Forest by-Product Valorization: Pilot-Scale Pinus radiata and Eucalyptus globulus Bark Mixture Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material
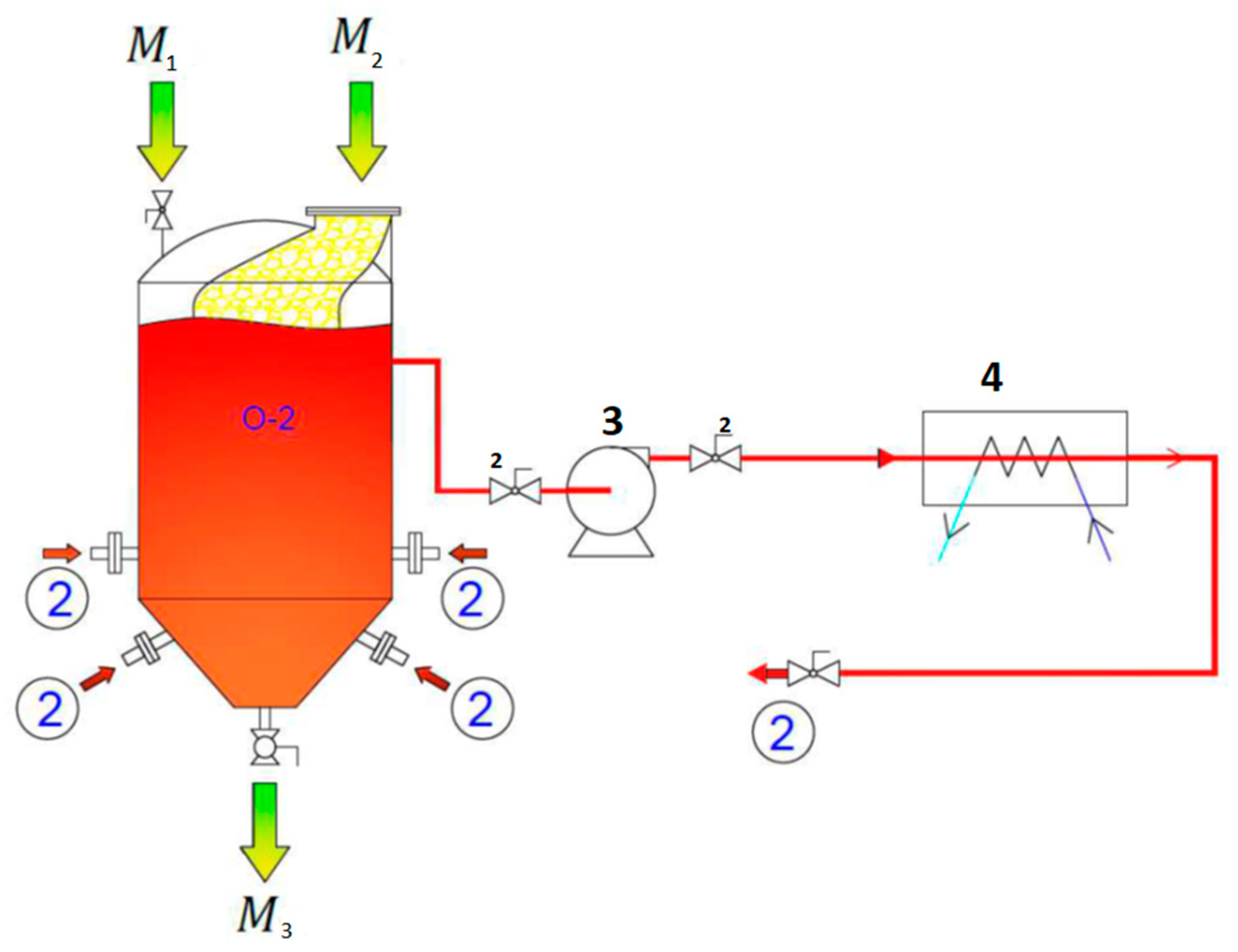
2.2. Pilot Scale Extraction
2.3. Stiasny Number
2.4. Molecular Weight Distribution by Gel Permeation Chromatography (GPC)
2.5. Fourier Transform Infrared Spectroscopy (FTIR) Assay
2.6. Mass Spectrometric Analysis by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight (MALDI-TOF)
2.7. Thermogravimetric Analysis (TGA)
2.8. Automated Bond Evaluation System (ABES) Test
2.9. Dynamic Mechanical Analysis Experiments
- (1)
- Stabilization at 35 °C;
- (2)
- Isotherm at 35 °C for 30 s;
- (3)
- Heating ramp at 2 °C/min up to 180 °C.
