Genome-Wide Analysis, Identification, and Characterization of the PFK Gene Family Members of Populus deltoides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tree Materials
2.2. Identification of Phosphofructokinase Family Genes in P. deltoides
2.3. Chromosomal Location and Collinearity Analyses of PdPFK Genes
2.4. Conserved Motifs and Gene Structure Analysis
2.5. Phylogenetic Analysis and Classification of the Phosphofructokinase Genes
2.6. RNA Extraction and Quantitative PCR (qPCR)
2.7. Measurement of Phosphofructokinase Content
2.8. Statistical Analysis
3. Results
3.1. Identification and Physicochemical Properties of Phosphofructokinase Gene Family in P. deltoides
3.2. Prediction of the Subcellular Localization of Phosphofructokinase Family Members of P. deltoides
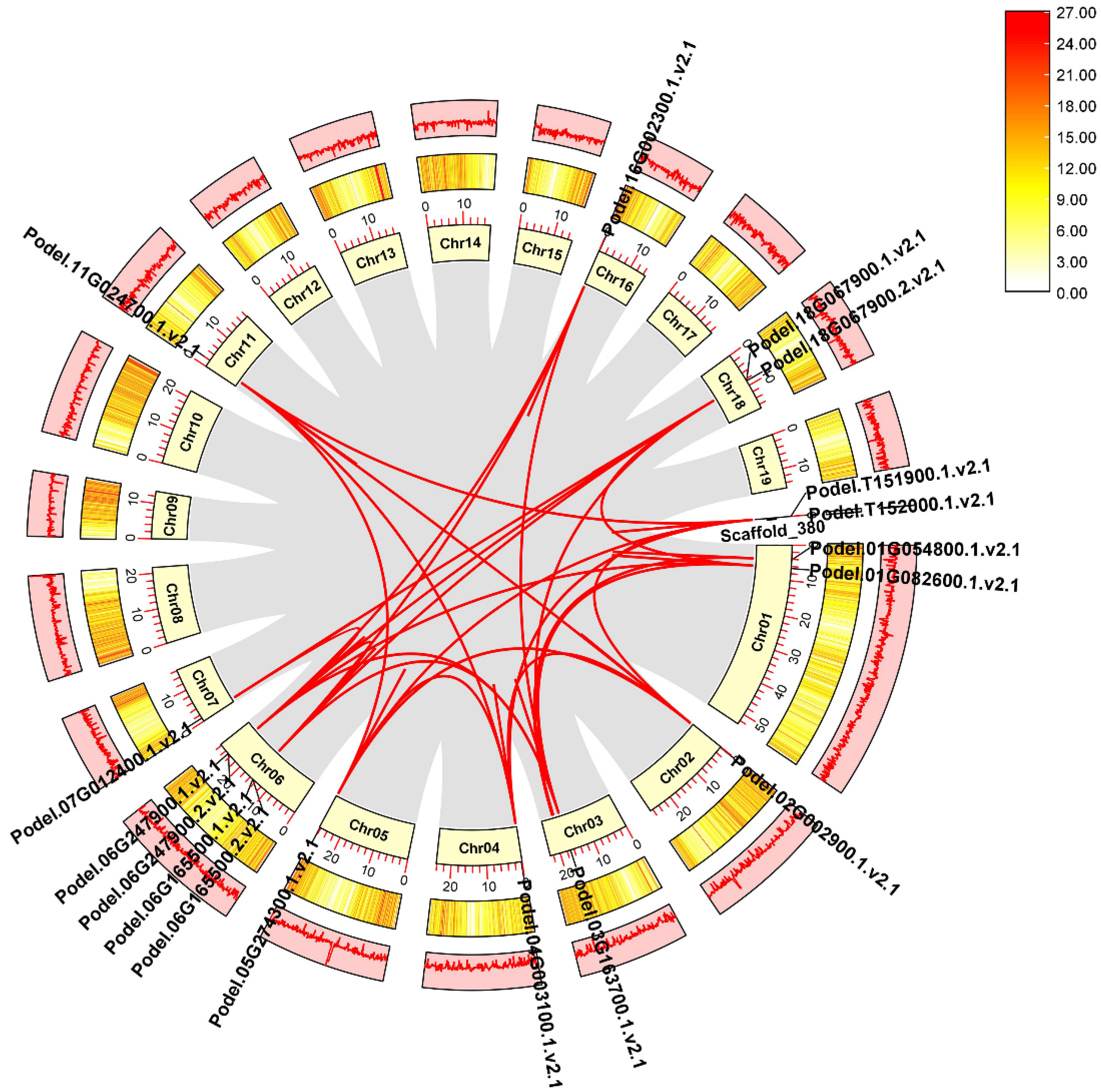
3.3. Chromosomal Location and Collinearity Analysis of PdPFP and PdPFK Gene Families
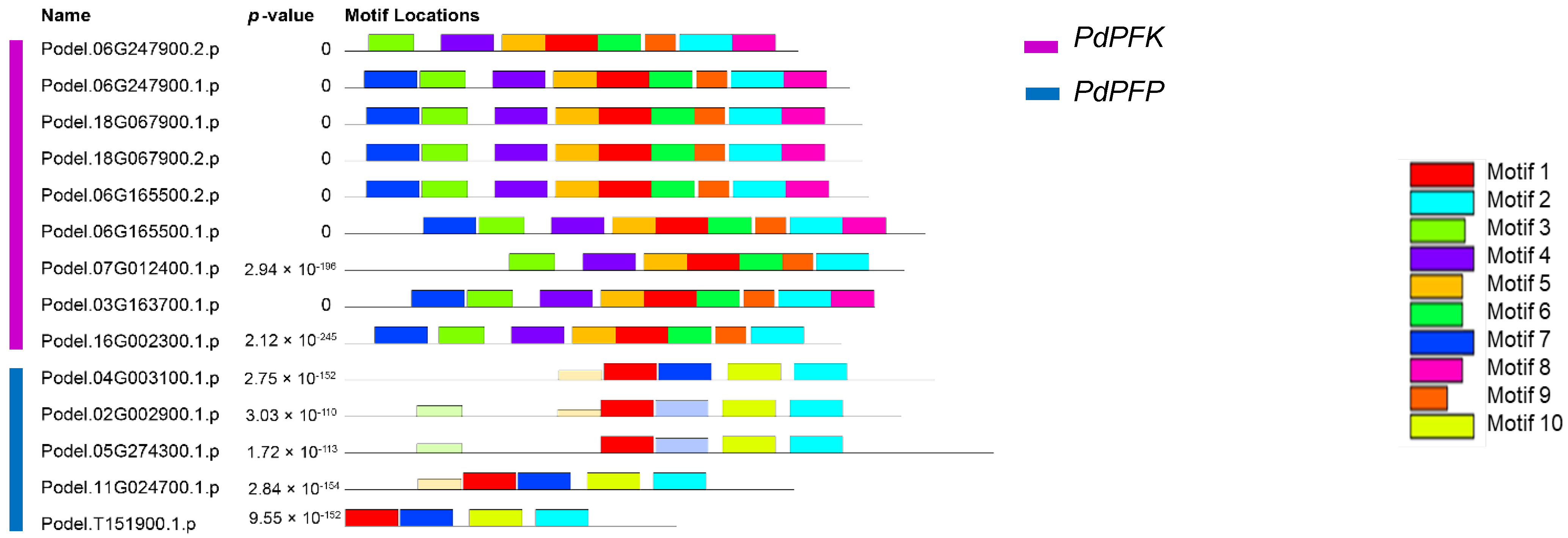
3.4. Conserved Motifs and Gene Structure Analysis of the PdPFP and PdPFK Gene Families
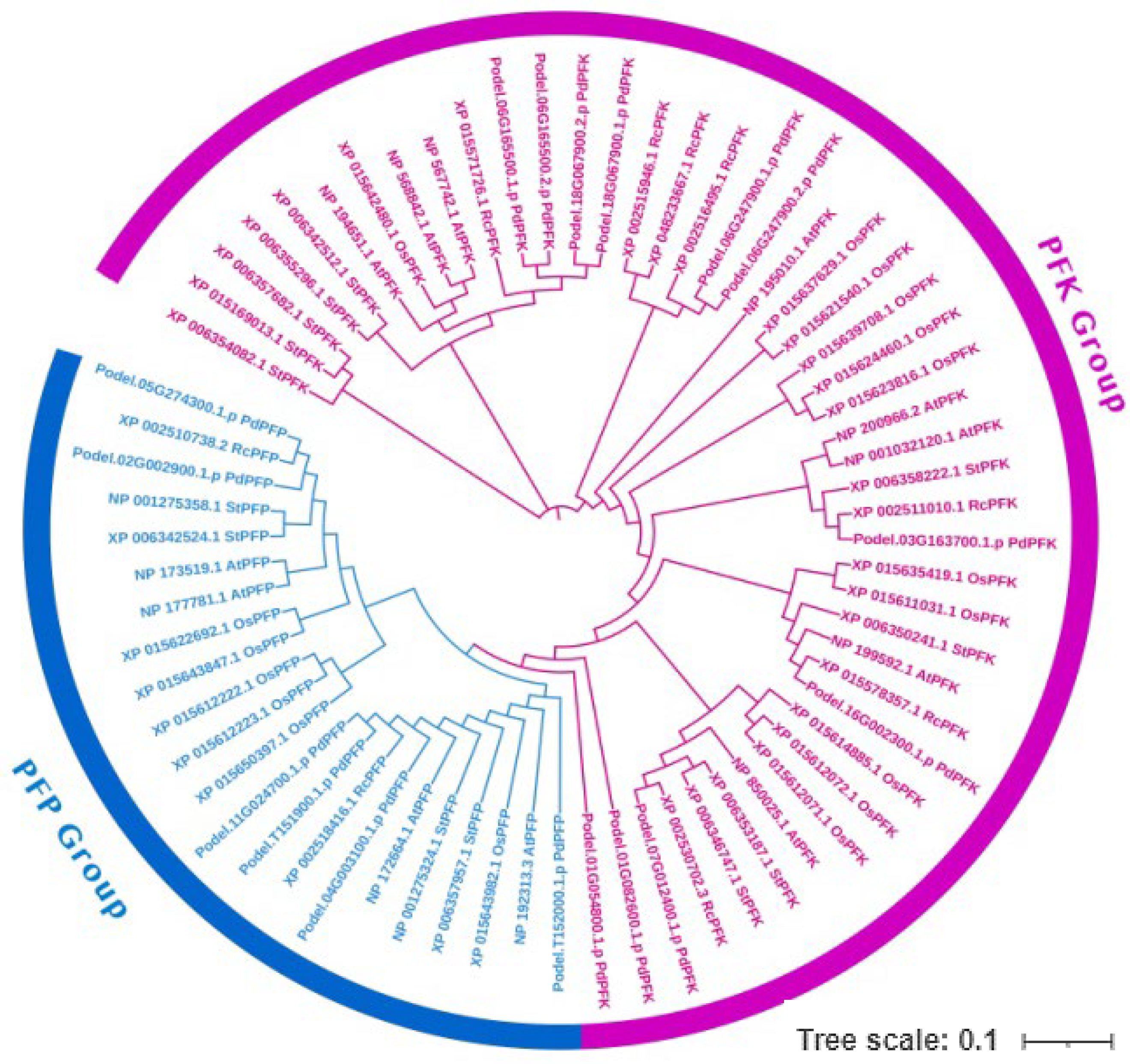
3.5. Phylogenetic Analysis of the PdPFP and PdPFK Gene Families
3.6. Analysis of PdPFP and PdPFK Expression Patterns
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plaxton, W.C. The organization and regulation of plant glycolysis. Annu. Rev. Plant Biol. 1996, 47, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Mertens, E.; Larondelle, Y.; Hers, H.-G. Induction of pyrophosphate: Fructose 6-phosphate 1-phosphotransferase by anoxia in rice seedlings. Plant Physiol. 1990, 93, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Claassen, P.A.; Budde, M.A.; de Ruyter, H.J.; van Calker, M.H.; van Es, A. Potential role of pyrophosphate: Fructose 6-phosphate phosphotransferase in carbohydrate metabolism of cold stored tubers of Solanum tuberosum cv Bintje. Plant Physiol. 1991, 95, 1243–1249. [Google Scholar] [CrossRef]
