Effects of Climate on Variation of Soil Organic Carbon and Alkali-Hydrolyzed Nitrogen in Subtropical Forests: A Case Study of Zhejiang Province, China
Abstract
:1. Introduction
2. Materials and Methods
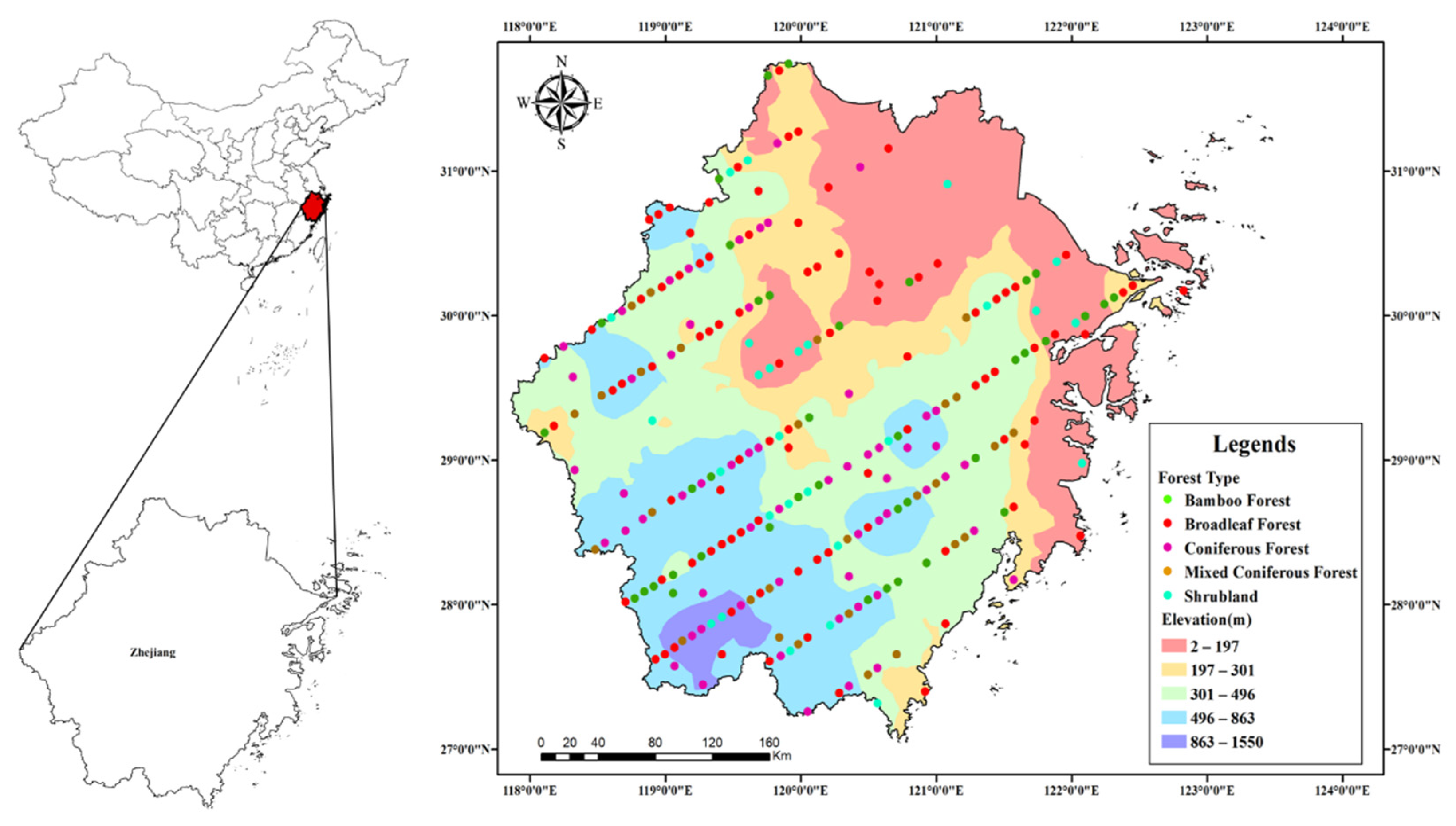
2.1. Description of Study Area
2.2. Sample Plots and Data Collection
2.3. Sample Processing and Analysis
2.4. Data Analysis
3. Results
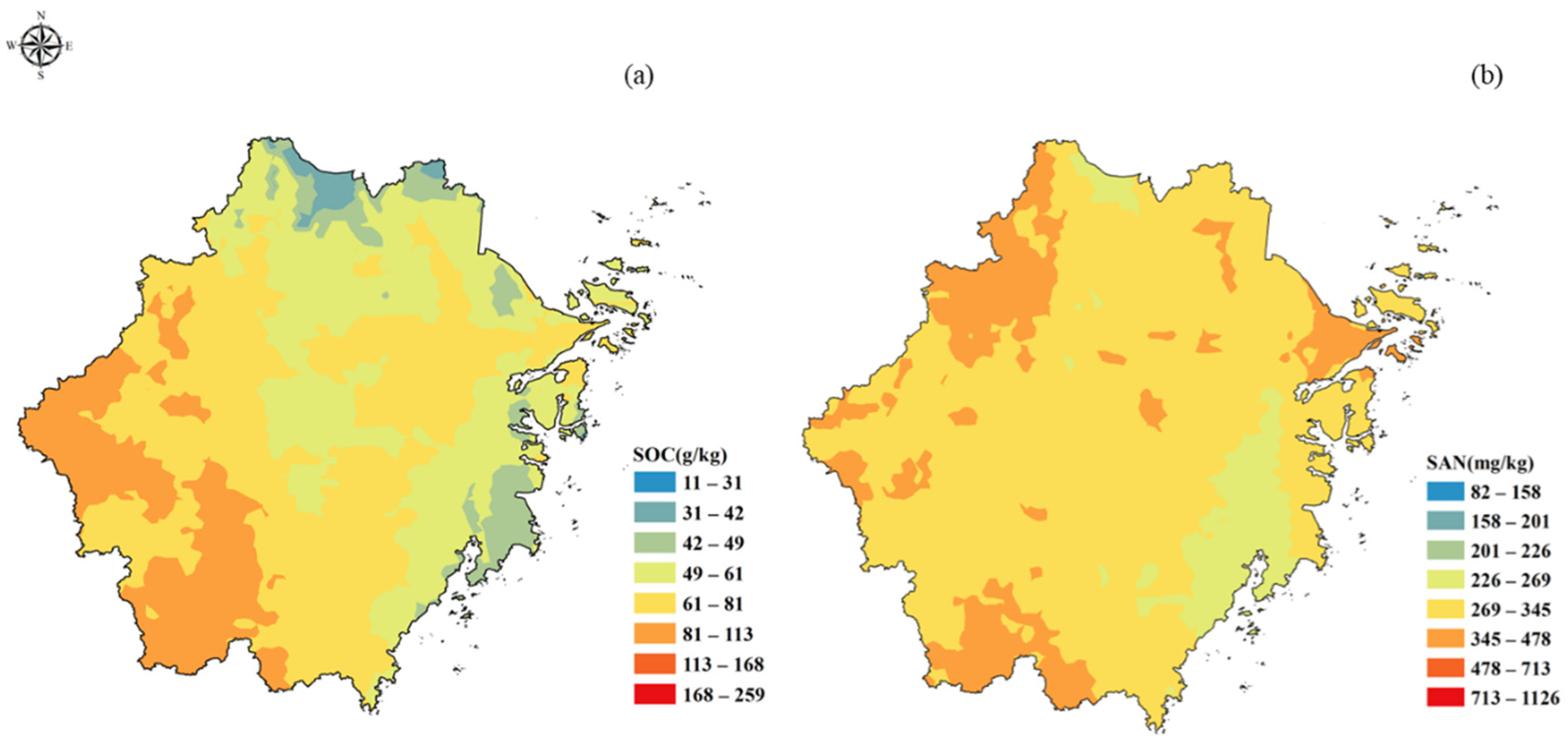
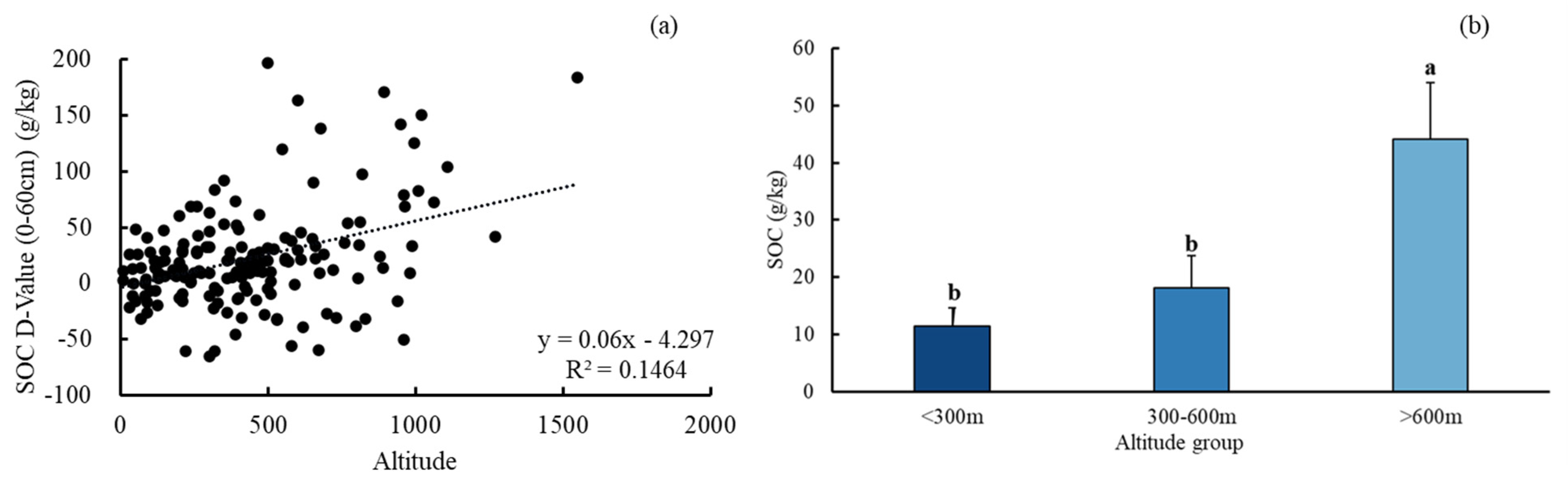
3.1. The Distribution of SOC and SAN in Subtropical Forests in Zhejiang Province
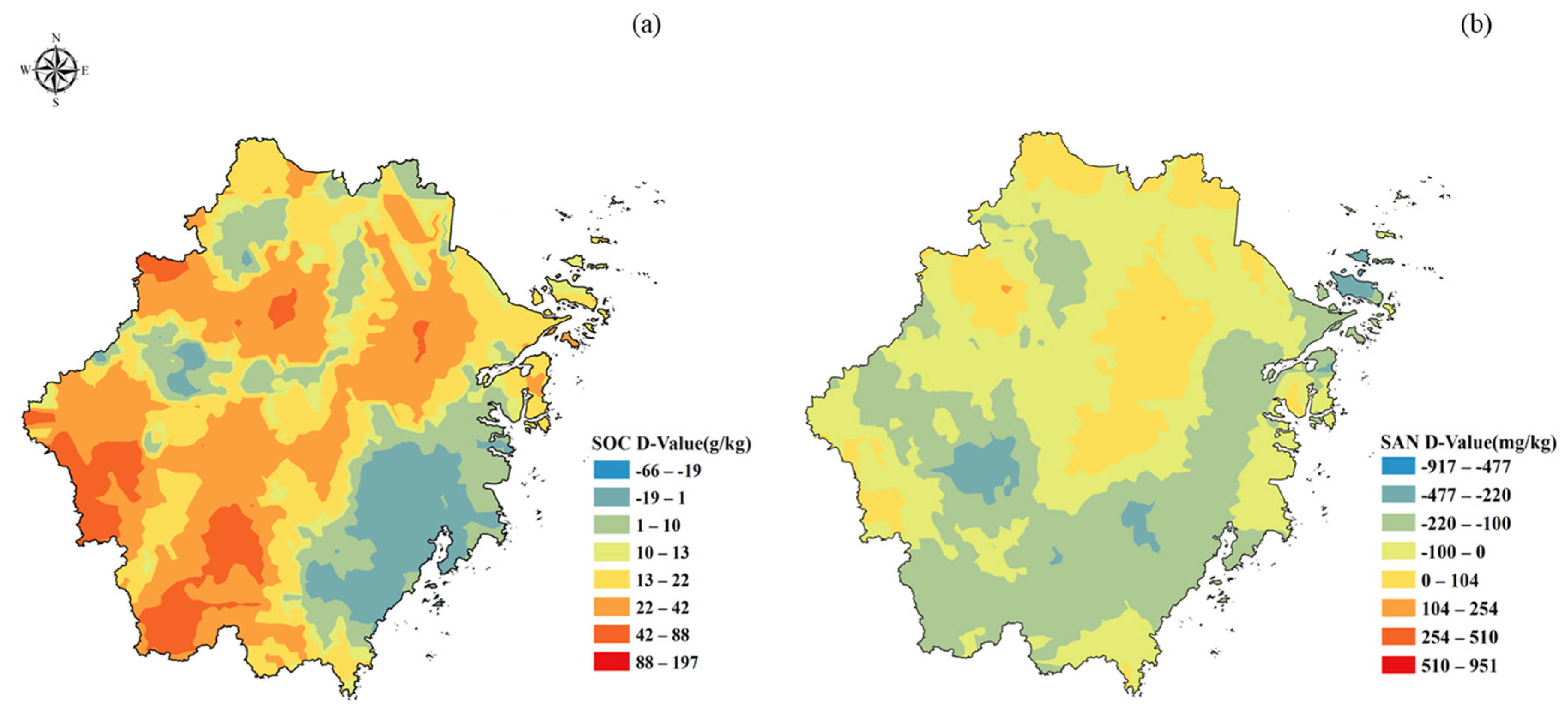
3.2. Variation Characteristics of SOC and SAN in Subtropical Forests
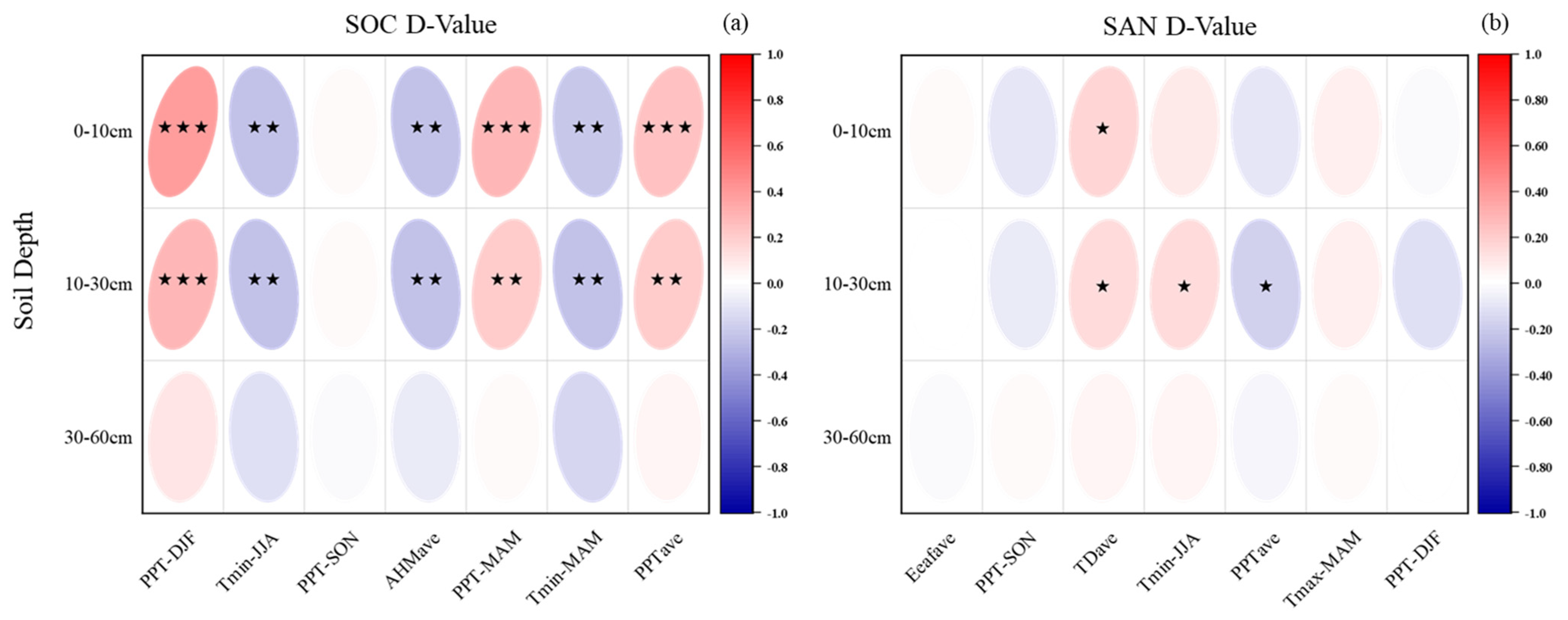
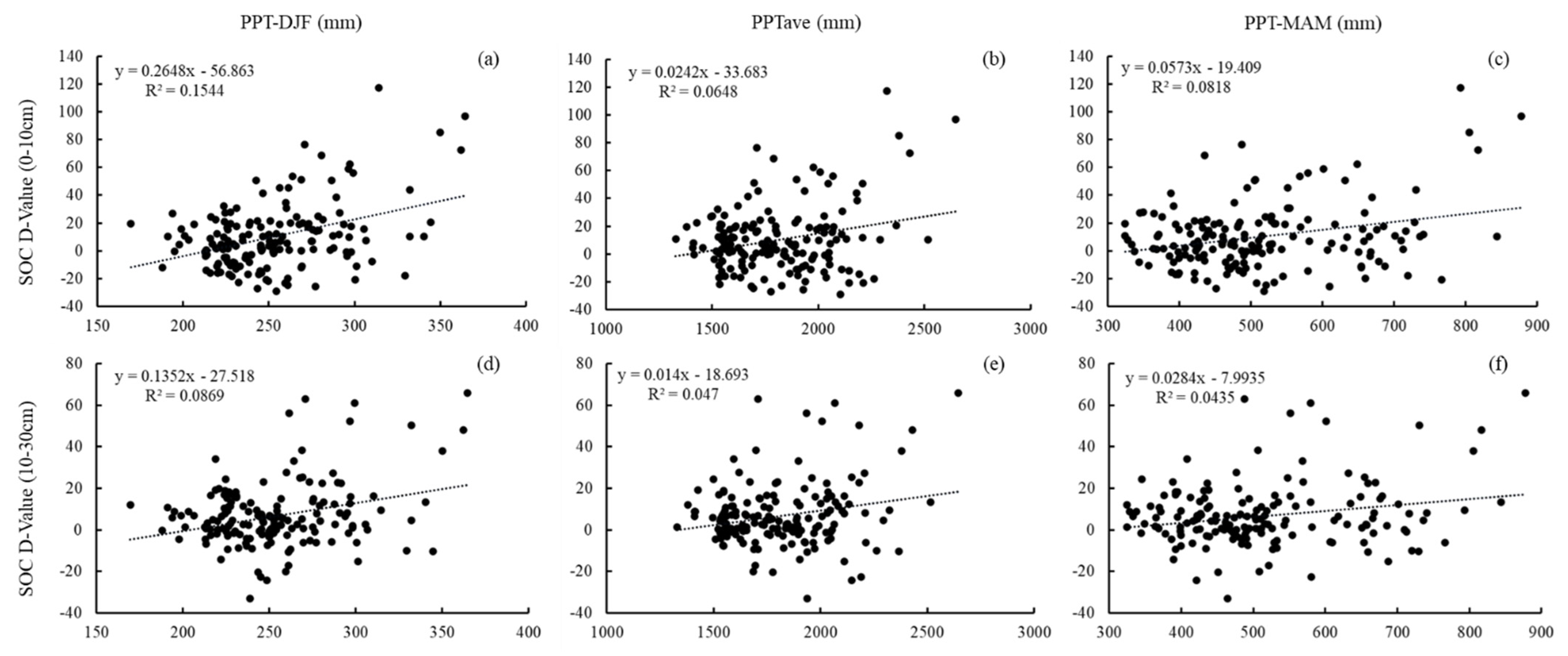
3.3. Effects of Climate Indicators on SOC and SAN Variation Characteristics in Subtropical Forests
4. Discussion
4.1. Distribution of SOC and SAN Variations in the Subtropical Forest System in the Zhejiang Province
4.2. Responses of SOC and SAN to Climate Factors in Subtropical Forests
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Annex I: Glossary. In Global Warming of 1.5 °C; Masson-Delmotte, V., Zhai, P.M., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., Moufou-ma-Okia, W., Péan, C., Pidcock, R., et al., Eds.; An IPCC Special Report on the Impacts of Global Warming of 1.5 °C Above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to Thethreat of Climate Change, Sustainable Development, and Efforts to Era-Dicate Poverty; World Meteorological Organization: Geneva, Switzerland, 2018; pp. 541–562. [Google Scholar]