2.10. Manufacture and Testing of Particleboard
3. Results and Discussion
3.1. Characterization of P. radiata and E. globulus Bark
3.2. Characterization of P. radiata and E. globulus Bark Extracts
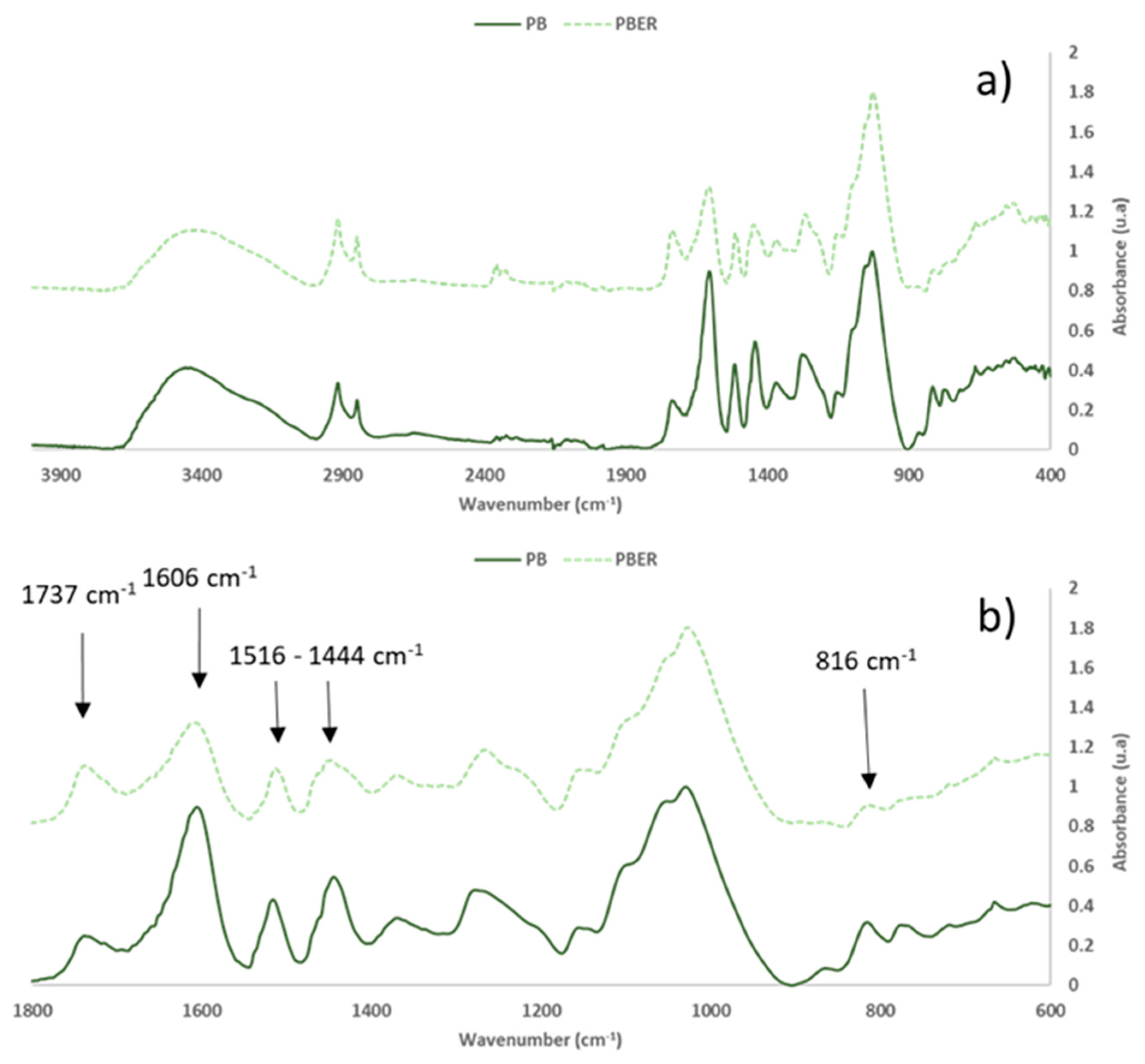
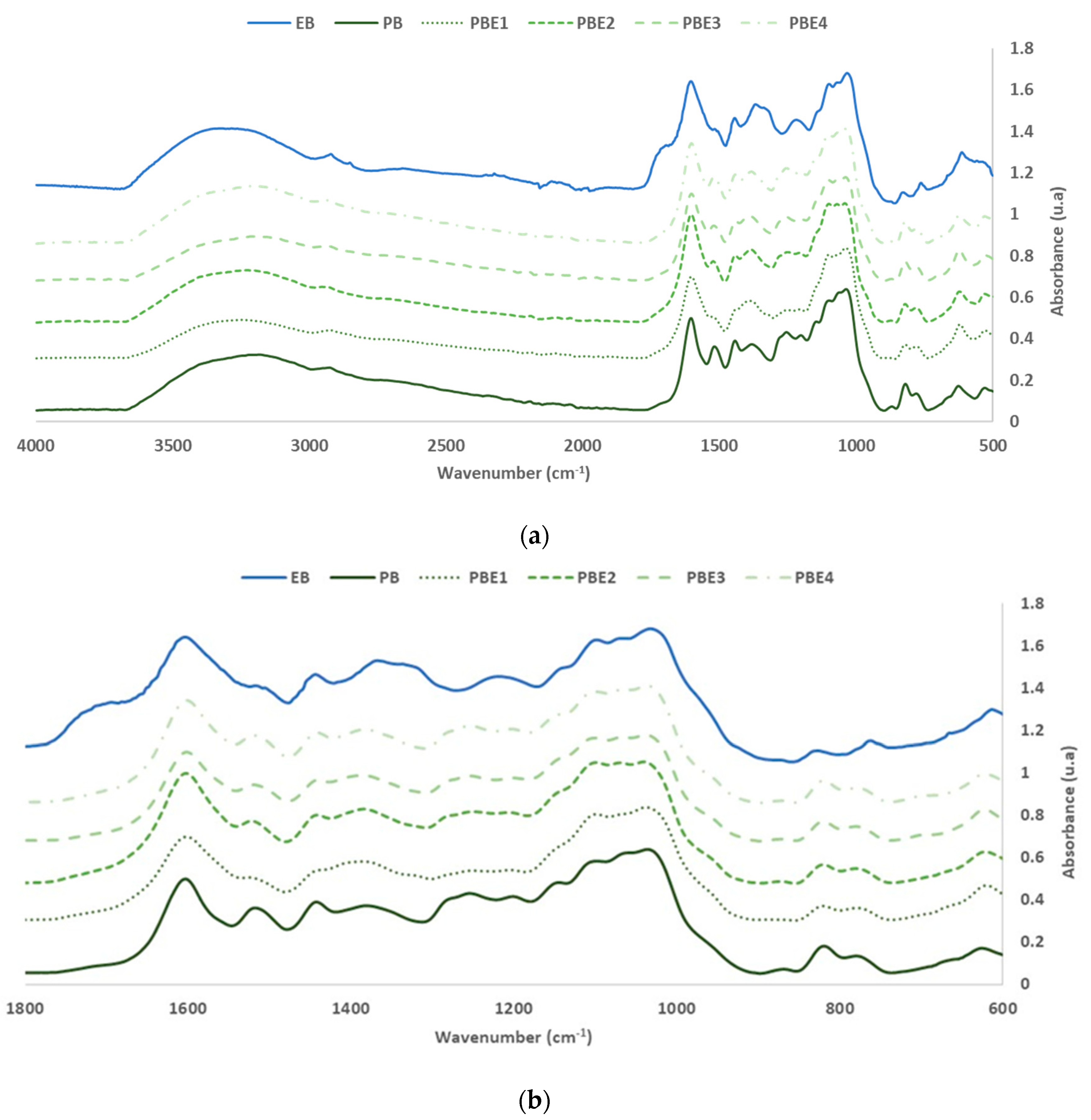
3.3. Characterization of Eucalyptus and Pine Bark Extracts by FTIR-ATR
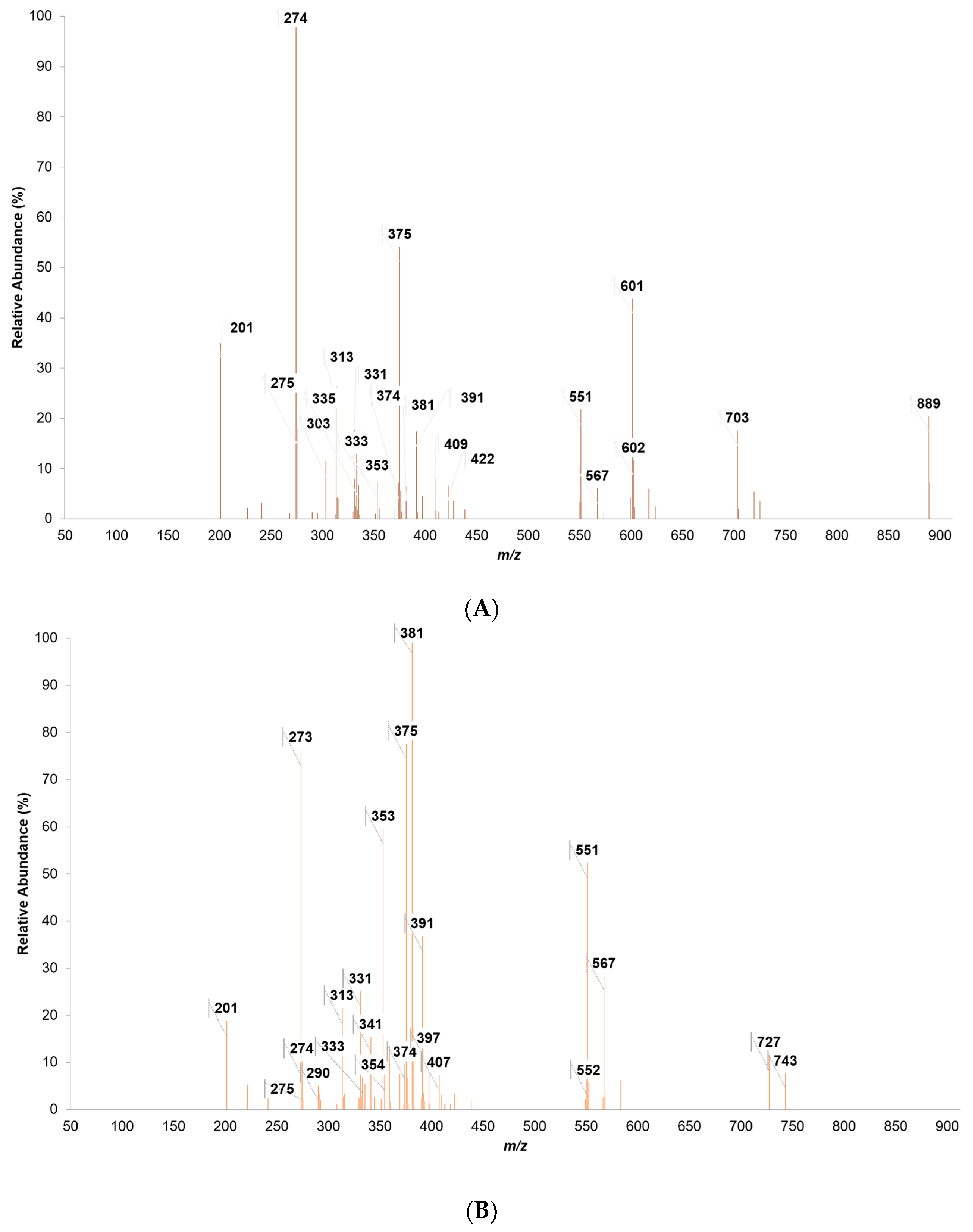
3.4. Characterization of Eucalyptus and Pine Bark Extracts by MALDI-TOF
3.5. Characterization of the Reactivity of P. radiata and E. globulus Bark Extracts
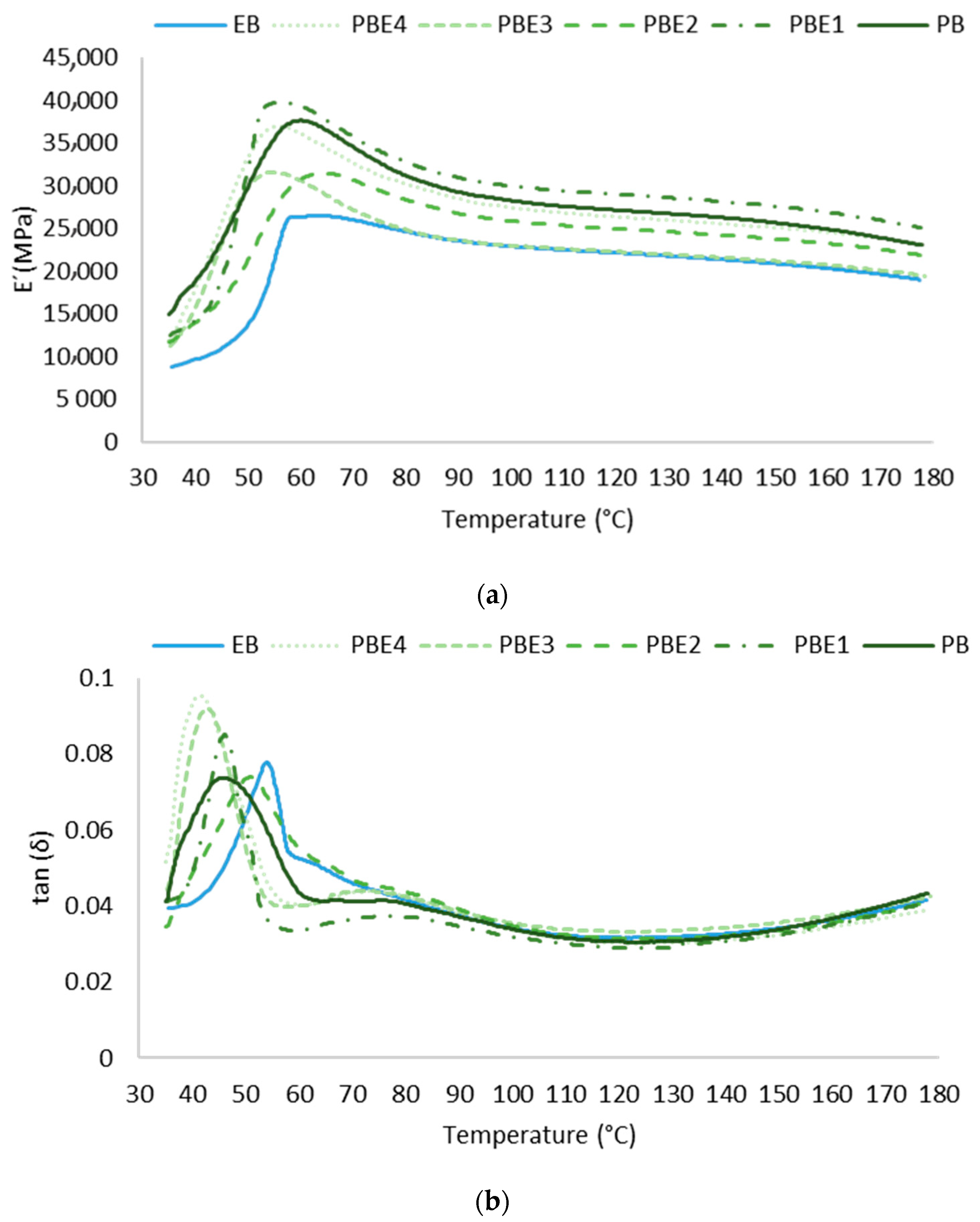
3.5.1. Evaluation of the Curing Reaction by DMA
3.5.2. Evaluation of the Curing Reaction by ABES
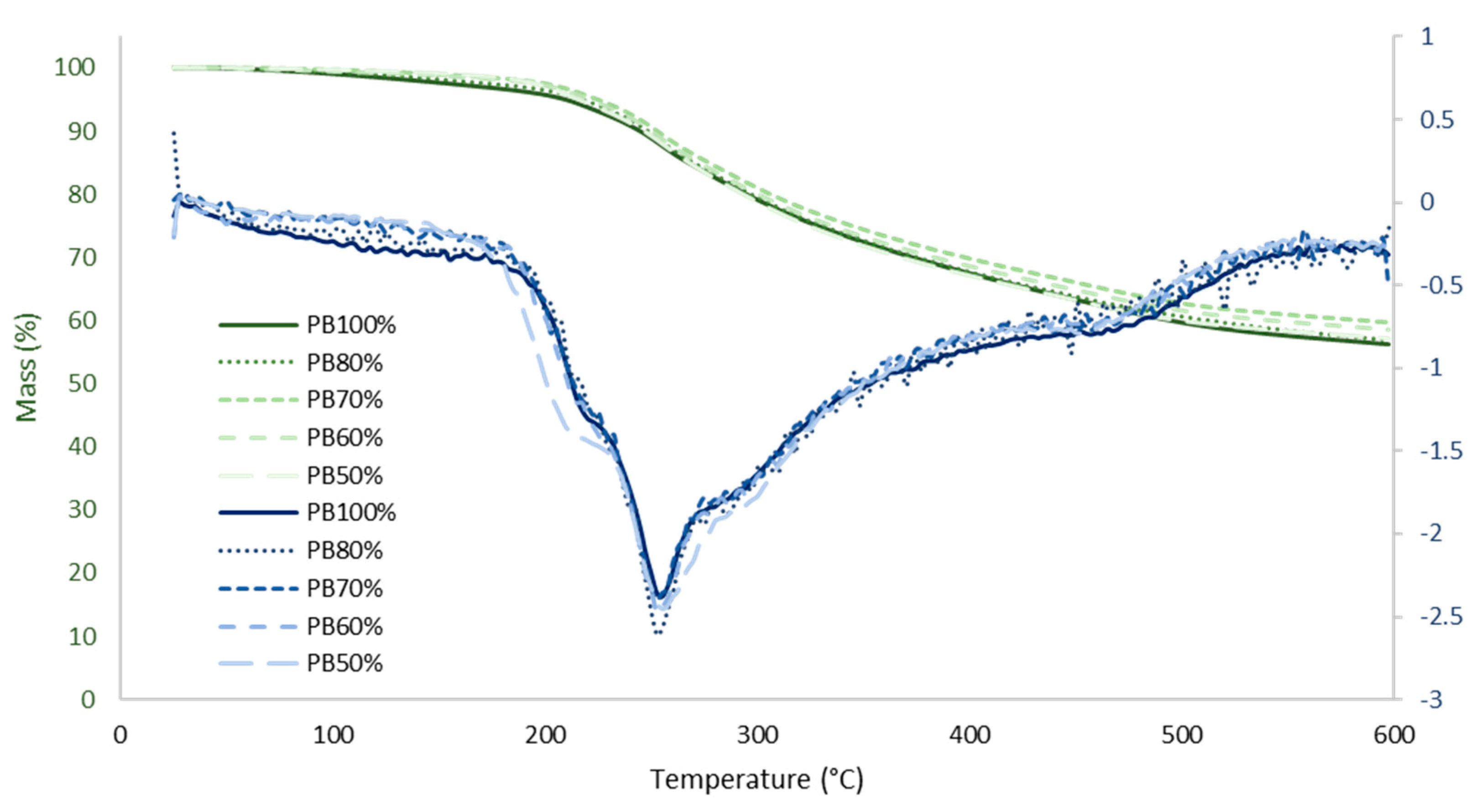
3.5.3. Evaluation of the Thermostability of Pine and Eucalyptus Extracts by TGA
3.5.4. Bioadhesive Formulation and Particleboard Performance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of Non-Timber by-Products from Maritime Pine (Pinus pinaster, Ait) for Particleboard Production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Santos, J.; Delgado, N.; Fuentes, J.; Fuentealba, C.; Vega-Lara, J.; García, D.E. Exterior Grade Plywood Adhesives Based on Pine Bark Polyphenols and Hexamine. Ind. Crops Prod. 2018, 122, 340–348. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.d.C.; Pereira, H. Potential of Eucalyptus globulus Industrial Bark as a Biorefinery Feedstock: Chemical and Fuel Characterization. Ind. Crops Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on Tannins: Extraction Processes, Applications and Possibilities. S. Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Santos, J.; Antorrena, G.; Freire, M.S.; Pizzi, A.; González-Álvarez, J. Environmentally Friendly Wood Adhesives Based on Chestnut (Castanea sativa) Shell Tannins. Eur. J. Wood Wood Prod. 2017, 75, 89–100. [Google Scholar] [CrossRef]
- García, D.E.; Fuentealba, C.A.; Salazar, J.P.; Pérez, M.A.; Escobar, D.; Pizzi, A. Mild Hydroxypropylation of Polyflavonoids Obtained under Pilot-Plant Scale. Ind. Crops Prod. 2016, 87, 350–362. [Google Scholar] [CrossRef]
- Abilleira, F.; Varela, P.; Cancela, Á.; Álvarez, X.; Sánchez, Á.; Valero, E. Tannins Extraction from Pinus pinaster and Acacia dealbata Bark with Applications in the Industry. Ind. Crops Prod. 2021, 164, 113394. [Google Scholar] [CrossRef]
- García, D.E.; Gavino, J.; Escobar, D.; Cancino, R.A. Maleinated Polyflavonoids and Lignin as Functional Additives for Three Kinds of Thermoplastics. Iran. Polym. J. 2017, 26, 295–304. [Google Scholar] [CrossRef]
- Peredo, K.; Escobar, D.; Vega-Lara, J.; Berg, A.; Pereira, M. Thermochemical Properties of Cellulose Acetate Blends with Acetosolv and Sawdust Lignin: A Comparative Study. Int. J. Biol. Macromol. 2016, 83, 403–409. [Google Scholar] [CrossRef]
