian basin using PRECIS simulations for A2 and B2 scenarios. In Proceedings of the EGU General Assembly Conference Abstracts, Vienna, Austria, 19–24 April 2009; p. 11794. [Google Scholar]
- Hlásny, T.; Mátyás, C.S.; Seidl, R.; Kulla, L.; Merganicova, K.; Trombik, J.; Dobor, L.; Barcza, Z.; Konôpka, B. Climate change increases the drought risk in Central European forests: What are the options for adaptation? Cent. Eur. J. 2014, 60, 5–18. [Google Scholar] [CrossRef]
- Antofie, T.; Naumann, G.; Spinoni, J.; Vogt, J. Estimating the water needed to end the drought or reduce the drought severity in the Carpathian region. Hydrol. Earth Syst. Sci. 2015, 19, 177–193. [Google Scholar] [CrossRef]
- Gálos, B.; Jacob, D.; Mátyás, C.S. Effects of Simulated Forest Cover Change on Projected Climate Change—A Case Study of Hungary. Acta Silv. Lignaria Hung. 2011, 7, 49–62. [Google Scholar]
- Szirmai, O.; Horel, J.; Neményi, A.; Pándi, I.; Gyuricza, C.S.; Czóbel, S.Z. Overview of the collections of the first agrobotanical garden of Hungary. Hung. Agric. Res. 2014, 23, 19–25. [Google Scholar]
- Orlóci, L.; Kiszel, P.; Solymosiné László, I.; Papp, L. Delectus Seminum Sporarum Plantarumque Horti Botanici Universitatis Hungariae. Eötvös Lórand Tudományegyetem. Botanikus Kertje Universitatis Scientiarum Hungariae de Lorand Eoetvoes Nuncupatae, 165th ed.; Füvészkert Alapítvány (Foundation): Budapest, Hungary, 2019; 36p. [Google Scholar]
- Stewart, D.; Oke, T.R. Local climate zones for urban temperature studies. Bull. Am. Meteorol. Soc. 2012, 93, 1879–1900. [Google Scholar] [CrossRef]
- Geopedia. Available online: https://geopedia.world/#T4_L107_x2124674.6907575_y6021482.165878462_s16_b2345 (accessed on 13 March 2023).
- WFO Plant List. Available online: https://wfoplantlist.org/plant-list/ (accessed on 15 February 2023).
- R Core Team R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: http://www.R-project.org/ (accessed on 15 March 2023).
- RStudio Team. RStudio: Integrated Development for R; RStudio Inc.: Boston, FL, USA, 2015; Available online: http://www.rstudio.com/ (accessed on 15 March 2023).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation. R Package Version 1.1.0. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 15 March 2023).
- Wickham, H.; Seidel, D. Scales: Scale Functions for Visualization. R Package Version 1.2.1. 2022. Available online: http://CRAN.R-project.org/package=scales (accessed on 15 March 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; 213p. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 2nd ed.; Prentice-Hall Internattional: London, UK, 1984; 718p. [Google Scholar]
- Navarro, D.J. Learning Statistics with R: A Tutorial for Psychology Students and Other Beginners; University of New South Wales: Sydney, NSW, Australia, 2015. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 15 March 2023).