- Yang, Y.; Shi, Y.; Sun, W.; Chang, J.; Zhu, J.; Chen, L.; Wang, X.; Guo, Y.; Zhang, H.; Yu, L.; et al. Terrestrial carbon sinks in China and around the world and their contribution to carbon neutrality. Sci. China Life Sci. 2022, 65, 861–895. [Google Scholar] [CrossRef]
- Batjes, N.H. Total carbon and nitrogen in the soils of the world. Eur. J. Soil Sci. 2014, 65, 10–21. [Google Scholar] [CrossRef]
- Morisada, K.; Ono, K.; Kanomata, H. Organic carbon stock in forest soils in Japan. Geoderma 2004, 119, 21–32. [Google Scholar] [CrossRef]
- Yang, Y.; Mohammat, A.; Feng, J.; Zhou, R.; Fang, J. Storage, patterns and environmental controls of soil organic carbon in China. Biogeochemistry 2007, 84, 131–141. [Google Scholar] [CrossRef]
- Martin, M.P.; Wattenbach, M.; Smith, P.; Meersmans, J.; Jolivet, C.; Boulonne, L.; Arrouays, D. Spatial distribution of soil organic carbon stocks in France. Biogeosciences 2011, 8, 1053–1065. [Google Scholar] [CrossRef] [Green Version]
- Viscarra Rossel, R.A.; Webster, R.; Bui, E.N.; Baldock, J.A. Baseline map of organic carbon in Australian soil to support national carbon accounting and monitoring under climate change. Glob. Change Biol. 2014, 20, 2953–2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Martín, J.A.; Álvaro-Fuentes, J.; Gonzalo, J.; Gil, C.; Ramos-Miras, J.J.; Grau Corbí, J.M.; Boluda, R. Assessment of the soil organic carbon stock in Spain. Geoderma 2016, 264, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Scharlemann, J.P.W.; Tanner, E.V.J.; Hiederer, R.; Kapos, V. Global soil carbon: Understanding and managing the largest terrestrial carbon pool. Carbon Manag. 2014, 5, 81–91. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Freeman, C.; Ostle, N.J. Microbial contributions to climate change through carbon cycle feedbacks. ISME J. 2008, 2, 805–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waldrop, M.P.; Firestone, M.K. Response of microbial community composition and function to soil climate change. Microb. Ecol. 2006, 52, 716–724. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Chen, H.; Liu, H.; Nie, M. Enzymic moderations of bacterial and fungal communities on short- and long-term warming impacts on soil organic carbon. Sci. Total Environ. 2022, 804, 150197. [Google Scholar] [CrossRef] [PubMed]
- Rui, J.; Li, J.; Wang, S.; An, J.; Liu, W.T.; Lin, Q.; Yang, Y.; He, Z.; Li, X. Responses of bacterial communities to simulated climate changes in alpine meadow soil of the Qinghai-Tibet plateau. Appl. Environ. Microbiol. 2015, 81, 6070–6077. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Aluko, O.O.; Yuan, G.; Li, J.; Liu, H. The responses of soil organic carbon and total nitrogen to chemical nitrogen fertilizers reduction base on a meta-analysis. Sci. Rep. 2022, 12, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Mo, X.; Meng, W.; Li, H.; Xu, W.; Huang, Z. Optimization of nitrogen, water and salinity for maximizing soil organic carbon in coastal wetlands. Glob. Ecol. Conserv. 2022, 36, e02146. [Google Scholar] [CrossRef]
- Yu, G.; Chen, Z.; Piao, S.; Peng, C.; Ciais, P.; Wang, Q.; Lia, X.; Zhu, X. High carbon dioxide uptake by subtropical forest ecosystems in the East Asian monsoon region. Proc. Natl. Acad. Sci. USA 2014, 111, 4910–4915. [Google Scholar] [CrossRef] [Green Version]
- Dong, J.; Zhou, K.; Jiang, P.; Wu, J.; Fu, W. Revealing horizontal and vertical variation of soil organic carbon, soil total nitrogen and C:N ratio in subtropical forests of southeastern China. J. Environ. Manag. 2021, 289, 112483. [Google Scholar] [CrossRef]
