1. Introduction
Nonlinear regression is part of statistical analysis methods to curvilinearly fit the observed data to follow a function of indicating predictor(s). Nonlinear regression takes a
y~
f(
x,
β) nonlinear function as the statistical model to attribute the corresponding observed response variable (
y) to the indicating predictor (
x), and foresters frequently employ it to analyze many natural behaviors’ inter-dependency in the forest and environment. Nonlinear regression is reliable for analyzing a tree’s xylem growth curve (i.e., fiber length, cell-wall thickness, and microfibril angle) [
1,
2] and determining its juvenile–mature periphery. Construction engineers also rely on nonlinear regression to empirically justify the service environment effects on the structural properties of a wooden building member [
3,
4], estimating its residual service life [
5,
6,
7], and even determining the time interval of people’s comfort in an environment that is neighbor to forest stands [
8]. A material’s strength test’s load–displacement curve resembles a curvilinear shape. Forest engineers apply nonlinear regression to develop an allometric equation in forest inventory to estimate biomass, carbon stocks, and wood production [
9,
10,
11,
12,
13,
14]. However, it is dispiriting that current remote sensing and sensors still insist on advocating an algorithm following the linear correlation between several measured predicting and dependent variables to estimate the aboveground biomass [
15]. This study developed alternative calculation methods [
16] related to nonlinear regression.
Tree trunk biomass is an essential parameter in estimating forest productivity [
17], growth [
18], and carbon sequestration [
19]. A stem’s cross-sectional shape and size change with the tree’s growth. An accurate description of the dynamic interrelation is necessary for describing a tree’s ability to absorb carbon from the atmosphere and store it in its trunks. Forest science traditionally simplifies a tree’s basal area and log cross-section as a circular shape [
20]. It has been widely used in forest inventory, annual cutting allowance determination, the wood market, and carbon sequestration assessments. Under only genetic influence during the tree growth periods, a healthy tree’s cambium cell differentiation to produce xylem proceeds at a similar rate along the tree circumference; thus, the annual-ring shape and tree trunk cross-section should be circular [
21]. The deviation from the circular form of a stem’s cross-sectional shape indicates the effect of external factors, such as local climate, environmental disturbance (e.g., light intensity and direction, water availability, wind force, and snow mound mass), biogeophysical aspects (e.g., ground position and trunk slope), competition, and succession during tree growth. Several standard 2D geometric shapes (ellipse, superellipse, or ovoid) may resemble these varied cross-sectional shapes. The peripheral circumference of a log’s cross-section can be digitized, and the data plot forms a curve that a mathematical equation can fit.
Digitization and curve fitting may also be run for each annual tree ring and the sapwood–heartwood transition. Wood is produced by many plants, including gymnosperms and angiosperms, and the different growth rates in each season (i.e., spring–summer–autumn–winter in temperate regions or rainy–drought in the tropical region) tag early and late wood cell gradation. Gradation creates annual tree rings. Dendrochronology reads annual tree-ring variations to analyze a tree’s archaeological and historical structures for reconstructing past climates and significantly contributes to environmental science development [
22]. Tree-ring chronologies, which may contain dimensional variations and circular shape deviations, provide high-frequency signals [
23] that record a tree’s responses to the silvicultural system [
24,
25] and its habitat’s environmental condition history (i.e., climate [
26,
27,
28,
29,
30,
31,
32,
33,
34], pollutants [
35,
36], and water–heat stress [
37]) during its growing periods yielding the xylem.
The outer part of the wood that lies between the cambium and the heartwood is called sapwood. Sapwood has living parenchymal cells (including vertically oriented longitudinal parenchymal cells and horizontally oriented ray cells) and is physiologically active in storing foods. About 10% of sapwood cells are alive on average, and they play a role in transporting water and minerals to the tree crown; therefore, they contain more water and are more permeable than heartwood. In addition to cambial age and axial height [
38], tree inclination [
39] affects the sapwood’s anatomical features. Heartwood formation results in the death of parenchymal cells. When the xylem becomes older, the cells die and produce heartwood, which is no longer involved in the physiological process but continues providing mechanical support. Heartwood tissue generally produces a specific color formation [
40] because of the deposits’ enhancement of staining extractive content. Contrast color indicates higher extractive content, significantly improving the wood’s natural durability. The degradation and incrustation of pores in the xylem vascular elements with extractive substances accompany heartwood formation, significantly reduce wood permeability, lead to water transport cessation, and prevent wood-decaying organism penetration [
41,
42,
43]. Scented extractive substance contents in heartwood, namely, terpenoids (i.e., monoterpenoids and sesquiterpenes), are synthesized, accumulated, and shaped for fragrance formation [
44]. Although the circumference of the sapwood–heartwood transition periphery does not follow a certain annual tree ring and may form an irregular pattern, some idealization to become a standard geometric section may be possible to be promoted.
This study discusses the theoretical basis and application of nonlinear regression and creates alternative computation tools [
16] to estimate a tree stem’s cross-sectional shape and its sapwood–heartwood transition by transforming rectangular coordinates (
x,
y) of the raw data points’ positions into polar coordinates (
r,
θ). Some 2D closed curved geometric shapes, such as circles, ellipses, or superellipses, may fit a log’s cross-sectional shape and sapwood–heartwood transition. The mathematical models promoted in this study may become a starting point to develop an algorithm to formulate the best-fit curve of a tree’s cross-sectional shape at each growth stage. Therefore, the signal recorded in the annual tree ring can be read more precisely by measuring its deviation from the standard geometric shape.
2. Mathematical Basis
The function idealizes the dependency of a quantity variation on another quantity. A function from the domain (x-set) to the codomain (y-set) connects each element of x to exactly an element of y. The f, g, and h letters may represent functions, and f(x), g(x), and h(x) represent the function value of the x-element. The set of all pairs of (x, f(x)) uniquely describes a function. Each point has a pair of coordinates, and all points are plotted in a rectangular (Cartesian) diagram to form a graph of the function. A rectangular diagram plots a point position as a linear distance from the origin about two or three mutually perpendicular axes. The origin is the center of the coordinates where the axes intersect {(0, 0) or (0, 0, 0)}, and a point plotted in the rectangular diagram is defined as the coordinate {(x, y) or (x, y, z)}. Each set of coordinates uniquely establishes the position of a point in a 2D plane or 3D space. Rectangular diagrams are well-known and used in many applications to graph a function.
Several functions (including interpolation of circular, ellipse, superellipse, or ovoid) may be more effectively expressed in nonlinear coordinate systems such as a polar than in a rectangular diagram. In a rectangular diagram, each x-point originating from the domain corresponds to two y-points on the graph of a circle, ellipse, superellipse, or ovoid; thus, they are not eligible for a function’s requirements. This non-function phenomenon prohibits the nonlinear regression application for fitting the observed data; therefore, this study suggests transforming the rectangular coordinate system into a polar coordinate system to solve these specific cases. The curve fitting of the circumference of a log and the leaves’ shape require nonlinear regression analysis methods at polar diagrams that consider the transformation of rectangular coordinate (x, y) position points into polar coordinates (r, θ). In polar coordinates, a point position is represented by a straight distance from the origin (0, 0) and an angle to the positive horizontal x-axis. The straight distance from the origin is the radius (r), and the counterclockwise rotation angle centered from the origin is θ.
In contrast to rectangular diagrams, the polar coordinate system allows an infinite number of sets of coordinates to describe any point (for example, the locations of points (r, θ), (r, θ + 2π), and (r, θ + 4π) are the same). The angle value (θ) is positive (+) to find the coordinates of the angle that rotated in the counterclockwise direction and a negative angle value (−) for the clockwise one. The radial coordinate (r) can also be positive or negative. Negative radial coordinates (−r) indicate that the angular coordinates are in the opposite quadrant of the destination point. A negative radius moves the point back to the destination point. Data transformation from the (x, y) rectangular coordinate system into the (r, θ) polar coordinate system converts the non-function form of a circle, ellipse, superellipse, or ovoid; then, may result in a function that could be well-fitted using nonlinear regression.