- Lim, H.; Cho, M.-H.; Bhoo, S.H.; Hahn, T.-R. Pyrophosphate: Fructose-6-phosphate 1-phosphotransferase is involved in the tolerance of Arabidopsis seedlings to salt and osmotic stresses. In Vitro Cell Dev. Biol.-Plant 2014, 50, 84–91. [Google Scholar] [CrossRef]
- Nielsen, M.F.; Caumo, A.; Chandramouli, V.; Schumann, W.C.; Cobelli, C.; Landau, B.R.; Vilstrup, H.; Rizza, R.A.; Schmitz, O. Impaired basal glucose effectiveness but unaltered fasting glucose release and gluconeogenesis during short-term hypercorti-solemia in healthy subjects. Am. J. Physiol.-Endocrinol. Metab. 2004, 286, E102–E110. [Google Scholar] [CrossRef]
- Dennis, D.T.; Greyson, M.F. Fructose 6-phosphate metabolism in plants. Physiol. Plant 1987, 69, 395–404. [Google Scholar] [CrossRef]
- Mustroph, A.; Sonnewald, U.; Biemelt, S. Characterisation of the ATP-dependent phosphofructokinase gene family from Ara-bidopsis thaliana. FEBS Lett. 2007, 581, 2401–2410. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, J.; Chen, Y.; Pan, H.; Ming, R. Identification and genes expression analysis of ATP-dependent phosphofructo-kinase family members among three Saccharum species. Funct. Plant Biol. 2012, 40, 369–378. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The catalytic direction of pyrophosphate: Fructose 6-phosphate 1-phosphotransferase in rice coleoptiles in anoxia. Physiol. Plant. 2002, 116, 345–350. [Google Scholar] [CrossRef]
- Lü, H.; Li, J.; Huang, Y.; Zhang, M.; Zhang, S.; Wu, J. Genome-wide identification, expression and functional analysis of the phosphofructokinase gene family in Chinese white pear (Pyrus bretschneideri). Gene 2019, 702, 133–142. [Google Scholar] [CrossRef]