- Vavilala, S.L.; Siddhesh, B.; Ghag, J.S.D. Chapter 9—Lignin: Understanding and Exploring Its Potential for Biofuel Production. In Woodhead Publishing Series in Energy, Advanced Bioprocessing for Alternative Fuels, Biobased Chemicals, and Bioproducts; Majid, H., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 165–186. [Google Scholar]
- Tanase, C.; Cosarcă, S.; Muntean, D.L. A Critical Review of Phenolic Compounds Extracted from the Bark of Woody Vascular Plants and Their Potential Biological Activity. Molecules 2019, 24, 1182. [Google Scholar] [CrossRef]
- Escobar-Avello, D.; Mardones, C.; Saéz, V.; Riquelme, S.; von Baer, D.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Pilot-Plant Scale Extraction of Phenolic Compounds from Grape Canes: Comprehensive Characterization by LC-ESI-LTQ-Orbitrap-MS. Food Res. Int. 2021, 143, 110265. [Google Scholar] [CrossRef]
- Yazaki, Y. Utilization of Flavonoid Compounds from Bark and Wood: A Review. Nat. Prod. Commun. 2015, 10, 513–520. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Freire, S.; López-Suevos, F.; Antorrena, G. Characteristics of Pinus pinaster Bark Extracts Obtained under Various Extraction Conditions. Holz Als Roh-Und Werkst. 2001, 59, 451–456. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of Maritime Pine (Pinus pinaster) Bark Tannins Extracted under Different Conditions by Spectroscopic Methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 879–903. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of Potential Applications for Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus globulus) Bark Extracts. Ind. Crops Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Vázquez, G.; Santos, J.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. Extraction of Antioxidants from Eucalyptus (Eucalyptus globulus) Bark. Wood Sci. Technol. 2012, 46, 443–457. [Google Scholar] [CrossRef]
- Yazaki, Y.; Hillis, W.E. Molecular Size Distribution of Radiata Pine Bark Extracts and Its Effect on Properties. Holzforschung 1980, 34, 125–130. [Google Scholar] [CrossRef]
- Costa, N.A. Evaluation of Bonding Performance of Amino Polymers Using ABES. J. Adhes. 2014, 37–41. [Google Scholar] [CrossRef]
- EN 323:1993; Wood-Based Panels—Determination of Density. European Commission: Brussels, Belgium, 1993.
- EN 319:1993; Particleboards and Fibreboards—Determination of Tensile Strength Perpendicular. European Commission: Brussels, Belgium, 1993.
- EN 312:2010; Particleboards—Specifications. European Commission: Brussels, Belgium, 2010.
- Grasel, F.D.S.; Ferrão, M.F.; Wolf, C.R. Development of Methodology for Identification the Nature of the Polyphenolic Extracts by FTIR Associated with Multivariate Analysis. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2016, 153, 94–101. [Google Scholar] [CrossRef]