2.1. Circle
Equation (1) represents the standard formula of a circular shape with center (
p,
q) and radius
r =
a in a Cartesian (rectangular) coordinate system. Equation (1) can be rearranged to become Equation (2), then transformed into Equation (3) to build the polar form. In its polar form, the circle curve is drawn from the
r =
f(
θ) function for 0 <
θ < 2π, where the
r cos
θ, r sin
θ, and
r2 substitute
x,
y, and
x2+
y2, respectively. Some algebra steps rearrange Equation (3) to become Equation (4), which one can solve for
r to become Equation (5):
If the origin position (0, 0) is inside the scanned log’s cross-section picture periphery and the radius (
r) value is positive, then the positive root term at Equation (5) is a more suitable solution for the nonlinear regression model than the negative ones. Equation (6) is a nonlinear regression model to fit the circle’s radius with a center (
p,
q) and radius
a, where
ri is the response variable,
θi is the predictor variable, and
εi is the residual. Equation (7) shows the circle’s center (
p,
q) lies at the (
r0,
θ0) polar coordinate, where
p =
r0 cos
θ0 and
q =
r0 sin
θ0. Equation (8) simplifies Equation (7):
Iteration methods developed by the Gauss–Newton [
45] or Levenberg–Marquardt [
46,
47] algorithms minimize the residual mean square to estimate the coefficient of regression parameters (
a,
ro, and
θo). The residual mean square, synonymous with the mean squared error (MSE), is the loss function that works acceptably for most regression cases. If
ri is the observed data point and
is its estimated value, the residual square of the nonlinear regression model (Equation (6)) is
, and its MSE is Equation (9). Iterations of all parameter values are applied to find the least MSE. The initial parameter values, the starting point in the iteration process, should be carefully chosen because the MSE curve may have several peaks and valleys. The visual assessment of the data plot may significantly contribute to the well-chosen initial parameter values, which have a higher probability of resulting in the least MSE in the global range than those at the local valley.
2.2. Ellipse
The ellipse shape model is more flexible than the circular model; thus, its goodness of fit is usually improved. If the semi-major axis (
a) and semi-minor axis (
b) of an ellipse are the same values, the ellipse forms a circular shape. The standard formula of an ellipse with semi-major
a, semi-major axis
b, and center at coordinate (
p, q) is presented in Equation (10). We arrange Equation (10) to become Equation (11), then substitute
r cos
θ to
x,
r sin
θ to
y, and
r2 to
x2 +
y2 to create Equation (12). Equation (12) is a quadratic equation; its solution is Equation (13). Equation (14) is another form of Equation (13). The
k parameter is added, followed by the transformation of
and
(Equation (15)) to consider the rotation. The nonlinear curve-fitting model (Equation (16)) following the simplification of Equation (15) is chosen to fit the log’s cross-sectional shape and sapwood–heartwood transition that includes the rotation. Equation (17) is the nonlinear regression of an ellipse model without rotation because the
kπ value is 0 (zero). Like the previous circular model, the nonlinear estimation can be employed following the iteration process with the least square loss function to calculate the best fit estimated parameters (
a,
b,
ro,
θo, and
k).
2.3. Superellipse
A superellipse, introduced by Gabriel Lamé [
48], is a closed curved where its plot in a rectangular diagram generally follows Equation (18). The superellipse equation is adaptive, embracing circular, ellipse, square, and rectangular shapes. The step-by-step algebraic procedure in Equations (19)–(20) changes the superellipse curve plot centered at (0, 0) at the rectangular diagram (Equation (18)) to be plotted at the polar diagram (Equation (21)), and then Equation (22) determines the rotated superellipse at a polar diagram. Nonlinear regression can be applied based on the superellipse model (Equations (23) and (24)) following similar procedures with the above circular and ellipse model parameters’ estimation.
2.4. Computer Modelling
2.4.1. Python
Python programming language is a high-level programming software [
49,
50] to develop data science and machine learning created by Guido Van Rossum, a programmer from the Netherlands [
49,
50,
51,
52]. The Python programming language has several elements, such as syntax [
53], the variable of which is a value or data [
54,
55] used in Python programming, data structure [
54,
56], functions in performing data processing [
51], modules consisting of combining functions and programming code, libraries containing data science and machine learning [
57], interpreted [
49,
53,
54], and cross-platform that can support Python on several platforms [
51]. In this study, Python was employed to develop a rotated ellipse graph. Building a program using Python programming language [
58] generally includes structures such as headers, comments, import statements, function definitions, and main programs [
59] as a basis which can later be developed into a special format intended for the program to be created. The general structures of Python code are:
Header or shebang line (optional): on Unix/Linux/Mac platforms, this line tells the shell that the file should be interpreted using the Python interpreter.
Comments (optional): this section provides comments or explanations about the code being written, but Python does not execute it.
Import statement (optional): this section imports modules used in the program.
Function definition (optional): in this section, we can define functions that will be used in the program.
Main program: in this section, we write the main code of the program that will be executed.
2.4.2. Qt
Qt is a cross-platform application development framework that provides a variety of components, tools, and programming languages to build applications [
60]. Qt aims to simplify and accelerate the application development process by offering a consistent and robust framework [
61]. It also focuses on the ease of designing attractive and responsive user interfaces to enhance user experience. In summary, Qt is a versatile application development framework that simplifies the cross-platform development process and designs attractive user interfaces. It offers benefits such as cross-platform capability, attractive user interfaces, high productivity, modular architecture, active community support, and commercial support options [
62].
3. Materials and Methods
A chainsaw-man team cut a
Maesopsis eminii tree from the Arboretum of Forestry and Environment Faculty, IPB University, Bogor, West Java (ID). Then, they cut the tree into several logs. The photographs of the randomly chosen logs’ cross-sections were taken and then scaled into the actual dimension (1:1). The points of the log’s circumference and the sapwood–heartwood transition periphery were digitized and plotted on a rectangular diagram. The data points count ranged from 80 to 150 for each periphery curve. Equations (25)–(27) transformed every data point’s rectangular coordinate (
x,
y) into a polar coordinate (
r,
θ). Since Microsoft Excel’s “=asin (number)” formula results in the value range of −π/2 ≤
θ ≤ π/2, Equation (28) adjusted the
θ value into its point position at the 1st, 2nd, 3rd, or 4th quadrant; for example, when the cell B2 contains
x, and cell C2 contains
y; Equation (28) is a formula to define the quadrant where the point lays. Equation (29) is the formula to determine the angle (
θ), where cell F2 contains the quadrant resulting from Equation (28), and cell E2 contains “=sin
θ” resulting from Equation (26). The radius (
r) unit is cm, while the angle (
θ) unit is radian.
All raw data points’ polar coordinates (ri, θi) were tabulated on a spreadsheet. Statsoft Statistica 12, a nonlinear estimation of advanced linear/nonlinear models, was employed to estimate the (a, b, ro, θo, and k) parameters in Equations (8), (16), (17) and (24); the loss function is the least square, and the iteration method is Levenberg–Marquardt. We chose the 10−10 maximum convergence criteria, 10,000 maximum iterations, and varied starting values. Visual assessment observed the raw data plot, and the Desmos calculator initially fitted the curve of the data plot. The Desmos graph, which nearly fits the raw data points, contributes to choosing the initial parameter values for the iteration starting values.
The model’s goodness of fit was evaluated using the coefficient of determination (R2), which indicated the proportion of the accounted variance. The higher value of R2 leads to a better-fit model. We also calculated the root of mean square error (RMSE) as the model’s goodness of fit measurement, whose smaller value indicates the better model. The observed and estimated data are plotted with the x = y line. The t-values measure the significant relationship between radius (r) and angle (θ), where the hypothesis represents the non-zero value of estimated parameters (a, b, ro, θo, and k). The null hypothesis concerning this model are H0: a = 0, b = 0, ro = 0, θo = 0, and k = 0, while the alternative hypothesis H1: a ≠ 0, b ≠ 0, ro ≠ 0, θo ≠ 0, and k ≠ 0. Since we determined the 95% confidence level, the null hypothesis is rejected, and the alternative hypothesis is accepted if the |t-value| > t0.025 or the p-value < 0.025.