- Stitt, M. Fructose-2, 6-bisphosphate as a regulatory molecule in plants. Annu. Rev. Plant Biol. 1990, 41, 153–185. [Google Scholar] [CrossRef]
- Hajirezaei, M.; Sonnewald, U.; Viola, R.; Carlisle, S.; Dennis, D.; Stitt, M. Transgenic potato plants with strongly decreased expression of pyrophosphate: Fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 1993, 192, 16–30. [Google Scholar] [CrossRef]
- Spilatro, S.R.; Anderson, J.M. Carbohydrate metabolism and activity of pyrophosphate: Fructose-6-phosphate phosphotrans-ferase in photosynthetic soybean (Glycine max, Merr.) suspension cells. Plant Physiol. 1988, 88, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-P.; Sung, S.-J.S.; Black, C.C. Sucrose metabolism in lima bean seeds. Plant Physiol. 1989, 89, 1106–1116. [Google Scholar] [CrossRef]
- Kruger, N.J.; Dennis, D.T. Molecular properties of pyrophosphate: Fructose-6-phosphate phosphotransferase from potato tuber. Arch. Biochem. Biophys. 1987, 256, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-H.; Shi, J.-N. Evidence that fructose 1, 6-bisphosphate specifically protects the α-subunit of pyrophosphate-dependent 6-phosphofructo-1-phosphotransferase against proteolytic degradation. FEBS Lett. 1999, 459, 448–452. [Google Scholar] [CrossRef]
- Guo, X.; Ronhovde, K.; Yuan, L.; Yao, B.; Soundararajan, M.P.; Elthon, T.; Zhang, C.; Holding, D.R. Pyrophosphate-dependent fructose-6-phosphate 1-phosphotransferase induction and attenuation of Hsp gene expression during endosperm modification in quality protein maize. Plant Physiol. 2012, 158, 917–929. [Google Scholar] [CrossRef]
- Podestá, F.E.; Plaxton, W.C. Fluorescence study of ligand binding to potato tuber pyrophosphate-dependent phosphofructo-kinase: Evidence for competitive binding between fructose-1, 6-bisphosphate and fructose-2, 6-bisphosphate. Arch. Biochem. Biophys. 2003, 414, 101–107. [Google Scholar] [CrossRef]