- Santos, J.; Escobar-Avello, D.; Magalhães, F.D.; Manuel, J.; González-álvarez, J.; De, L.H. High-Value Compounds Obtained from Grape Canes (Vitis Vinifera L.) by Steam Pressure Alkali Extraction. Food Bioprod. Process. 2022, 133, 153–167. [Google Scholar] [CrossRef]
- Poletto, M.; Ornaghi Júnior, H.L.; Zattera, A.J. Native Cellulose: Structure, Characterization and Thermal Properties. Materials 2014, 7, 105. [Google Scholar] [CrossRef] [PubMed]
- Yemele, M.C.N.; Koubaa, A.; Cloutier, A.; Soulounganga, P.; Wolcott, M. Effect of Bark Fiber Content and Size on the Mechanical Properties of Bark/HDPE Composites. Compos. Part A Appl. Sci. Manuf. 2010, 41, 131–137. [Google Scholar] [CrossRef]
- Kačík, F.; Ďurkovič, J.; Kačíková, D. Chemical Profiles of Wood Components of Poplar Clones for Their Energy Utilization. Energies 2012, 5, 5243–5256. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative Visualization of Lignocellulose Components in Transverse Sections of Moso Bamboo Based on FTIR Macro- and Micro-Spectroscopy Coupled with Chemometrics. Biotechnol. Biofuels 2018, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.; Pereira, J.; Escobar-Avello, D.; Ferreira, I.; Vieira, C.; Magalh, D.; Martins, J.M.; Carvalho, L.H. Grape Canes (Vitis Vinifera L.) Applications on Packaging and Particleboard Industry: New Bioadhesive Based on Grape Extracts and Citric Acid. Polymers 2022, 14, 1137. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and Quantitative Analysis of Lignocellulosic Biomass Using Infrared Techniques: A Mini-Review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Vázquez, G.; Fontenla, E.; Santos, J.; Freire, M.S.; González-Álvarez, J.; Antorrena, G. Antioxidant Activity and Phenolic Content of Chestnut (Castanea Sativa) Shell and Eucalyptus (Eucalyptus globulus) Bark Extracts. Ind. Crops Prod. 2008, 28, 279–285. [Google Scholar] [CrossRef]
- Yazaki, Y.; Collins, P.J.; Iwashina, T. Extractives from Blackbutt {Eucalyptus pilularis) Wood Which Affect Gluebond Quality of Phenolic Resins. Holzforschung 1993, 47, 412–418. [Google Scholar] [CrossRef]
- Hospodarova, V.; Singovszka, E.; Stevulova, N. Characterization of Cellulosic Fibers by FTIR Spectroscopy for Their Further Implementation to Building Materials. Am. J. Anal. Chem. 2018, 09, 303–310. [Google Scholar] [CrossRef]
- Özgenç, Ö.; Durmaz, S.; Kustas, S. Chemical Analysis of Tree Barks Using ATR-FTIR Spectroscopy and Conventional Techniques. BioResources 2017, 12, 9143–9151. [Google Scholar] [CrossRef]