The basic pattern model algorithm transforms the provision of formulas from ellipse formulas into computer models using Python scripts provided by libraries from the Wolfram engine. Variable declaration, initialization, process, and output by applying the formula are below:
![Forests 14 01102 i006 Forests 14 01102 i006]()
4. Results and Discussions
4.1. Circle Model
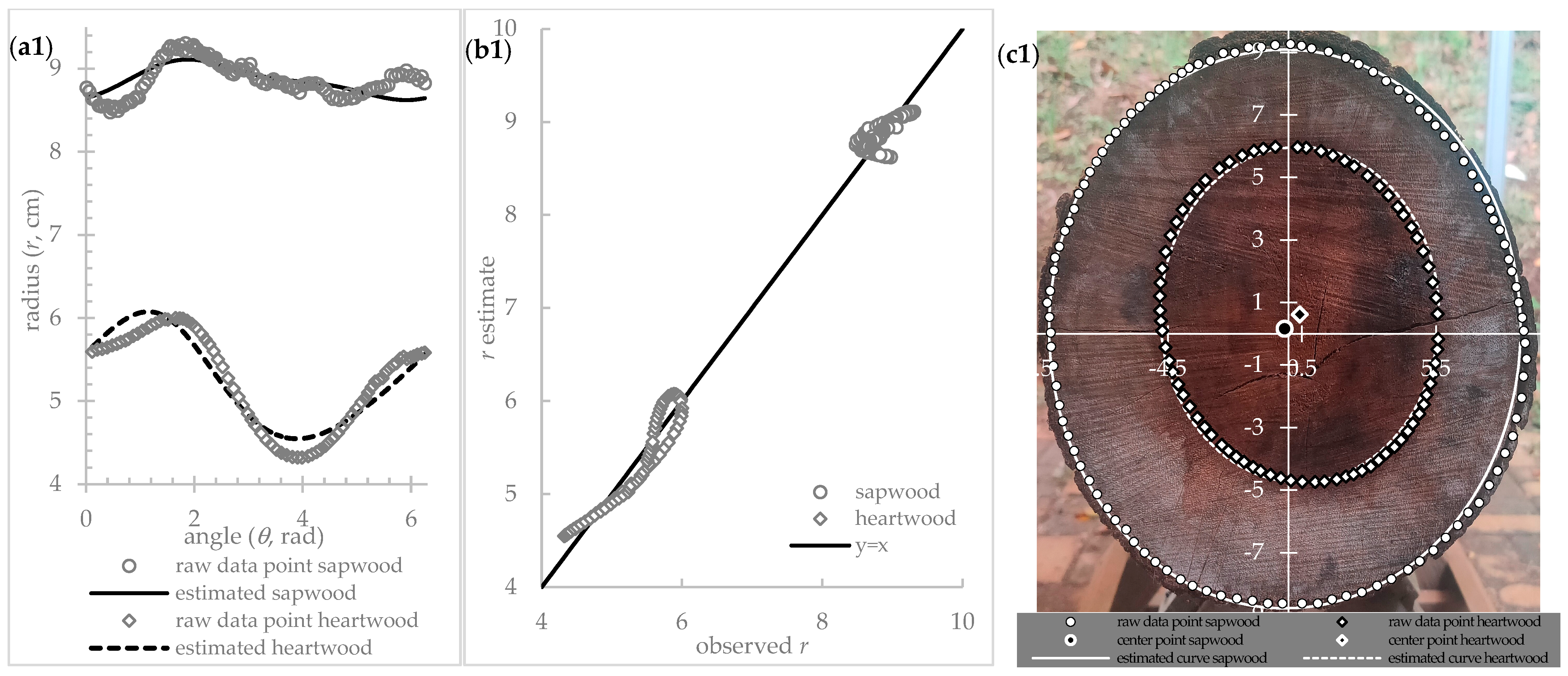
Figure 1 shows a nonlinear regression curve fitting for all pairs of angles (
θ) and radius (
r) data points following the circular model (Equation (8)) with medium to high precision (R
2 = 0.4686 for the log cross-section outer circumference and R
2 = 0.8910 for sapwood–heartwood transition). The least-square loss function, which runs following the Levenberg–Marquardt iteration algorithm [
63,
64], could produce reliable parameter estimates.
Table 1 summarizes the circle curve’s estimated parameters (
a, ro, and
θo) based on the Equation (6) model, which best fits the sapwood and heartwood transition.
Table 2 summarizes the analysis of variance (ANOVA). All estimated parameters have a
p-value less than 0.025, and their |
t-value| is more than
t0.025; thus, the null hypothesis is rejected, and the alternative hypothesis is accepted. The sapwood and heartwood transition geometric shapes are circular and have radius
ri and center coordinates (
ro, θo) (
Figure 1c).
Table 1, b section, shows the significant estimated parameter values which form the curve of circular shapes having 5.28 cm and 8.86 radii. The heartwood center’s polar coordinate (−0.74 cm, −2.17 rad) and the log center’s polar coordinate (0.21 cm, 2.29 rad) are not at the same points, and they are not at the pith position (
Figure 1c). The different center positions indicate that the sapwood–heartwood formation does not follow the tree’s annual ring and strengthen Bamber’s report [
65] that the heartwood formation is the product of the developmental process rather than a deterioration in cell function with age. The sapwood–heartwood color transition is sometimes unclear, and it must be identified based on the fundamental change, such as the death of the vertical parenchyma and the ray cells [
66]. The center shift phenomenon is also consistent with Taylor et al.’s hypothesis that the amount of sapwood converted into heartwood is required to balance the foliar and energy demands [
67].
A circular curve-fitting following the Equation (6) model successfully idealizes the sapwood–heartwood transition (
Figure 1). The high coefficient of the determination value (R
2 = 0.8910) confirms that the high proportion of variable variation in the sapwood–heartwood transition radius (
ri) can be predictable from the angle (
θ). This R
2 value of sapwood–heartwood transition regression is even higher than the R
2 of the log cross-section outer periphery regression. The low MSE value also emphasizes the circular curve’s high goodness of fit (
Table 2, b section). This circular model, therefore, is sufficiently reliable to be advocated for determining the sapwood–heartwood transition. This circular model can estimate the sapwood and heartwood area, and then the heartwood to sapwood proportion can also be calculated.
This study justifies that the shape simplification following the nonlinear circle model can fit the outer log periphery well enough. This developing method provides a precise quantitative number to determine a log’s idealized cross-sectional shape and size.
Table 1, a section, shows the estimated parameter values, which form the best-fit circular curve to the raw data. The more convincing goodness of fit, demonstrated by the high coefficient of determination (R
2) and small root mean square error (RMSE) value, improves the forester’s confidence in continuing using the circular shape assumption to measure the log’s cross-sectional area. The coefficient of determination (R
2) indicates the proportion of variance accounted for is 0.469 for the log cross-section outer periphery.
Table 2 shows the mean square error is 0.03 (its root is 0.16), much smaller than the mean square corrected total.
The deviation from a circular form in the stem’s cross-section shape may reasonably be ignored. The bias between circular and ellipse shapes has long been considered small [
18]. Researchers have chosen a circle as the geometric shape of the log’s cross-section and tree-ring periphery for more than 75 years in multidisciplinary ecological research. The circular basal area of the tree is traditionally calculated in practice based on the diameter at breast height (
Dbh) [
68,
69,
70,
71].
Dbh is generally the average value of the maximum diameter (major axis) and the minimum diameter (minor axis) at 1.3 m height.
As stated before, the circular shape is sufficient to fit the outer periphery of a log. However, the tree often physiologically adjusts the cambium cell differentiation rates during the xylem forming as a reaction of the physiological responses (i.e., intercellular signaling [
72], sucrose signaling [
73], long non-coding RNAs (lncRNAs) [
74], the receptor-like kinase PXY and its ligand CLE41 [
75], transcriptional regulator AHL15 [
76],) to the local environmental stress [
77,
78]; thus, the variation in the outer log circumference may exist. Some alternative geometric shape models, such as ellipse and superellipse, are also discussed in this study to fit the variation in a log’s cross-section shape and sapwood–heartwood transition.
4.2. Ellipse Model
A tree stem’s diameter at the top (
Dt) is usually smaller than the diameter at breast height (
Dbh). Its three-dimensional shape may resemble a truncated cone [
79], sometimes called a conical frustum, as the best-fit idealized standard geometric form. The tree’s or log’s tapered stem volume equations, empirically proposed by foresters, are Equations (30)–(34) [
80]. Equation (31) is the best-known formula, where
b1 denotes the breast-height form factor. Those empirical equations may slightly deviate from the exact formula for calculating the volume of an idealized conical frustum (Equation (35a)). If the ratio of the top diameter per the bottom diameter (
Dt/
Db) is defined as a taper (1/
α), its substitution to Equation (35a) results in Equation (35b). An individual tree stem’s taper is assumed to be constant; therefore, the breast-height form factor (
b1) is a constant that expresses π(1 +
α2 +
α)/12 and
Db transformation to
Dbh. Equation (35c) agrees with Equation (30) and generally confirms that foresters had recognized a conical frustum shape that matched the trunk shape of an individual tree.
where
V = stem volume;
D = diameter at the breast height (
Dbh);
Dt = diameter at the top;
Db = diameter at the bottom;
h = tree height or log length;
b0,
b1,
b2, and
b3 = estimated regression coefficients; 1/α = taper.
Slicing a conic section may result in a circle, ellipse, parabolic, or hyperbolic plane. Cutting a tree’s stem, which resembles a conical frustum, into several logs may result in a circle or ellipse cross-sectional shape. A perpendicular to the longitudinal axis cut resulted in a circle, while an oblique cut resulted in an ellipse cross-section. There is a higher probability that the chainsaw man or harvester made an oblique cut, resulting in an ellipse cross-sectional shape. Therefore, elliptical cross-section logs are more numerous, prevalent, and easier to find than circular ones.
In addition to the indication of an oblique cut, the better-fit ellipse curve may show the environmental factors affecting the secondary growth of the tree. The resultant of wind, snow, and unsymmetric crown self-weight generate the non-eccentric compressive load the stem receives. Their combination with the bending moment triggers buckling, severely reducing its load-bearing capacity [
79,
81,
82,
83,
84,
85]. The buckling generates compressive stress in half of the stem and tensile stress in the other half. A tree’s stem reacts to the gradual stress by adjusting the cambium cell differentiation rates, producing the reaction wood, and may form an almost ellipse shape in cross-section. The gradual stress and strain in the tree stem give rise to long-duration tensile or compressive loading, and creep and relaxation directly affect the vascular cambium [
86].