- Theodorou, M.; Cornel, F.; Duff, S.; Plaxton, W. Phosphate starvation-inducible synthesis of the alpha-subunit of the pyro-phosphate-dependent phosphofructokinase in black mustard suspension cells. J. Biol. Chem. 1992, 267, 21901–21905. [Google Scholar] [CrossRef]
- Wong, J.H.; Kiss, F.; Wu, M.-X.; Buchanan, B.B. Pyrophosphate fructose-6-P 1-phosphotransferase from tomato fruit: Evidence for change during ripening. Plant Physiol. 1990, 94, 499–506. [Google Scholar] [CrossRef]
- Huang, S.; Colmer, T.D.; Millar, A.H. Does anoxia tolerance involve altering the energy currency towards PPi? Trends Plant Sci. 2008, 13, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Van Schaftingen, E.; Hers, H.-G. Fructose 2, 6-bisphosphate in relation with the resumption of metabolic activity in slices of Jerusalem artichoke tubers. FEBS Lett. 1983, 164, 195–200. [Google Scholar] [CrossRef]
- Fahrenkrog, A.M.; Neves, L.G.; Resende, M.F., Jr.; Dervinis, C.; Davenport, R.; Barbazuk, W.B.; Kirst, M. Population genomics of the eastern cottonwood (Populus deltoides). Ecol. Evol. 2017, 7, 9426–9440. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wu, H.; Zhang, J.; Pan, Z.; Zhao, W.; Li, Z.; Tong, C. Genome assembly of Salicaceae Populus deltoides (Eastern Cottonwood) I-69 based on nanopore sequencing and Hi-C technologies. J. Hered. 2021, 112, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for inter-active analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Debarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for de-tection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL): An online tool for phylogenetic tree display and annotation. Bioinformatics 2007, 23, 127–128. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pettengill, E.A.; Parmentier-Line, C.; Coleman, G.D. Evaluation of qPCR reference genes in two genotypes of Populus for use in photoperiod and low-temperature studies. BMC Res. Notes 2012, 5, 366. [Google Scholar]
- Eddy, S.R. Bioinformatics Review. Bioinformatics 1998, 14, 755–763. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Shen, X.; Xia, Z.; Zhou, X.; Chen, X.; Lu, C. Genome-wide survey of the phosphofructokinase family in cassava and functional characterization in response to oxygen-deficient stress. BMC Plant Biol. 2021, 21, 375. [Google Scholar] [CrossRef] [PubMed]
- Mehari, T.G.; Xu, Y.; Umer, M.J.; Hui, F.; Cai, X.; Zhou, Z.; Hou, Y.; Wang, K.; Wang, B.; Liu, F. Genome-Wide Identification and Expression Analysis Elucidates the Potential Role of PFK Gene Family in Drought Stress Tolerance and Sugar Metabolism in Cotton. Front. Genet. 2022, 13, 922024. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Manna, M.; Thakur, T.; Gautam, V.; Salvi, P. Imperative role of sugar signaling and transport during drought stress responses in plants. Physiol. Plant. 2021, 171, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Basson, C.; Groenewald, J.-H.; Kossmann, J.; Cronjé, C.; Bauer, R. Upregulation of pyrophosphate: Fructose 6-phosphate 1-phosphotransferase (PFP) activity in strawberry. Transgenic Res. 2011, 20, 925–931. [Google Scholar] [CrossRef]
- Lim, H.; Cho, M.-H.; Jeon, J.-S.; Bhoo, S.H.; Kwon, Y.-K.; Hahn, T.-R. Altered expression of pyrophosphate: Fruc-tose-6-phosphate 1-phosphotransferase affects the growth of transgenic Arabidopsis plants. Mol. Cells 2009, 27, 641–649. [Google Scholar] [CrossRef]