- Dai, W.; Fu, W.; Jiang, P.; Zhao, K.; Li, Y.; Tao, J. Spatial pattern of carbon stocks in forest ecosystems of a typical subtropical region of southeastern China. For. Ecol. Manag. 2018, 409, 288–297. [Google Scholar] [CrossRef]
- Fang, H.; Ji, B.; Deng, X.; Ying, J.; Zhou, G.; Shi, Y.; Xu, L.; Tao, J.; Zhou, Y.; Li, C.; et al. Effects of topographic factors and aboveground vegetation carbon stocks on soil organic carbon in Moso bamboo forests. Plant Soil 2018, 433, 363–376. [Google Scholar] [CrossRef]
- Wang, T.; Wang, G.; Innes, J.L.; Seely, B.; Chen, B. ClimateAP: An application for dynamic local downscaling of historical and future climate data in Asia Pacific. Front. Agric. Sci. Eng. 2017, 4, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Huang, X.; Zhou, Y.; Zhang, J.; Zhang, X. Discrepancies in karst soil organic carbon in southwest china for different land use patterns: A case study of Guizhou Province. Int. J. Environ. Res. Public Health 2019, 16, 4199. [Google Scholar] [CrossRef] [Green Version]
- Nóbrega, G.N.; Ferreira, T.O.; Artur, A.G.; de Mendonça, E.S.; Raimundo, R.A.; Teixeira, A.S.; Otero, X.L. Evaluation of methods for quantifying organic carbon in mangrove soils from semi-arid region. J. Soils Sediments 2015, 15, 282–291. [Google Scholar] [CrossRef]
- Agricultural Chemistry Committee of China. Determination of soil organic matter. In Soil and Agricultural Chemistry Analysis; Agricultural Chemistry Committee of China: Beijing, China, 2000; pp. 30–34. [Google Scholar]
- Mirzaee, S.; Ghorbani-Dashtaki, S.; Mohammadi, J.; Asadi, H.; Asadzadeh, F. Spatial variability of soil organic matter using remote sensing data. Catena 2016, 145, 118–127. [Google Scholar] [CrossRef]