- Traore, M.; Kaal, J. Differentiation between Pine Woods According to Species and Growing Location Using FTIR-ATR. Wood Sci. Technol. 2018, 52, 487–504. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.J. Curing Behavior and Viscoelastic Properties of Pine and Wattle Tannin-Based Adhesives Studied by Dynamic Mechanical Thermal Analysis and FT-IR-ATR Spectroscopy. J. Adhes. Sci. Technol. 2003, 17, 1369–1383. [Google Scholar] [CrossRef]
- Tondi, G.; Petutschnigg, A. Middle Infrared (ATR FT-MIR) Characterization of Industrial Tannin Extracts. Ind. Crops Prod. 2015, 65, 422–428. [Google Scholar] [CrossRef]
- Ricci, A.; Parpinello, G.P.; Olejar, K.J.; Kilmartin, P.A.; Versari, A. Attenuated Total Reflection Mid-Infrared (ATR-MIR) Spectroscopy and Chemometrics for the Identification and Classification of Commercial Tannins. Appl. Spectrosc. 2015, 69, 1243–1250. [Google Scholar] [CrossRef]
- Soto, R.; Freer, J.; Baeza, J. Evidence of Chemical Reactions between Di- and Poly-Glycidyl Ether Resins and Tannins Isolated from Pinus radiata D. Don Bark. Bioresource 2005, 96, 95–101. [Google Scholar] [CrossRef]
- Jerez, M.; Sineiro, J.; Guitian, E.; Nunez, M.J. Identification of Polymeric Procyanidins from Pine Bark by Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 23, 4013–4018. [Google Scholar] [CrossRef]
- Abdalla, S.; Pizzi, A.; Ayed, N.; Charrier-El Bouhtoury, F.; Charrier, B.; Bahabri, F.; Ganash, A. MALDI-TOF Analysis of Aleppo Pine (Pinus halepensis) Bark Tannin. BioResources 2014, 9, 3396–3406. [Google Scholar] [CrossRef]
- Akbar, J.; Iqbal, M.S.; Massey, S.; Masih, R. Kinetics and Mechanism of Thermal Degradation of Pentose- and Hexose-Based Carbohydrate Polymers. Carbohydr. Polym. 2012, 90, 1386–1393. [Google Scholar] [CrossRef]




| Sample | Extraction Raw Material | Extraction Agent | ||
|---|---|---|---|---|
| P. radiata Bark (%) | E. globulus Bark (%) | NaOH (%) | Na2SO3 (%) | |
| PB | 100 | 0 | 1 | 1 |
| EB | 0 | 100 | 1 | 1 |
| PBE1 | 50 | 50 | 1 | 1 |
| PBE2 | 60 | 40 | 1 | 1 |
| PBE3 | 70 | 30 | 1 | 1 |
| PBE4 | 80 | 20 | 1 | 1 |
| PB | PBER | EB | EBER | Group | Range | ||||
|---|---|---|---|---|---|---|---|---|---|
| cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | ||
| 3457 | 41.4 | 3417 | 30.7 | 3437 | 33.7 | 3418 | 20.9 | -OH stretch | 3300−3400 |
| 2919 | 33.7 | 2919 | 36.6 | 2920 | 22.8 | 2919 | 14.4 | -CH2- asymmetric stretch | 2916−2936 |
| 2851 | 25.0 | 2851 | 27.2 | 2852 | 17.1 | 2852 | 11.6 | -CH2- symmetric stretch | 2843−2863 |
| 1737 | 24.9 | 1738 | 30.7 | 1730 | 33.5 | 1731 | 16.2 | C=O stretch in unconjugated ketones, carbonyls and in ester groups (hemicellulose) | 1738 |
| 1606 | 89.8 | 1607 | 52.3 | 1613 | 58.3 | 1601 | 37.0 | Aromatic skeletal vibration and C=O stretch (lignin) | 1595 |
| 1516 | 43.1 | 1513 | 29.2 | 1508 | 9.9 | 1505 | 12.6 | CAR=CAR (Pp cd.) | 1500−1600 |