An inclined tree produces reaction wood and distorts the circularly uniform cambium differentiation. The cambiums of a buckled or inclined tree have a sloping orientation to the normal vertical direction; thus, the gravitational stimulus influences the auxin hormone distribution and creates the abnormal woody tissue, namely reaction wood. Gymnosperms produce the reaction wood on the lower side of leaning stems, called compression wood; meanwhile, a dicotyledonous angiosperms’ reaction wood is formed on the leaning stem’s upper side and is referred to as tension wood [
87,
88,
89]. The non-uniform growth rate of reaction wood places the pith rather to the edge, and the cross-section deviates from the circular to become more similar to an ellipse than a circle. The competition for light, space, water, and nutrition significantly affects the xylem cell differentiation [
90,
91] and also contributes to deviating from a perfectly circular cross-section.
Because of its more adaptive parameter, the ellipse model resulted in a better-fit shape to a log cross-section than a circle. A circle is a special form of an ellipse if the major axis has the same value as the minor axis. The curve would form a circle if the nonlinear regression following an ellipse model resulted in similar values of semi-major (
a) and semi-minor (
b).
Figure 2(a1,b1,c1) is the graph of the ellipse curve-fit result following the Equation (17) model, while
Figure 2(a2,b2,c2) is the graph of the rotated ellipse curve-fit result following the Equation (16) model.
Table 3 summarizes the estimated parameters of the ellipse model, while
Table 4 summarizes those of the rotated model. As expected, the ellipse model significantly improves the goodness of fit, shown by the increasing coefficient of determination (R
2) value. The ellipse model’s R
2 values are 0.523 and 0.902 for fitting the log cross-section shape and sapwood–heartwood transition, respectively, while those of the rotated ellipse are 0.7317 and 0.9998. A rotated ellipse better fits the raw data than the non-rotated one. Both ellipse models’ goodness of fit is higher than the circular model. The analysis of variances of the ellipse (
Table 5) and the rotated ellipse (
Table 6) curve fitting models also indicate the goodness of fit enhancement. The MSE values of both ellipse models are smaller than that of the circle model.
Similar to the circle model’s results, the log and sapwood–heartwood transition centers are in different locations. The rotated ellipse model estimated that the log’s center is (0.208 cm, 2.293 rad) and the heartwood center is (0.748 cm, 0.979 rad). Meanwhile, the non-rotated ellipse model resulted in (−0.1918 cm, −0.8796 rad) and (−0.7403 cm, −2.1344 rad) polar coordinate locations for log and heartwood center, respectively. The sapwood–heartwood transition did not coincide with a tree ring.
4.3. Superellipse Model
If the superellipse curve’s center is the origin (0, 0), its formula at the rectangular diagram is Equation (18). Converting the rectangular diagram to a polar diagram,
r cos
θ substitution to
x, and
r sin
θ to
y, result in Equation (19). Equation (22) includes the
kπ angle rotation into the superellipse formula. Applying nonlinear regression to fit the superellipse models (Equations (23) and (24)) resulted in a less precise curve than the ellipse or circular models.
Figure 3 indicates the systematic error of the prediction curve compared to the original data. This unsatisfactory result may appear because the superellipse’s center is assumed at the origin (0, 0). The solution model needs to find the best-fit estimated superellipse’s central location. Since the nonlinear regression of the superellipse is unreliable enough to determine the best-fit parameters (
Table 7 and
Table 8), justified by the lower analysis of variance (
Table 9 and
Table 10), we propose another method to accommodate the curve-fitting of the superellipse curve. Intrinsic regression can be employed to fit the superellipse models, and we will discuss it in a future study.
4.4. Computer Modelling
The rotated ellipse model is the best-fit model, and it also covers a circle and a regular ellipse. Therefore, this study chooses to develop computer programming for the rotated ellipse model. The executable program script is below, and
Figure 4 displays its user interface.
![Forests 14 01102 i007 Forests 14 01102 i007]()
Figure 4.
User interface display of computer programming for a rotated ellipse graph at the polar diagram system.
Figure 4.
User interface display of computer programming for a rotated ellipse graph at the polar diagram system.
5. Conclusions
This study discusses transforming the rectangular coordinate system into a polar coordinate system to solve the non-function of the log’s cross-section shape and sapwood–heartwood periphery. This transformation successfully solves the difficulty of non-function and paves the way to function; thus, the nonlinear regression methods smoothly solve it. The transformation of raw data points’ positions of a closed curve from the rectangular coordinate (x, y) into the polar coordinates (r, θ) raises the possibility of fitting the log circumference and sapwood–heartwood transition using nonlinear regression. The circular shape model is sufficient to fit the outer periphery of the logs and sapwood–heartwood transition, while ellipse models significantly improve their goodness of fit. The rotated ellipse model is the best fit among others. The deviation from the circular shape indicates the environmental effect on vascular cambium differentiation. Foresters have good choices: (1) continue using the circular model as the simplest one, or (2) change to the rotated ellipse model because it is the best fit to estimate the tree stem’s cross-sectional shape; therefore, it can more precisely determine the basal area, tree volume, and tree trunk biomass. This study developed computer modelling for the rotated ellipse graph in the polar diagram system using Python scripts provided by libraries from the Wolfram engine.
Author Contributions
Conceptualization, E.T.B. and A.D.; methodology, E.T.B. and A.D.; software, A.D.; validation, E.T.B., G.R.P. and Z.K.; formal analysis, E.T.B. and A.D.; investigation, E.T.B.; resources, Z.K. and A.D.; data curation, E.T.B.; writing—original draft preparation, E.T.B.; writing—review and editing, E.T.B. and A.D.; visualization, E.T.B., G.R.P. and Z.K.; supervision, E.T.B. and A.D.; project administration, A.D. and G.R.P.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw and processed data required to reproduce these findings are available in the Mendeley data (doi:10.17632/j7jdp5n39s.2) [
92].
Acknowledgments
The authors express their gratitude and appreciation to IPB University (Bogor Agricultural University) (ID), Pakuan University (ID), and the Directorate General of Higher Education—Ministry of Education and Culture (ID) for the finance, facilities, and opportunity to conduct this research. The authors also thank Fahmi Noor Fiqri and Reza Rizaldi for their technical support during data analysis and computer programming.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bahtiar, E.T.; Darwis, A. Exponential curve modification by linear and nonlinear function to fit the fiber length of teakwood (Tectona grandis). J. Biol. Sci. 2014, 14, 183–194. [Google Scholar] [CrossRef]