- Krivoruchko, K.; Gribov, A. Evaluation of empirical Bayesian kriging. Spat. Stat. 2019, 32, 100368. [Google Scholar] [CrossRef]
- Sivanandam, P.; Lucieer, A. Tree Detection and Species Classification in a Mixed Species Forest Using Unoccupied Aircraft System (UAS) RGB and Multispectral Imagery. Remote Sens. 2022, 14, 4963. [Google Scholar] [CrossRef]
- Wang, T.; Kang, F.; Cheng, X.; Han, H.; Bai, Y.; Ma, J. Spatial variability of organic carbon and total nitrogen in the soils of a subalpine forested catchment at Mt. Taiyue, China. Catena 2017, 155, 41–52. [Google Scholar] [CrossRef]
- Podwojewski, P.; Poulenard, J.; Zambrana, T.; Hofstede, R. Overgrazing Effects on Vegetation Cover and Properties of Volcanic Ash Soil in the Páramo of Llangahua and La Esperanza (Tungurahua, Ecuador). Soil Use Manag. 2002, 18, 45–55. [Google Scholar] [CrossRef]
- Ao, L.; Zhao, M.; Li, X.; Sun, G. Different Urban Forest Tree Species Affect the Assembly of the Soil Bacterial and Fungal Community. Microb. Ecol. 2022, 83, 447–458. [Google Scholar] [CrossRef]
- Sui, X.; Li, M.; Frey, B.; Wang, M.; Weng, X.; Wang, X.; Chen, F.; Li, X.; Du, Z.; Yang, L.; et al. Climax forest has a higher soil bacterial diversity but lower soil nutrient contents than degraded forests in temperate northern China. Ecol. Evol. 2022, 12, e9535. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, D.; Liang, G.; Qiu, Q.; Liu, J.; Zhou, G.; Liu, S.; Chu, G.; Yan, J. Effects of precipitation on soil organic carbon fractions in three subtropical forests in southern China. J. Plant Ecol. 2015, 9, 10–19. [Google Scholar] [CrossRef] [Green Version]