| 1444 | 54.6 | 1449 | 33.3 | 1441 | 24.5 | -- | -- | C=C and C-H bond O-H in plane deformation (lignin and hemicellulose) | 1450−1453 |
| 1463 | 33.5 | 1463 | 26.8 | -- | -- | 1418 | 17.6 | CH- deformation; asymmetric in -CH3 and -CH2- (cellulose) | 1430−1485 |
| 1370 | 33.9 | 1370 | 25.9 | 1368 | 29.0 | 1370 | 23.7 | CH deformation (cellulose and hemicellulose) | 1372 |
| -- | -- | -- | -- | 1318 | 26.0 | 1319 | 18.8 | Ph-CHR-OH deformation | 1260−1350 |
| 1278 | 48.0 | 1266 | 38.7 | -- | -- | -- | -- | ||
| -- | -- | 1226 | 28.1 | 1235 | 26.6 | 1238 | 15.3 | Syringyl ring and C=C stretch in lignin and xylan | 1235 |
| 1155 | 29.0 | 1154 | 28.6 | -- | -- | -- | -- | Involves C-O stretching of C-OH/C-O-C (cellulose) | 1160 |
| 1101 | 60.3 | 1100 | 53.5 | 1100 | 53.6 | 1103 | 47.1 | C-O-C stretch (cellulose and hemicellulose) | 1105 |
| 1055 | 52.4 | 1054 | 84.7 | -- | -- | -- | -- | C-O-C aromatic ethers symmetric stretch (pyranose ring) | 1010−1050 |
| 1030 | 100.0 | 1028 | 100.4 | 1024 | 100.0 | 1027 | 100.0 | C-O, C-C, and C-C-O stretch (cellulose, hemicellulose, and lignin) | 1025−1035 |
| 816 | 31.9 | 813 | 10.7 | -- | -- | -- | -- | C-O-C aromatic ethers, symmetric stretch | 810−850 |
| 775 | 30.4 | -- | -- | C-C alkane skeletal vibrations | 720−750 | ||||
| Nomenclature | Extraction Yield (%) | Stiasny Number (%) | Mn (Da) | Mw (Da) | PD |
|---|---|---|---|---|---|
| EB | 7.50 ± 0.38 | 30.42 ± 1.1 | 596 ± 3.1 | 1217 ± 5.5 | 2.04 ± 0.2 |
| PEB1 | 8.41 ± 0.32 | 61.25 ± 0.5 | 161 ± 2.1 | 275 ± 3.5 | 1.04 ± 0.3 |
| PEB2 | 9.30 ± 0.16 | 56.80 ± 1.2 | 214 ± 5.1 | 675 ± 7.8 | 3.16 ± 0.7 |
| PEB3 | 10.09 ± 0.09 | 58.29 ± 0.7 | 172 ± 3.6 | 318 ± 2.9 | 1.85 ± 0.1 |
| PEB4 | 12.21 ± 0.10 | 66.31 ± 0.9 | 181 ± 1.8 | 363 ± 3.9 | 2.01 ± 0.3 |
| PB | 14.30 ± 0.28 | 79.24 ± 1.6 | 461 ± 6.2 | 909 ± 10.6 | 1.97 ± 0.6 |
| PB | EB | PBE1 (50% PB 50% EB) | PBE2 (60% PB 40% EB) | PBE3 (70% PB 30% EB) | PBE4 (80% PB 20% EB) | Group | Range | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | cm−1 | Intensity | ||
| 3179 | 60.4 | 3454 | 36.2 | 3250 | 45.9 | 3204 | 48.2 | 3188 | 50.8 | 3203 | 57.5 | -OH stretch | 3300–3400 |
| 2926 | 46.4 | 2920 | 16.0 | 2924 | 33.0 | 2954 | 33.0 | 2932 | 39.5 | 2931 | 43.0 | -CH2- asymmetric stretch | 2916–2936 |
| 1718 | 6.8 | 1715 | 41.7 | 1718 | 9.3 | 1718 | 10.4 | 1718 | 7.3 | 1718 | 8.5 | C=O Pp Hs [23,24] | 1710–1720 |
| 1604 | 100.0 | 1605 | 100.0 | 1602 | 100.0 | 1603 | 100.0 | 1602 | 100.0 | 1602 | 100.0 | CAR=CAR (Pp.) | 1500–1600 |
| 1518 | 69.4 | 1517 | 31.4 | 1525 | 51.3 | 1523 | 56.7 | 1520 | 63.3 | 1522 | 65.7 | C=C asymmetric stretching aromatic (Pp) [37] | 1510 |
| 1443 | 75.7 | 1444 | 36.7 | 1442 | 59.7 | 1442 | 62.1 | 1441 | 68.5 | 1442 | 68.5 | C-H bending of CH2 groups (Pp cd.) | 1450–1455 |