- Cahyono, T.D.; Wahyudi, I.; Priadi, T.; Febrianto, F.; Darmawan, W.; Bahtiar, E.T.; Ohorella, S.; Novriyanti, E. The quality of 8 and 10 years old samama wood (Anthocephalus macrophyllus). J. Indian Acad. Wood Sci. 2015, 12, 22–28. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Erizal, E.; Hermawan, D.; Nugroho, N.; Hidayatullah, R. Experimental Study of Beam Stability Factor of Sawn Lumber Subjected to Concentrated Bending Loads at Several Points. Forests 2022, 13, 1480. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Arinana; Nugroho, N.; Nandika, D. Daily Cycle of Air Temperature and Relative Humidity Effect to Creep Deflection of Wood Component of Low-cost House in Cibeureum-Bogor, West Java, Indonesia. Asian J. Sci. Res. 2014, 7, 501–512. [Google Scholar] [CrossRef][Green Version]
- Bahtiar, E.T.; Nugroho, N.; Rahman, M.M.; Arinana; Kartika Sari, R.; Wirawan, W.; Hermawan, D. Estimation the remaining service-lifetime of wooden structure of geothermal cooling tower. Case Stud. Constr. Mater. 2017, 6, 91–102. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Nugroho, N.; Arinana; Darwis, A. Pendugaan Sisa Umur Pakai Kayu Komponen Cooling Tower di Pembangkit Listrik Tenaga Panas Bumi (PLTP) Unit II Kamojang (Estimating the Remaining Life of Wood Cooling Tower Component in Geothermal Power Plant Unit II Kamojang). J. Tek. Sipil 2012, 19, 103–113. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Nugroho, N.; Hermawan, D.; Wirawan, W. Khuschandra Triangle bracing system to reduce the vibration level of cooling tower—Case study in PT Star Energy Geothermal (Wayang Windu) Ltd—Indonesia. Case Stud. Constr. Mater. 2018, 8, 248–257. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Nugroho, N.; Karlinasari, L.; Surjokusumo, S. Human Comfort Period Outside and Inside Bamboo Stands. J. Environ. Sci. Technol. 2014, 7, 245–265. [Google Scholar] [CrossRef]
- Tashi, S.; Keitel, C.; Singh, B.; Adams, M. Allometric equations for biomass and carbon stocks of forests along an altitudinal gradient in the eastern Himalayas. For. Int. J. For. Res. 2017, 90, 445–454. [Google Scholar] [CrossRef]
- Vargas-Larreta, B.; López-Sánchez, C.A.; Corral-Rivas, J.J.; López-Martínez, J.O.; Aguirre-Calderón, C.G.; Álvarez-González, J.G. Allometric Equations for Estimating Biomass and Carbon Stocks in the Temperate Forests of North-Western Mexico. Forests 2017, 8, 269. [Google Scholar] [CrossRef]
- Chave, J.; Réjou-Méchain, M.; Búrquez, A.; Chidumayo, E.; Colgan, M.S.; Delitti, W.B.C.; Duque, A.; Eid, T.; Fearnside, P.M.; Goodman, R.C.; et al. Improved allometric models to estimate the aboveground biomass of tropical trees. Glob. Chang. Biol. 2014, 20, 3177–3190. [Google Scholar] [CrossRef] [PubMed]
- López-Cruz, S.d.C.; Aryal, D.R.; Velázquez-Sanabria, C.A.; Guevara-Hernández, F.; Venegas-Sandoval, A.; Casanova-Lugo, F.; La O-Arias, M.A.; Venegas-Venegas, J.A.; Reyes-Sosa, M.B.; Pinto-Ruiz, R.; et al. Effect of Prescribed Burning on Tree Diversity, Biomass Stocks and Soil Organic Carbon Storage in Tropical Highland Forests. Forests 2022, 13, 2164. [Google Scholar] [CrossRef]
- Návar, J. Allometric equations for tree species and carbon stocks for forests of northwestern Mexico. For. Ecol. Manag. 2009, 257, 427–434. [Google Scholar] [CrossRef]
- Ortega-Rodriguez, D.R.; Hevia, A.; Sánchez-Salguero, R.; Bermudez Dobbertin, S.; Rosero-Alvarado, J.; Chavesta, M.; Tomazello-Filho, M. Novel Yield Model of Pinus patula Schltdl. & Cham. Growth near the Ecological Limit in Northwestern Peruvian Andes. Forests 2022, 13, 2109. [Google Scholar] [CrossRef]
- Kanmegne Tamga, D.; Latifi, H.; Ullmann, T.; Baumhauer, R.; Bayala, J.; Thiel, M. Estimation of Aboveground Biomass in Agroforestry Systems over Three Climatic Regions in West Africa Using Sentinel-1, Sentinel-2, ALOS, and GEDI Data. Sensors 2022, 23, 349. [Google Scholar] [CrossRef]
- Suzuki, J. Nonlinear Regression. In Statistical Learning with Math and Python; Springer Nature Singapore: Singapore, 2021; pp. 133–169. [Google Scholar]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of the evolution of dendrometric concepts for even-aged stands. Forestry 2008, 81, 13–31. [Google Scholar] [CrossRef]
- Pretzsch, H. The course of tree growth. Theory and reality. For. Ecol. Manag. 2020, 478, 118508. [Google Scholar] [CrossRef]
- Fotis, A.T.; Murphy, S.J.; Ricart, R.D.; Krishnadas, M.; Whitacre, J.; Wenzel, J.W.; Queenborough, S.A.; Comita, L.S. Above-ground biomass is driven by mass-ratio effects and stand structural attributes in a temperate deciduous forest. J. Ecol. 2018, 106, 561–570. [Google Scholar] [CrossRef]
- Bettinger, P.; Boston, K.; Siry, J.P.; Grebner, D.L. Valuing and Characterizing Forest Conditions. In Forest Management and Planning; Elsevier: Amsterdam, The Netherlands, 2017; pp. 21–63. [Google Scholar]
- Lupi, C.; Rossi, S.; Vieira, J.; Morin, H.; Deslauriers, A. Assessment of xylem phenology: A first attempt to verify its accuracy and precision. Tree Physiol. 2014, 34, 87–93. [Google Scholar] [CrossRef]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 2012. [Google Scholar]
- Bontemps, J.-D.; Esper, J. Statistical modelling and RCS detrending methods provide similar estimates of long-term trend in radial growth of common beech in north-eastern France. Dendrochronologia 2011, 29, 99–107. [Google Scholar] [CrossRef]
- Jelonek, T.; Kopaczyk, J.; Neumann, M.; Tomczak, A.; Pazdrowski, W.; Grzywiński, W.; Klimek, K.; Naskrent, B.; Kuźmiński, R.; Szwed, T. How Wood Quality Can Be Shaped: Results of 70 Years of Experience. Forests 2022, 13, 2103. [Google Scholar] [CrossRef]
- Höwler, K.; Seidel, D.; Krenn, T.; Berthold, D.; Ehbrecht, M.; Müller, J.; Kietz, B. Evaluation of Softwood Timber Quality—A Case Study on Two Silvicultural Systems in Central Germany. Forests 2022, 13, 1910. [Google Scholar] [CrossRef]
- Pavão, D.C.; Jevšenak, J.; Silva, L.B.; Elias, R.B.; Silva, L. Climate–Growth Relationships in Laurus azorica—A Dominant Tree in the Azorean Laurel Forest. Forests 2023, 14, 166. [Google Scholar] [CrossRef]
- Choi, J.; Tian, N.; Gan, J.; Pelkki, M.; Mhotsha, O. Growth Response of Red Oaks to Climatic Conditions in the Lower Mississippi Alluvial Valley: Implications for Bottomland Hardwood Restoration with a Changing Climate. Climate 2022, 11, 10. [Google Scholar] [CrossRef]
- Winitsky, A.G.; Meko, D.M.; Taylor, A.H.; Biondi, F. Species Sensitivity to Hydrologic Whiplash in the Tree-Ring Record of the High Sierra Nevada. Environments 2023, 10, 12. [Google Scholar] [CrossRef]
- Cabral-Alemán, C.; Villanueva-Díaz, J.; Quiñonez-Barraza, G.; Gómez-Guerrero, A. Resilience of Pinus durangensis Martínez in Extreme Drought Periods: Vertical and Horizontal Response of Tree Rings. Atmosphere 2022, 14, 43. [Google Scholar] [CrossRef]
- Milenin, A.I.; Popova, A.A.; Shestibratov, K.A. Effect of Type of Forest Growth Conditions and Climate Elements on the Dynamics of Radial Growth in English Oak (Quercus robur L.) of Early and Late Phenological Forms. Forests 2022, 14, 11. [Google Scholar] [CrossRef]
- Panyushkina, I.P.; Leavitt, S.W.; Meko, D.M.; Black, B.A.; Jull, A.J.T.; Van de Water, P.; Squire, J.; Testa, N.R. Douglas Fir Multiproxy Tree-Ring Data Glimpse MIS 5 Environment in the U.S. Pacific Northwest. Forests 2022, 13, 2161. [Google Scholar] [CrossRef]
- Roibu, C.-C.; Palaghianu, C.; Nagavciuc, V.; Ionita, M.; Sfecla, V.; Mursa, A.; Crivellaro, A.; Stirbu, M.-I.; Cotos, M.-G.; Popa, A.; et al. The Response of Beech (Fagus sylvatica L.) Populations to Climate in the Easternmost Sites of Its European Distribution. Plants 2022, 11, 3310. [Google Scholar] [CrossRef]
- Zhirnova, D.F.; Belokopytova, L.V.; Krutovsky, K.V.; Kholdaenko, Y.A.; Babushkina, E.A.; Vaganov, E.A. Spatial-Coherent Dynamics and Climatic Signals in the Radial Growth of Siberian Stone Pine (Pinus sibirica Du Tour) in Subalpine Stands along the Western Sayan Mountains. Forests 2022, 13, 1994. [Google Scholar] [CrossRef]
- Khandu, Y.; Polthanee, A.; Isarangkool Na Ayutthaya, S. Dendroclimatic Reconstruction of Mean Annual Temperatures over Treeline Regions of Northern Bhutan Himalayas. Forests 2022, 13, 1794. [Google Scholar] [CrossRef]