- Mao, F.; Du, H.; Zhou, G.; Zheng, J.; Li, X.; Xu, Y.; Huang, Z.; Yin, S. Simulated net ecosystem productivity of subtropical forests and its response to climate change in Zhejiang Province, China. Sci. Total Environ. 2022, 838, 155993. [Google Scholar] [CrossRef]
- Davidson, E.A.; Verchot, L.V.; Henrique Cattânio, J.; Ackerman, I.L.; Carvalho, J.E.M. Effects of soil water content on soil respiration in forests and cattle pastures of eastern Amazonia. Biogeochemistry 2000, 48, 53–69. [Google Scholar] [CrossRef]
- Walker, T.W.N.; Kaiser, C.; Strasser, F.; Herbold, C.W.; Leblans, N.I.W.; Woebken, D.; Janssens, I.A.; Sigurdsson, B.D.; Richter, A. Microbial temperature sensitivity and biomass change explain soil carbon loss with warming. Nat. Clim. Change 2018, 8, 885–889. [Google Scholar] [CrossRef]
- Cao, H.; Chen, R.; Wang, L.; Jiang, L.; Yang, F.; Zheng, S.; Wang, G.; Lin, X. Soil pH, total phosphorus, climate and distance are the major factors influencing microbial activity at a regional spatial scale. Sci. Rep. 2016, 6, 25815. [Google Scholar] [CrossRef] [Green Version]
- Klimek, B. Scots pine roots modify the short-term effects of temperature and moisture on soil bacteria and fungi. Appl. Ecol. Environ. Res. 2013, 11, 173–188. [Google Scholar] [CrossRef]
- Perreault, L.; Forrester, J.A.; Wurzburger, N.; Mladenoff, D.J. Emergent properties of downed woody debris in canopy gaps: A response of the soil ecosystem to manipulation of forest structure. Soil Biol. Biochem. 2020, 151, 108053. [Google Scholar] [CrossRef]
- Kravchenko Irina, K.; Tikhonova Ekaterina, N.; Ulanova Ruzalia, V.; Menko Ekaterina, V.; Sukhacheva Marina, V. Effect of temperature on litter decomposition, soil microbial community structure and biomass in a mixed-wood forest in European Russia. Curr. Sci. 2019, 116, 765–772. [Google Scholar] [CrossRef]