| 1381 | 71.8 | 1368 | 49.2 | 1385 | 69.7 | 1384 | 67.6 | 1386 | 74 | 1382 | 71.5 | C-C stretch | 1380–1400 |
| 1283 | 77.6 | 1336 | 45.2 | 1280 | 54.95 | 1281 | 61.2 | 1282 | 67.4 | 1285 | 67.2 | Ph-CHR-OH deformation | 1260–1350 |
| 1254 | 84.8 | -- | -- | 1251 | 58.3 | 1250 | 65.2 | 1255 | 73.4 | 1254 | 75.0 | C-O-C aromatic ethers, asymmetric stretch | 1210–1310 |
| -- | -- | 1222 | 29.0 | -- | -- | -- | -- | -- | -- | -- | -- | C–O stretch Pp Hs [23,24] | 1240–1250 |
| 1202 | 81.5 | 1200 | 25.6 | 1205 | 59.2 | 1207 | 64.5 | 1201 | 71 | 1203 | 71.7 | Ph-OH stretch | 1180–1260 |
| 1145 | 96.3 | 1142 | 44.1 | 1148 | 86.5 | 1146 | 82.9 | 1144 | 93.1 | 1148 | 85.4 | Involves C-O stretching of C-OH/C-O-C | 1160 |
| 1098 | 119.0 | 1095 | 83.8 | 1099 | 127.6 | 1099 | 109.8 | 1101 | 115.6 | 1100 | 108.6 | C-O-C stretch | 1105 |
| 1063 | 127.7 | 1066 | 91.9 | 1071 | 126.6 | 1070 | 109.6 | 1067 | 116.7 | 1065 | 111.0 | C-O primary alcohol stretching (Pp) | 1060–1070 |
| 1036 | 131.4 | 1029 | 109.8 | 1038 | 136.1 | 1041 | 110.5 | 1038 | 119.1 | 1037 | 114.6 | C-O, C-C, and C-C-O stretch | 1025–1035 |
| -- | -- | 869 | 2.5 | -- | -- | -- | -- | -- | -- | -- | -- | Aromatic C–H out-of-plane bending Pp Hs [23,24] | 860–870 |
| 819 | 28.9 | 825 | 18.5 | 820 | 14.9 | 820 | 17.1 | 820 | 22.2 | 820 | 21.6 | C-O-C aromatic ethers, symmetric stretch | 810–850 |
| 780 | 18.5 | 762 | 35.0 | 779 | 15.0 | 780 | 13.5 | 780 | 16.5 | 779 | 14.4 | C-C alkane skeletal vibrations | 720–750 |
| M+Na+ (exp.) (Da) | M+Na+ (calc.) (Da) | Tentative Identification | PB (% R.A.) | EB (% R.A.) | PB/EB (% R.A.) |
|---|---|---|---|---|---|
| 201.5 | 201 | Sugar + Na-2H+/Stilbene | 35 | 19 | 3 |
| 273.9 | 274 | Fisetinidol | 100 | 76 | 9 |
| 274.9 | 275 | Fisetinidol + 1H+ | 18 | 10 | -- |
| 290.4 | 303 | Catechin | -- | 5 | -- |
| 303.3 | 304 | Taxifolin-1H+ | 12 | -- | -- |
| 313.1 | 313 | Catechin + Na+ | 27 | 22 | 4 |
| 331.1 | 331 | Gallocatechin + Na + 2H+ | 21 | 25 | 15 |
| 333.1 | 333 | Gallocatechin + Na + 4H+ | 13 | 7 | 2 |
| 335.1 | 335 | Gallocatechin + 2Na-(OH−) | -- | 6 | -- |
| 335.1 | 335 | Gallocatechin + Na + 6H+ | 7 | -- | -- |
| 341.3 | 341 | Isorhamnetin + 2H+ | -- | 15 | -- |
| 353.3 | 353 | Sugar + Galic Acid + Na-2H+ | 7 | 60 | -- |
| 354.3 | 353 | Sugar + Galic Acid + Na-2H+ | -- | -- | 8 |
| 354.3 | 354 | Sugar + Galic Acid + Na + −H+ | -- | 7 | -- |
| 369.2 | 369 | Catechin + SO3 − 1H+ | -- | -- | 100 |
| 370.2 | 370 | Catechin + SO3 | -- | -- | 17 |
| 374.0 | 374 | Fisetinidol + Na + SO3 − 3H+ | 7 | 10 | -- |
| 375.1 | 375 | Fisetinidol + Na + SO3 − 2H+ | 54 | 78 | 20 |
| 381.3 | 381 | Quercetin + SO3 − H+ | 7 | 100 | 44 |
| 391.1 | 391 | Catechin + Na+ SO3 − 2H+ | 17 | 37 | 8 |
| 397.3 | 397 | Isorhamnetin + SO3 + 1H+ | -- | 12 | 82 |