- Ballikaya, P.; Song, W.; Bachmann, O.; Guillong, M.; Wang, X.; Cherubini, P. Chemical Elements Recorded by Quercus mongolica Fisch. ex Ledeb. Tree Rings Reveal Trends of Pollution History in Harbin, China. Forests 2023, 14, 187. [Google Scholar] [CrossRef]
- Kharuk, V.I.; Petrov, I.A.; Im, S.T.; Golyukov, A.S.; Dvinskaya, M.L.; Shushpanov, A.S. Pollution and Climatic Influence on Trees in the Siberian Arctic Wetlands. Water 2023, 15, 215. [Google Scholar] [CrossRef]
- Sensuła, B.; Wilczyński, S. Dynamics Changes in Basal Area Increment, Carbon Isotopes Composition and Water Use Efficiency in Pine as Response to Water and Heat Stress in Silesia, Poland. Plants 2022, 11, 3569. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Link, R.; Li, R.; Deng, L.; Schuldt, B.; Jiang, X.; Zhao, R.; Zheng, J.; Li, S.; et al. Influence of Cambial Age and Axial Height on the Spatial Patterns of Xylem Traits in Catalpa bungei, a Ring-Porous Tree Species Native to China. Forests 2019, 10, 662. [Google Scholar] [CrossRef]
- Soheili, F.; Abdul-Hamid, H.; Almasi, I.; Heydari, M.; Tongo, A.; Woodward, S.; Naji, H.R. How Tree Decline Varies the Anatomical Features in Quercus brantii. Plants 2023, 12, 377. [Google Scholar] [CrossRef]
- Yang, H.; An, W.; Gu, Y.; Peng, J.; Jiang, Y.; Li, J.; Chen, L.; Zhu, P.; He, F.; Zhang, F.; et al. Integrative Metabolomic and Transcriptomic Analysis Reveals the Mechanism of Specific Color Formation in Phoebe zhennan Heartwood. Int. J. Mol. Sci. 2022, 23, 13569. [Google Scholar] [CrossRef]
- Sperry, J.S.; Perry, A.H.; Sullivan, J.E.M. Pit Membrane Degradation and Air-Embolism Formation in Ageing Xylem Vessels of Populus tremuloides Michx. J. Exp. Bot. 1991, 42, 1399–1406. [Google Scholar] [CrossRef]
- Fujii, T.; Suzuki, Y.; Kuroda, N. Bordered Pit Aspiration in the Wood of Cryptomeria Japonica in Relation to Air Permeability. IAWA J. 1997, 18, 69–76. [Google Scholar] [CrossRef]
- Tarelkina, T.V.; Galibina, N.A.; Moshnikov, S.A.; Nikerova, K.M.; Moshkina, E.V.; Genikova, N.V. Anatomical and Morphological Features of Scots Pine Heartwood Formation in Two Forest Types in the Middle Taiga Subzone. Forests 2022, 13, 91. [Google Scholar] [CrossRef]
- Yang, H.; An, W.; Wang, F.; Gu, Y.; Guo, H.; Jiang, Y.; Peng, J.; Liu, M.; Chen, L.; Zhang, F.; et al. Integrated Transcriptomic, Metabolomic, and Physiological Analyses Reveal New Insights into Fragrance Formation in the Heartwood of Phoebe hui. Int. J. Mol. Sci. 2022, 23, 14044. [Google Scholar] [CrossRef] [PubMed]
- Gratton, S.; Lawless, A.S.; Nichols, N.K. Approximate Gauss–Newton Methods for Nonlinear Least Squares Problems. SIAM J. Optim. 2007, 18, 106–132. [Google Scholar] [CrossRef]
- Kanzow, C.; Yamashita, N.; Fukushima, M. Levenberg–Marquardt methods with strong local convergence properties for solving nonlinear equations with convex constraints. J. Comput. Appl. Math. 2004, 172, 375–397. [Google Scholar] [CrossRef]
- Bergou, E.H.; Diouane, Y.; Kungurtsev, V. Convergence and Complexity Analysis of a Levenberg–Marquardt Algorithm for Inverse Problems. J. Optim. Theory Appl. 2020, 185, 927–944. [Google Scholar] [CrossRef]
- Lamé, G. Examen des différentes Méthodes employées pour résoudre les Problèmes de Géométrie. By G. Lamé. Pp. 124. 1818. (Courcier.) Reprinted by A. Hermann, Paris. Math. Gaz. 1904, 3, 64. [Google Scholar] [CrossRef][Green Version]
- Banerjee, S.; Seth, S.; Dey, T.; Pal, D. Python Programming Language and Its Scope in Future. Int. Res. J. Mod. Eng. Technol. Sci. 2022, 4, 2582–5208. [Google Scholar]
- Srinath, K.R. Python-The Fastest Growing Programming Language. Int. Res. J. Eng. Technol. 2017, 4, 354–357. [Google Scholar]
- Thangarajah, V. Python Current Trend Applications—An Overview. Int. J. Adv. Eng. Res. 2019, 6, 6–12. [Google Scholar]
- Chen, X.; Liu, W. The Value of Python Programming in General Education and Comprehensive Quality Improvement of Medical Students Based on a Retrospective Cohort Study. J. Healthc. Eng. 2022, 2022, 4043992. [Google Scholar] [CrossRef]
- Munawar, K.; Naveed, M.S. The Impact of Language Syntax on the Complexity of Programs: A Case Study of Java and Python. Int. J. Innov. Sci. Technol. 2022, 4, 683–695. [Google Scholar] [CrossRef]
- Nikula, U.; Sajaniemi, J.; Tedre, M.; Wray, S. Python and Roles of Variables in Introductory Programming: Experiences from Three Educational Institutions. J. Inf. Technol. Educ. Res. 2007, 6, 199–214. [Google Scholar] [CrossRef]
- Cheon, M.; Lee, O.; Mun, C.; Ha, H. A Study on the Factors Affecting Intention of Learning Python Programming: For Non-majors in University. Int. J. Inf. Educ. Technol. 2022, 12, 414–420. [Google Scholar] [CrossRef]
- Li, Y. Python Data Analysis and Attribute Information Extraction Method Based on Intelligent Decision System. Mob. Inf. Syst. 2022, 2022, 2495166. [Google Scholar] [CrossRef]
- Cielen, D.; Meysman, A.; Ali, M. Introducing Data Science: Big Data, Machine Learning, and More, Using Python Tools; Simon and Schuster: New York, NY, USA, 2016; ISBN 16334300309781633430037. [Google Scholar]
- Pejovic, P. Application of Python Programming Language in Measurements. Facta Univ.—Ser. Electron. Energ. 2019, 32, 1–23. [Google Scholar] [CrossRef]
- Kathiravelu, P.; Sarker, D.M.O.F. Python Network Programming Cookbook_Practical Solutions to Overcome Real-World Networking Challenges; Packt Publishing: Birmingham, UK, 2017; ISBN 9781786463999. [Google Scholar]
- Akinkuolie, B.B.; Lin, C.; Yuan, S. A cross-platform mobile learning system using QT SDK Framework. In Proceedings of the 2011 Fifth International Conference on Genetic and Evolutionary Computing, Kitakyushu, Japan, 29 August–1 September 2011. [Google Scholar] [CrossRef]
- Myllärniemi, V.; Kujala, S.; Raatikainen, M. Development as a journey: Factors supporting the adoption and use of software frameworks. J. Softw. Eng. Res. Dev. 2018, 6, 6. [Google Scholar] [CrossRef]
- Smołka, J.; Matacz, B.; Łukasik, E.; Skublewska-paszkowska, M. Performance analysis of mobile applications developed with different programming tools. MATEC Web Conf. 2019, 252, 05022. [Google Scholar] [CrossRef][Green Version]
- Levenberg, K. A method for the solution of certain non-linear problems in least squares. Q. Appl. Math. 1944, 2, 164–168. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Bamber, R.K. Heartwood, its function and formation. Wood Sci. Technol. 1976, 10, 1–8. [Google Scholar] [CrossRef]
- Chattaway, M.M. The Sapwood-Heartwood Transition. Aust. For. 1952, 16, 25–34. [Google Scholar] [CrossRef]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability—A review. Wood Fiber Sci. 2022, 34, 587–611. [Google Scholar]
- Li, S.; Fang, L.; Sun, Y.; Xia, L.; Lou, X. Development of Measuring Device for Diameter at Breast Height of Trees. Forests 2023, 14, 192. [Google Scholar] [CrossRef]
- Cusack, D.; Dietterich, L.; Valdes, E. Tropical Tree Species Identity and Diameter at Breast Height in a Throughfall-Reduction Drying Experiment in Four Lowland Panamanian Forests; Biological and Environmental Research (BER): Washington, DC, USA, 2023. [Google Scholar]
- Sumnall, M.J.; Albaugh, T.J.; Carter, D.R.; Cook, R.L.; Hession, W.C.; Campoe, O.C.; Rubilar, R.A.; Wynne, R.H.; Thomas, V.A. Estimation of individual stem volume and diameter from segmented UAV laser scanning datasets in Pinus taeda L. plantations. Int. J. Remote Sens. 2023, 44, 217–247. [Google Scholar] [CrossRef]
- Terryn, L.; Calders, K.; Åkerblom, M.; Bartholomeus, H.; Disney, M.; Levick, S.; Origo, N.; Raumonen, P.; Verbeeck, H. Analysing individual 3D tree structure using the R package ITSMe. Methods Ecol. Evol. 2023, 14, 231–241. [Google Scholar] [CrossRef]