- Faraway, J.J. Linear Models with R; Chapman & Hall/CRC: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2005; 229p. [Google Scholar]
- Jacoby, W.G. LOESS: A nonparametric, graphical tool for depicting relationships between variables. Elect. Stud. 2000, 19, 577–613. [Google Scholar] [CrossRef]
- Pekár, S.; Brabec, M. Modern Analysis of Biological Data. Generalized Linear Models in R; Masaryk University Press: Brno, Czech Republic, 2016; 226p. [Google Scholar]
- Rutishauser, T.; Schleip, C.; Sparks, T.H.; Nordli, Ø.; Menzel, A.; Wanner, H.; Jeanneret, F.; Luterbacher, J. Temperature sensitivity of Swiss and British plant phenology from 1753 to 1958. Clim. Res. 2009, 39, 179–190. [Google Scholar] [CrossRef]
- Vitasse, Y.; Lenz, A.; Körner, C. The interaction between freezing tolerance and phenology in temperate deciduous trees. Front. Plant Sci. 2014, 5, 541. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Huang, J.-G.; Hänninen, H.; Li, X.; Berninger, F. Climate warming prolongs the time interval between leaf-out and flowering in temperate trees: Effects of chilling, forcing and photoperiod. J. Ecol. 2021, 109, 1319–1330. [Google Scholar] [CrossRef]
- Ibáñez, I.; Primack, R.B.; Miller-Rushing, A.J.; Ellwood, E.; Higuchi, H.; Lee, S.D.; Kobori, H.; Silander, J.A. Forecasting phenology under global warming. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3247–3260. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, S.; Zotz, G.; Asshoff, R.; Körner, C. Responses of deciduous forest trees to severe drought in Central Europe. Tree Physiol. 2005, 25, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.S.; Campioli, M.; Vitasse, Y.; De Boeck, H.J.; Van den Berge, J.; AbdElgawad, H.; Asard, H.; Piao, S.; Deckmyn, G.; Janssens, I.A. Variation in leaf flushing date influences autumnal senescence and next year’s flushing date in two temperate tree species. Proc. Natl. Acad. Sci. USA 2014, 111, 7355–7360. [Google Scholar] [CrossRef]
- Keenan, T.F.; Richardson, A.D. The timing of autumn senescence is affected by the timing of spring phenology: Implications for predictive models. Glob. Chang. Biol. 2015, 21, 2634–2641. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ohta, T.; Irasawa, M.; Nakamura, T. Climate change and extension of the Ginkgo biloba L. growing season in Japan. Glob. Chang. Biol. 2003, 9, 1634–1642. [Google Scholar] [CrossRef]
- Peñuelas, J.; Rutishauser, T.; Filella, I. Phenology feedbacks on climate change. A longer growing season as a result of climate change will in turn affect climate through biogeochemical and biophysical effects. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Keenan, T.F.; Migliavacca, M.; Ryu, Y.; Sonnentag, O.; Toomey, M. Climate change, phenology, and phenological control of vegetation feedbacks to the climate system. Agric. Meteorol. 2013, 169, 156–173. [Google Scholar] [CrossRef]
- Tan, X.; Gao, L.; Wang, W.; Zhang, W.; Wei, J.; Wang, J.; Li, L.; Zhou, Q.; Liang, H.; Liu, Y. Modelling alteration of leaf coloration peak date in Cotinus coggygria in a high-elevation karst region. Agric. Meteorol. 2022, 323, 109044. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verbényiné Neumann, K.; Baltazár, T.; Saláta, D.; Szirmai, O.; Czóbel, S. Comparative Study of the Phenology of Seven Native Deciduous Tree Species in Two Different Mesoclimatic Areas in the Carpathian Basin. Forests 2023, 14, 885. https://doi.org/10.3390/f14050885
Verbényiné Neumann K, Baltazár T, Saláta D, Szirmai O, Czóbel S. Comparative Study of the Phenology of Seven Native Deciduous Tree Species in Two Different Mesoclimatic Areas in the Carpathian Basin. Forests. 2023; 14(5):885. https://doi.org/10.3390/f14050885
Chicago/Turabian StyleVerbényiné Neumann, Krisztina, Tivadar Baltazár, Dénes Saláta, Orsolya Szirmai, and Szilárd Czóbel. 2023. "Comparative Study of the Phenology of Seven Native Deciduous Tree Species in Two Different Mesoclimatic Areas in the Carpathian Basin" Forests 14, no. 5: 885. https://doi.org/10.3390/f14050885
APA StyleVerbényiné Neumann, K., Baltazár, T., Saláta, D., Szirmai, O., & Czóbel, S. (2023). Comparative Study of the Phenology of Seven Native Deciduous Tree Species in Two Different Mesoclimatic Areas in the Carpathian Basin. Forests, 14(5), 885. https://doi.org/10.3390/f14050885