| 398.3 | 398 | Isorhamnetin + SO3 + 2H+ | -- | -- | 15 |
| 399.3 | 399 | Isorhamnetin + SO3 + 3H+ | -- | -- | 6 |
| 407.0 | 409 | Gallocatechin + Na + SO3 | -- | 7 | -- |
| 409.2 | 409 | Gallocatechin + Na + SO3 | 11 | -- | -- |
| 422.4 | Sugar + Galic Acid + 5Na − 2H+ | 7 | -- | -- | |
| 551.1 | 551 | 2 Fisetidin − 3H+ | 22 | 52 | 5 |
| 552.1 | 552 | 2 Fisetidin − 2H+ | -- | 6 | |
| 567.1 | 567 | Fisetinidol + Catechin − 3H+ | 6 | 28 | 3 |
| 601.3 | 601 | 2 Catechin + Na − 2H+ | 44 | -- | -- |
| 602.3 | 602 | 2 Catechin + Na − H+ | 12 | -- | -- |
| 703.2 | 703 | Quercetin + Fisetinidol + 1H++SO3 | 18 | -- | -- |
| 727.1 | 727 | 2 Sugar + 2 Galolyl + SO3 − 1H+ − OH− | -- | 12 | -- |
| 743.1 | 744 | 2 Sugar + 2 Galolyl + SO3 − 1H+ | -- | 8 | -- |
| 889.3 | 889 | 3 Catechin − 4H+ | 20 | -- | -- |
| Nomenclature | Extraction Raw Material | E′ Max (MPa) | Temperature Tan (δ) (°C) | Temperature E′ Max (°C) | ∆E′ (MPa) | |
|---|---|---|---|---|---|---|
| PB (%) | EB (%) | |||||
| PEB1 | 50 | 50 | 2208 | 41.6 | 46.6 | 1568 |
| PEB2 | 60 | 40 | 2000 | 42.8 | 45.5 | 1498 |
| PEB3 | 70 | 30 | 1802 | 50.7 | 54.7 | 1397 |
| PEB4 | 80 | 20 | 1954 | 45.9 | 47.6 | 1440 |
| PB | 100 | 0 | 2126 | 45.9 | 51.9 | 1511 |
| EB | 0 | 100 | 1610 | 54.1 | 55.7 | 1265 |
| Extract Sample | GlyoxaL (%) on Extract Dry Basis | Density (kg/m3) | Dry IB Strength (MPa) |
|---|---|---|---|
| PB | 10 | 641 ± 15 | 0.33 ± 0.02 |
| PBE1 | 10 | 683 ± 13 | 0.31 ± 0.02 |
| EN 312 [22] Requirement (type P1) | ≥0.28 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, J.; Escobar-Avello, D.; Fuentealba, C.; Cabrera-Barjas, G.; González-Álvarez, J.; Martins, J.M.; Carvalho, L.H. Forest by-Product Valorization: Pilot-Scale Pinus radiata and Eucalyptus globulus Bark Mixture Extraction. Forests 2023, 14, 895. https://doi.org/10.3390/f14050895
Santos J, Escobar-Avello D, Fuentealba C, Cabrera-Barjas G, González-Álvarez J, Martins JM, Carvalho LH. Forest by-Product Valorization: Pilot-Scale Pinus radiata and Eucalyptus globulus Bark Mixture Extraction. Forests. 2023; 14(5):895. https://doi.org/10.3390/f14050895
Chicago/Turabian StyleSantos, Jorge, Danilo Escobar-Avello, Cecilia Fuentealba, Gustavo Cabrera-Barjas, Julia González-Álvarez, Jorge M. Martins, and Luisa H. Carvalho. 2023. "Forest by-Product Valorization: Pilot-Scale Pinus radiata and Eucalyptus globulus Bark Mixture Extraction" Forests 14, no. 5: 895. https://doi.org/10.3390/f14050895
APA StyleSantos, J., Escobar-Avello, D., Fuentealba, C., Cabrera-Barjas, G., González-Álvarez, J., Martins, J. M., & Carvalho, L. H. (2023). Forest by-Product Valorization: Pilot-Scale Pinus radiata and Eucalyptus globulus Bark Mixture Extraction. Forests, 14(5), 895. https://doi.org/10.3390/f14050895