- Lim, H.-M.; Cho, J.-I.; Lee, S.; Cho, M.-H.; Bhoo, S.H.; An, G.; Hahn, T.-R.; Jeon, J.-S. Identification of a 20-bp regulatory element of the Arabidopsis pyrophosphate: Fructose-6-phosphate 1-phosphotransferase α2 gene that is essential for expression. Plant Cell Rep. 2007, 26, 683–692. [Google Scholar] [CrossRef]
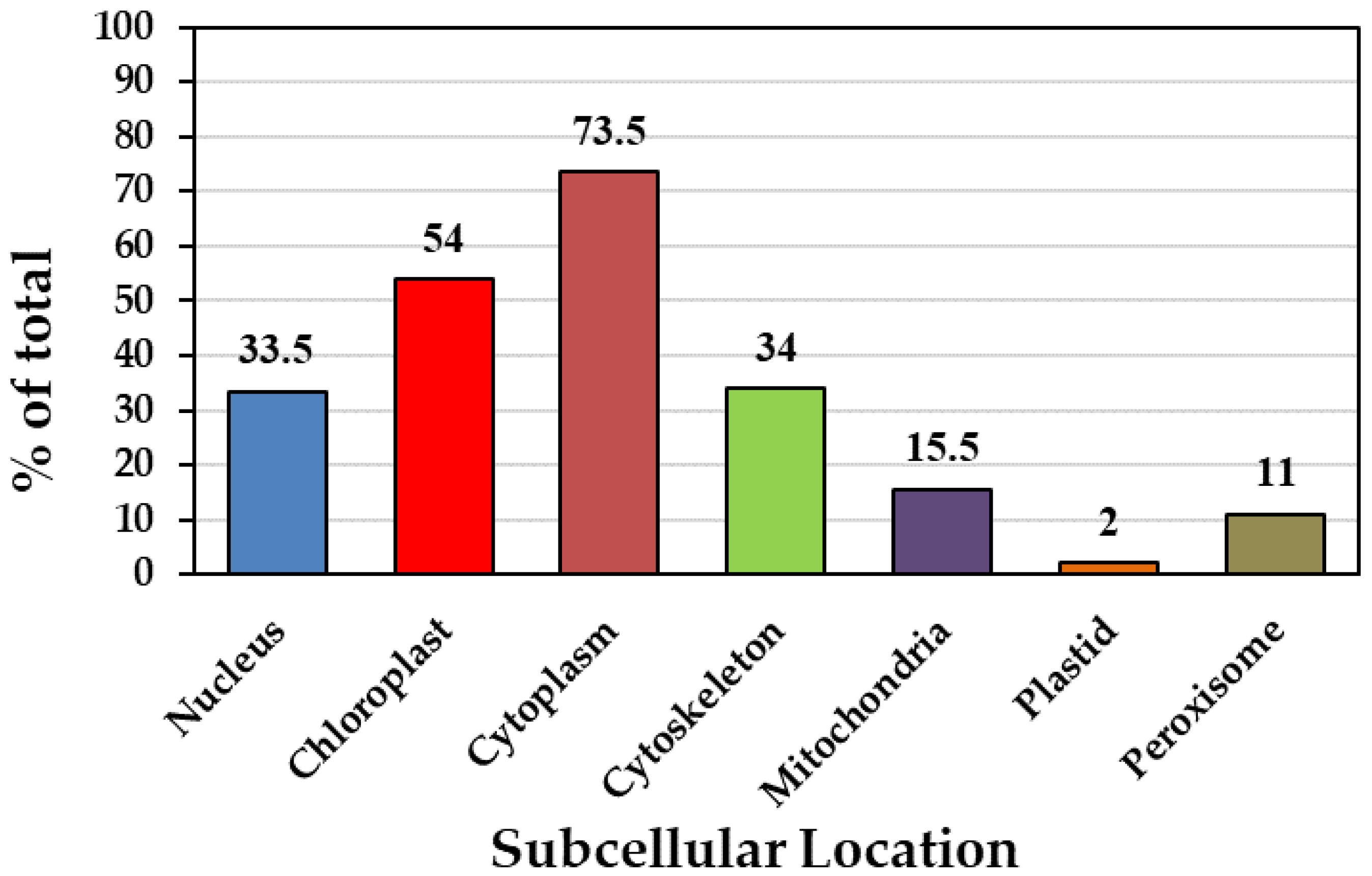



| Gene Name | Transcript ID | CDS (bp) | Protein Length (aa) | Molecular Weight (KDa) | Theoretical PI | Gravy | Predicted Localization |
|---|---|---|---|---|---|---|---|
| Podel.01G054800.1.p | Podel.01G054800.1 | 672 | 223 | 25.08 | 8.59 | −0.437 | Nucleus |
| Podel.01G082600.1.p | Podel.01G082600.1 | 261 | 86 | 9.74 | 8.21 | 0.029 | Chloroplast |
| Podel.02G002900.1.p | Podel.02G002900.1 | 1590 | 529 | 57.94 | 6.78 | −0.103 | Cytoplasm |
| Podel.03G163700.1.p | Podel.03G163700.1 | 1515 | 504 | 55.83 | 7.65 | −0.127 | Cytoplasm |
| Podel.04G003100.1.p | Podel.04G003100.1 | 1686 | 516 | 61.28 | 6.00 | −0.131 | Peroxisome |
| Podel.05G274300.1.p | Podel.05G274300.1 | 1854 | 617 | 67.24 | 6.39 | −0.115 | Cytoplasm |
| Podel.06G165500.1.p | Podel.06G165500.1 | 1659 | 552 | 60.69 | 6.16 | −0.217 | Chloroplast |
| Podel.06G165500.2.p | Podel.06G165500.2 | 1497 | 498 | 54.92 | 5.73 | −2.242 | Cytoplasm |
| Podel.06G247900.1.p | Podel.06G247900.1 | 1443 | 480 | 52.72 | 8.74 | −0.222 | Cytoplasm |
| Podel.06G247900.2.p | Podel.06G247900.2 | 1296 | 431 | 47.45 | 9.07 | −0.201 | Chloroplast |
| Podel.07G012400.1.p | Podel.07G012400.1 | 1599 | 532 | 58.34 | 6.83 | −0.112 | Cytoplasm |
| Podel.11G024700.1.p | Podel.11G024700.1 | 1284 | 427 | 47.20 | 6.24 | −0.22 | Cytoplasm |
| Podel.16G002300.1.p | Podel.16G002300.1 | 1419 | 472 | 52.08 | 5.82 | −0.161 | Cytoskeleton |
| Podel.18G067900.1.p | Podel.18G067900.1 | 1479 | 492 | 54.57 | 5.73 | −0.294 | Cytoskeleton |