- Turley, E.K.; Etchells, J.P. Laying it on thick: A study in secondary growth. J. Exp. Bot. 2022, 73, 665–679. [Google Scholar] [CrossRef]
- Narutaki, A.; Kahar, P.; Shimadzu, S.; Maeda, S.; Furuya, T.; Ishizaki, K.; Fukaki, H.; Ogino, C.; Kondo, Y. Sucrose Signaling Contributes to the Maintenance of Vascular Cambium by Inhibiting Cell Differentiation. Plant Cell Physiol. 2023, 64, pcad039. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, H.; Chen, B.; Grünhofer, P.; Schreiber, L.; Lin, J.; Zhao, Y. Genome-wide analysis of long non-coding RNAs in shoot apical meristem and vascular cambium in Populus tomentosa. J. Plant Physiol. 2022, 275, 153759. [Google Scholar] [CrossRef]
- Lebovka, I.; Hay Mele, B.; Liu, X.; Zakieva, A.; Schlamp, T.; Gursanscky, N.R.; Merks, R.M.; Großeholz, R.; Greb, T. Computational modeling of cambium activity provides a regulatory framework for simulating radial plant growth. elife 2023, 12, e66627. [Google Scholar] [CrossRef]
- Rahimi, A.; Karami, O.; Lestari, A.D.; de Werk, T.; Amakorová, P.; Shi, D.; Novák, O.; Greb, T.; Offringa, R. Control of cambium initiation and activity in Arabidopsis by the transcriptional regulator AHL15. Curr. Biol. 2022, 32, 1764–1775.e3. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, H.; Wu, H.; Shen, W.; Lin, J.; Zhao, Y. Seasonal changes in cambium activity from active to dormant stage affect the formation of secondary xylem in Pinus tabulaeformis Carr. Tree Physiol. 2022, 42, 585–599. [Google Scholar] [CrossRef]
- Larysch, E.; Stangler, D.F.; Puhlmann, H.; Rathgeber, C.B.K.; Seifert, T.; Kahle, H.-P. The 2018 hot drought pushed conifer wood formation to the limit of its plasticity: Consequences for woody biomass production and tree ring structure. Plant Biol. 2022, 24, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- Karlinasari, L.; Bahtiar, E.T.; Kadir, A.S.A.; Adzkia, U.; Nugroho, N.; Siregar, I.Z. Structural Analysis of Self-Weight Loading Standing Trees to Determine Its Critical Buckling Height. Sustainability 2023, 15, 6075. [Google Scholar] [CrossRef]
- Burkhart, H.E.; Tomé, M. Tree-Stem Volume Equations. In Modeling Forest Trees and Stands; Springer: Dordrecht, The Netherlands, 2012; pp. 43–64. [Google Scholar]
- Zahn, J.J. Re-examination of ylinen and other column equations. J. Struct. Eng. 1992, 118, 2716–2728. [Google Scholar] [CrossRef]
- Nie, Y.; Wei, Y.; Huang, L.; Liu, Y.; Dong, F. Influence of slenderness ratio and sectional geometry on the axial compression behavior of original bamboo columns. J. Wood Sci. 2021, 67, 36. [Google Scholar] [CrossRef]
- Nugroho, N.; Bahtiar, E.T. Buckling formulas for designing a column with Gigantochloa apus. Case Stud. Constr. Mater. 2021, 14, e00516. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Malkowska, D.; Trujillo, D.; Nugroho, N. Experimental study on buckling resistance of Guadua angustifolia bamboo column. Eng. Struct. 2021, 228, 111548. [Google Scholar] [CrossRef]
- Bahtiar, E.T.; Denih, A.; Putra, G.R. Multi-culm bamboo composites as sustainable materials for green constructions: Section properties and column behavior. Results Eng. 2023, 17, 100911. [Google Scholar] [CrossRef]
- Kojs, P.; Miodek, A.; Miodek, A.P.; Włoch, W. Vascular Cambium—Between the Hammer and the Anvil: A Tensile Stress Hypothesis on the Mechanism of Radial Growth of Broadleaved Trees. Forests 2023, 14, 823. [Google Scholar] [CrossRef]
- Du, S.; Yamamoto, F. An Overview of the Biology of Reaction Wood Formation. J. Integr. Plant Biol. 2007, 49, 131–143. [Google Scholar] [CrossRef]
- Gardiner, B.; Barnett, J.; Saranpää, P.; Gril, J. (Eds.) The Biology of Reaction Wood; Springer Series in Wood Science; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 978-3-642-10813-6. [Google Scholar]
- Telewski, F.W. Flexure Wood: Mechanical Stress Induced Secondary Xylem Formation. In Secondary Xylem Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 73–91. [Google Scholar]
- Wodzicki, T.J. Natural factors affecting wood structure. Wood Sci. Technol. 2001, 35, 5–26. [Google Scholar] [CrossRef]
- Ghosh, S.; Nelson, J.F.; Cobb, G.M.C.; Etchells, J.P.; de Lucas, M. Light regulates xylem cell differentiation via PIF in Arabidopsis. Cell Rep. 2022, 40, 111075. [Google Scholar] [CrossRef] [PubMed]
- Bahtiar, E.T. Data set for article: Developing a Model for Curve-Fitting a Tree Stem’s Cross-Sectional Shape and Sapwood–Heartwood Transition in a Polar Diagram System Using Nonlinear Regression. Mendeley Data 2023. [Google Scholar] [CrossRef]
Figure 1.
(a) Nonlinear curve fitting for the explicit function model of circle r = f(θ) (Equation (8)), (b) Estimated r vs. observed r plotted together with y = x line, (c) The circle fitting at the actual log’s cross-section.
Figure 1.
(a) Nonlinear curve fitting for the explicit function model of circle r = f(θ) (Equation (8)), (b) Estimated r vs. observed r plotted together with y = x line, (c) The circle fitting at the actual log’s cross-section.
Figure 2.
Nonlinear curve fitting for r = f(θ) (a1–c1) ellipse (Equation (17)) and (a2–c2) rotated ellipse (Equation (16)): (a1,a2) the explicit function model, (b1,b2) Estimated r vs. observed r plotted together with y = x line, (c1,c2) The ellipse fitting at the actual log’s cross-section.
Figure 2.
Nonlinear curve fitting for r = f(θ) (a1–c1) ellipse (Equation (17)) and (a2–c2) rotated ellipse (Equation (16)): (a1,a2) the explicit function model, (b1,b2) Estimated r vs. observed r plotted together with y = x line, (c1,c2) The ellipse fitting at the actual log’s cross-section.
Figure 3.
(a1,a2) Nonlinear curve fitting for the explicit function model r = f(θ) of (a1–c1) superellipse (Equation (23)) and (a2–c2) rotated superellipse (Equation (24)), (b1,b2) Estimated r vs. observed r plotted together with y = x line, (c1,c2) The superellipse fitting at the actual log’s cross-section.
Figure 3.
(a1,a2) Nonlinear curve fitting for the explicit function model r = f(θ) of (a1–c1) superellipse (Equation (23)) and (a2–c2) rotated superellipse (Equation (24)), (b1,b2) Estimated r vs. observed r plotted together with y = x line, (c1,c2) The superellipse fitting at the actual log’s cross-section.
Table 1.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the circle model in Equation (8).
Table 1.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the circle model in Equation (8).
| Parameter | Estimate | Standard Error | t-Value | p-Value | 95% Lower Conf. Limit | 95% Upper Conf. Limit |
|---|
| a. Outer log circumference |
| ro | 0.2099 | 0.0215 | 9.7612 | 0.0000 | 0.1672 | 0.2525 |
| θo | 2.2891 | 0.1040 | 22.0127 | 0.0000 | 2.0830 | 2.4952 |
| a | 8.8597 | 0.0154 | 577.0298 | 0.0000 | 8.8292 | 8.8901 |
| b. Sapwood–Heartwood transition |
| ro | −0.7447 | 0.0305 | −24.4118 | 0.0000 | −0.8054 | −0.6839 |
| θo | −2.1697 | 0.0396 | −54.8502 | 0.0000 | −2.2486 | −2.0909 |
| a | 5.2821 | 0.0212 | 248.6318 | 0.0000 | 5.2397 | 5.3244 |
Table 2.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the circle model in Equation (8).
Table 2.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the circle model in Equation (8).
| Effect | Sum Square | df | Mean Square | F-Value | p-Value |
|---|
| a. Outer log circumference |
| Regression | 8666.97 | 3 | 2888.99 | 112,537.32 | 0.0000 |
| Residual | 2.75 | 107 | 0.03 | | |
| Total | 8669.71 | 110 | | | |
| Corrected Total | 5.17 | 109 | | | |
| Regression vs. Corrected Total | 8666.97 | 3 | 2888.99 | 60,924.84 | 0.0000 |
| b. Sapwood–Heartwood transition |
| Regression | 2151.38 | 3 | 717.13 | 20,682.72 | 0.0000 |
| Residual | 2.57 | 74 | 0.03 | | |
| Total | 2153.95 | 77 | | | |
| Corrected Total | 23.54 | 76 | | | |
| Regression vs. Corrected Total | 2151.38 | 3 | 717.13 | 2315.32 | 0.0000 |
Table 3.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the ellipse model in Equation (17).