- Wang, Q.; He, N.; Liu, Y.; Li, M.; Xu, L. Strong pulse effects of precipitation events on soil microbial respiration in temperate forests. Geoderma 2016, 275, 67–73. [Google Scholar] [CrossRef]
| Full Name | Abbreviation |
|---|---|
| mean annual temperature (°C), | MAT |
| mean warmest month temperature (°C) | MWMT |
| mean coldest month temperature (°C) | MCMT |
| temperature difference between MWMT and MCMT, or continentality (°C), | TD |
| mean annual precipitation (mm), | PPT |
| annual heat: moisture index (MAT + 10)/(PPT/1000) | AHM |
| extreme minimum temperature over 30 years | EMT |
| Hargreaves reference evaporation | Eref |
| Hargreaves climatic moisture deficit | CMD |
| mean temperature (°C) | Tave |
| mean maximum temperature (°C) | Tmax |
| mean minimum temperature (°C) | Tmin |
| December (from the previous year for an individual year), January, and February | DJF |
| March, April, and May | MAM |
| June, July, and August | JJA |
| September, October, and November | SON |
| Average value | ave |
| Depth (cm) | Number | Mean | Median | Maximum | Minimum | SD | CV (%) | |
|---|---|---|---|---|---|---|---|---|
| SOC content | 0–10 | 244 | 35.94 | 30.40 | 132.99 | 1.76 | 22.58 | 62.82 |
| 10–30 | 244 | 20.97 | 17.44 | 92.67 | 0.67 | 15.25 | 72.74 | |
| 30–60 | 244 | 13.71 | 10.95 | 70.11 | 0.37 | 11.15 | 81.28 | |
| SAN content | 0–10 | 244 | 145.42 | 118.86 | 606.23 | 17.39 | 91.32 | 62.79 |
| 10–30 | 244 | 101.61 | 82.17 | 554.20 | 13.94 | 75.16 | 73.97 | |
| 30–60 | 244 | 74.91 | 62.80 | 328.71 | 10.46 | 49.12 | 65.57 |
| Depth (cm) | Number | Mean | Median | Maximum | Minimum | SD | |