| Podel.18G067900.2.p | Podel.18G067900.2 | 1479 | 492 | 54.57 | 5.73 | −0.294 | Cytoskeleton |
| Podel.T151900.1.p | Podel.T151900.1 | 948 | 315 | 34.77 | 6.56 | −0.121 | Mitochondria |
| Podel.T152000.1.p | Podel.T152000.1 | 699 | 232 | 24.50 | 5.71 | −0.189 | Chloroplast |
| Motif | Symbol | Length | Amino Acid Sequence Information |
|---|---|---|---|
| motif 1 |  | 50 | INAAHVEAESVENGIGLVKLMGRYSGFIAMEATLASRDVDCCLIPESPFY |
| motif 2 |  | 50 | YIDPTYMIRAVPPNASDNVYCTLLAQSAVHGAMAGYTGFTSGVVNGRQPY |
| motif 3 |  | 43 | HFRRAGPRQKVYFEPDEVRACIVTCGGLCPGLNTVIREIVYSL |
| motif 4 |  | 50 | LTPKVVNDIHKRGGTILGTSRGGHDTSKIVDSIQDRGINQVYIIGGDGTQ |
| motif 5 |  | 41 | EIRRRGLKVAVAGIPKTIDNDIPVIDRSFGFDTAVEEAQRA |
| motif 6 |  | 41 | LEGKGGLFEYIEKRLKENGHMVIVIAEGAGQELLSESMQSD |
| motif 7 |  | 50 | DVPHLTDYIPBLPTYSNPLQDNPAYSVVKQYFVHVDDSVPQKIVVHKDSP |
| motif 8 |  | 41 | IPFYRINEKQNKVVITDRMWARLLSSTNQPSFISNKEVIED |
| motif 9 |  | 29 | ASGNKLLQDVGLWISQGIKDYFSRQKKMA |
| motif 10 |  | 50 | NQSLQLFEFLPPAIQEQLMLERDPHGNVQVAKIETEKMLIQMVETELEKR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-L.; Denison, M.I.J.; Lim, H.; Chung, H.; Oh, C. Genome-Wide Analysis, Identification, and Characterization of the PFK Gene Family Members of Populus deltoides. Forests 2023, 14, 1104. https://doi.org/10.3390/f14061104
Kim T-L, Denison MIJ, Lim H, Chung H, Oh C. Genome-Wide Analysis, Identification, and Characterization of the PFK Gene Family Members of Populus deltoides. Forests. 2023; 14(6):1104. https://doi.org/10.3390/f14061104
Chicago/Turabian StyleKim, Tae-Lim, Michael Immanuel Jesse Denison, Hyemin Lim, Hoyong Chung, and Changyoung Oh. 2023. "Genome-Wide Analysis, Identification, and Characterization of the PFK Gene Family Members of Populus deltoides" Forests 14, no. 6: 1104. https://doi.org/10.3390/f14061104