Table 3.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the ellipse model in Equation (17).
| Parameter | Estimate | Standard Error | t-Value | p-Value | 95% Lower Conf. Limit | 95% Upper Conf. Limit |
|---|
| a. Outer log circumference |
| ro | 0.208 | 0.021 | 10.1510 | 0.0000 | 0.1676 | 0.2489 |
| a | 8.788 | 0.025 | 350.7989 | 0.0000 | 8.7388 | 8.8381 |
| b | 8.933 | 0.026 | 345.4442 | 0.0000 | 8.8819 | 8.9844 |
| θo | 2.293 | 0.100 | 22.9810 | 0.0000 | 2.0948 | 2.4903 |
| b. Sapwood–Heartwood periphery |
| ro | 0.748 | 0.029 | 25.5898 | 0.0000 | 0.690 | 0.807 |
| a | 5.197 | 0.035 | 147.6168 | 0.0000 | 5.127 | 5.268 |
| b | 5.366 | 0.035 | 151.1844 | 0.0000 | 5.295 | 5.436 |
| θo | 0.979 | 0.037 | 26.2172 | 0.0000 | 0.904 | 1.053 |
Table 4.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the rotated ellipse model in Equation (16).
Table 4.
The best fit estimated parameters of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the rotated ellipse model in Equation (16).
| Parameter | Estimate | Standard Error | t-Value | p-Value | 95% Lower Conf. Limit | 95% Upper Conf. Limit |
|---|
| a. Outer log circumference |
| ro | −0.1918 | 0.0158 | −12.1445 | 0.0000 | −0.2231 | −0.1605 |
| a | 8.7051 | 0.0187 | 465.8943 | 0.0000 | 8.6680 | 8.7421 |
| b | 9.0159 | 0.0192 | 470.5462 | 0.0000 | 8.9779 | 9.0539 |
| k | 2.1767 | 0.0161 | 135.1281 | 0.0000 | 2.1448 | 2.2086 |
| θo | −0.8796 | 0.0803 | −10.9550 | 0.0000 | −1.0388 | −0.7204 |
| b. Sapwood–Heartwood transition |
| ro | −0.7403 | 0.0014 | −526.8547 | 0.0000 | −0.7431 | −0.7375 |
| a | 5.0235 | 0.0017 | 2932.1950 | 0.0000 | 5.0200 | 5.0269 |
| b | 5.5353 | 0.0018 | 3122.5152 | 0.0000 | 5.5318 | 5.5388 |
| k | 2.1943 | 0.0009 | 2462.1008 | 0.0000 | 2.1926 | 2.1961 |
| θo | −2.1344 | 0.0020 | −1083.8741 | 0.0000 | −2.1384 | −2.1305 |
Table 5.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the ellipse model in Equation (17).
Table 5.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the ellipse model in Equation (17).
| Effect | Sum Square | df | Mean Square | F-Value | p-Value |
|---|
| a. Outer log circumference | | | | | |
| Regression | 8667.25 | 4 | 2166.81 | 93077.35 | 0.0000 |
| Residual | 2.47 | 106 | 0.02 | | |
| Total | 8669.71 | 110 | | | |
| Corrected Total | 5.17 | 109 | | | |
| Regression vs. Corrected Total | 8667.25 | 4 | 2166.81 | 45695.10 | 0.0000 |
| b. Sapwood–heartwood transition | | | | | |
| Regression | 2151.65 | 4 | 537.91 | 17081.85 | 0.0000 |
| Residual | 2.30 | 73 | 0.03 | | |
| Total | 2153.95 | 77 | | | |
| Corrected Total | 23.54 | 76 | | | |
| Regression vs. Corrected Total | 2151.65 | 4 | 537.91 | 1736.71 | 0.0000 |
Table 6.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the rotated ellipse model in Equation (16).
Table 6.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve based on the rotated ellipse model in Equation (16).
| Effect | Sum Square | df | Mean Square | F-Value | p-Value |
|---|
| a. Outer log circumference | | | | | |
| Regression | 8668.33 | 5 | 1733.67 | 131,252.79 | 0.0000 |
| Residual | 1.39 | 105 | 0.01 | | |
| Total | 8669.71 | 110 | | | |
| Corrected Total | 5.17 | 109 | | | |
| Regression vs. Corrected Total | 8668.33 | 5 | 1733.67 | 36,560.64 | 0.0000 |
| b. Sapwood–heartwood transition | | | | | |
| Regression | 2153.94 | 5 | 430.79 | 5,463,867.57 | 0.0000 |
| Residual | 0.01 | 72 | 0.00 | | |
| Total | 2153.95 | 77 | | | |
| Corrected Total | 23.54 | 76 | | | |
| Regression vs. Corrected Total | 2153.94 | 5 | 430.79 | 1390.85 | 0.0000 |
Table 7.
The best fit estimated parameters of the sapwood (a) and heartwood (b) periphery curve-fit based on the superellipse model in Equation (23).
Table 7.
The best fit estimated parameters of the sapwood (a) and heartwood (b) periphery curve-fit based on the superellipse model in Equation (23).
| Parameter | Estimate | Standard Error | t-Value | p-Value | 95% Lower Conf. Limit | 95% Upper Conf. Limit |
|---|
| a. Outer log circumference |
| a | 8.8362 | 0.0483 | 182.7632 | 0.0000 | 8.7404 | 8.9320 |
| n | 1.9574 | 0.0370 | 52.9616 | 0.0000 | 1.8841 | 2.0306 |
| b | 8.9933 | 0.0504 | 178.3683 | 0.0000 | 8.8933 | 9.0932 |
| b. Sapwood–Heartwood transition |
| a | 5.1503 | 0.1509 | 34.1304 | 0.0000 | 4.8497 | 5.4510 |
| n | 2.0653 | 0.2201 | 9.3855 | 0.0000 | 1.6269 | 2.5038 |
| b | 5.3045 | 0.1537 | 34.5169 | 0.0000 | 4.9983 | 5.6107 |
Table 8.
The best fit estimated parameters of the sapwood (a) and heartwood (b) periphery curve-fit based on the rotated superellipse model in Equation (24).
Table 8.
The best fit estimated parameters of the sapwood (a) and heartwood (b) periphery curve-fit based on the rotated superellipse model in Equation (24).
| Parameter | Estimate | Standard Error | t-Value | p-Value | 95% Lower Conf. Limit | 95% Upper Conf. Limit |
|---|
| a. Outer log circumference |
| k | 1.11 | 0.02 | 69.64 | 0.0000 | 1.08 | 1.14 |
| a | 8.74 | 0.04 | 236.25 | 0.0000 | 8.66 | 8.81 |
| n | 2.29 | 0.05 | 48.12 | 0.0000 | 2.19 | 2.38 |
| b | 8.53 | 0.07 | 123.70 | 0.0000 | 8.39 | 8.66 |
| b. Sapwood–Heartwood transition |
| k | −0.81 | 0.07 | −12.3972 | 0.0000 | −0.94 | −0.68 |
| a | 5.05 | 0.15 | 34.1796 | 0.0000 | 4.76 | 5.35 |
| n | 1.99 | 0.20 | 10.1658 | 0.0000 | 1.60 | 2.37 |
| b | 5.48 | 0.15 | 37.0000 | 0.0000 | 5.18 | 5.77 |
Table 9.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the superellipse model in Equation (23).
Table 9.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the superellipse model in Equation (23).
| Effect | Sum Square | df | Mean Square | F-Value | p-Value |
|---|
| a. Outer log circumference | | | | | |
| Regression | 8664.92 | 3 | 2888.31 | 64511.86 | 0.0000 |
| Residual | 4.79 | 107 | 0.05 | | |
| Total | 8669.71 | 110 | | | |
| Corrected Total | 5.17 | 109 | | | |
| Regression vs. Corrected Total | 8664.92 | 3 | 2888.31 | 60910.48 | 0.0000 |
| b. Sapwood–heartwood transition | | | | | |
| Regression | 2130.67 | 3 | 710.22 | 2257.49 | 0.0000 |
| Residual | 23.28 | 74 | 0.31 | | |
| Total | 2153.95 | 77 | | | |
| Corrected Total | 23.54 | 76 | | | |
| Regression vs. Corrected Total | 2130.67 | 3 | 710.22 | 2293.03 | 0.0000 |
Table 10.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the rotated superellipse model in Equation (24).
Table 10.
The analysis of variance of the outer log circumference (a) and the sapwood–heartwood transition (b) curve-fit based on the rotated superellipse model in Equation (24).
| Effect | Sum Square | df | Mean Square | F-Value | p-Value |
|---|
| a. Outer log circumference | | | | | |
| Regression | 8666.23 | 4 | 2166.56 | 65930.26 | 0.0000 |
| Residual | 3.48 | 106 | 0.03 | | |
| Total | 8669.71 | 110 | | | |
| Corrected Total | 5.17 | 109 | | | |
| Regression vs. Corrected Total | 8666.23 | 4 | 2166.56 | 45689.75 | 0.0000 |
| b. Sapwood–heartwood transition | | | | | |
| Regression | 2132.14 | 4 | 533.04 | 1784.61 | 0.0000 |
| Residual | 21.80 | 73 | 0.30 | | |
| Total | 2153.95 | 77 | | | |
| Corrected Total | 23.54 | 76 | | | |
| Regression vs. Corrected Total | 2132.14 | 4 | 533.04 | 1720.96 | 0.0000 |
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).