|---|---|---|---|---|---|---|---|
| SOC difference | 0–10 | 165 | 10.00 | 6.83 | 117.53 | −29.17 | 23.71 |
| 10–30 | 165 | 6.61 | 4.70 | 65.75 | −52.67 | 16.13 | |
| 30–60 | 165 | 4.98 | 3.11 | 57.93 | −48.12 | 12.84 | |
| SAN difference | 0–10 | 244 | −58.43 | −55.37 | 405.03 | −416.94 | 107.25 |
| 10–30 | 244 | −29.16 | −29.62 | 501.84 | −357.19 | 99.32 | |
| 30–60 | 244 | −1.41 | −2.67 | 270.33 | −324.49 | 61.98 |
| Depth (cm) | ★★★★★★★ | ★★★★★★ | ★★★★★ | ★★★★ | ★★★ | ★★ | ★ | |
|---|---|---|---|---|---|---|---|---|
| SOC difference | 0–60 | PPT-DJF | Tmin-JJA | PPT-SON | AHMave | PPT-MAM | Tmin-MAM | PPTave |
| SAN difference | 0–60 | Erefav | PPT-SON | TDave | Tmin-JJA | PPTave | Tmax-MAM | PPT-DJF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, X.; Zhou, T.; Liu, S.; Sun, X.; Zhou, Y.; Xu, L.; Xie, B.; Ying, J.; Shi, Y. Effects of Climate on Variation of Soil Organic Carbon and Alkali-Hydrolyzed Nitrogen in Subtropical Forests: A Case Study of Zhejiang Province, China. Forests 2023, 14, 914. https://doi.org/10.3390/f14050914
Cheng X, Zhou T, Liu S, Sun X, Zhou Y, Xu L, Xie B, Ying J, Shi Y. Effects of Climate on Variation of Soil Organic Carbon and Alkali-Hydrolyzed Nitrogen in Subtropical Forests: A Case Study of Zhejiang Province, China. Forests. 2023; 14(5):914. https://doi.org/10.3390/f14050914
Chicago/Turabian StyleCheng, Xuekun, Tao Zhou, Shuhan Liu, Xiaobo Sun, Yufeng Zhou, Lin Xu, Binglou Xie, Jianping Ying, and Yongjun Shi. 2023. "Effects of Climate on Variation of Soil Organic Carbon and Alkali-Hydrolyzed Nitrogen in Subtropical Forests: A Case Study of Zhejiang Province, China" Forests 14, no. 5: 914. https://doi.org/10.3390/f14050914




