Soil Nutrient, Salinity, and Alkalinity Responses of Dendrocalamopsis oldhami in High-Latitude Greenhouses Depending on Planting Year and Nitrogen Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Methods for Determining Soil Salt Ions and Nutrients (Soil and Bamboo Shoot)
2.4. Statistical Analysis
3. Results
3.1. Growth of D. oldhami in Different Planting Years (2017–2019)
3.2. Soil Properties in Different Planting Years (2017–2019)
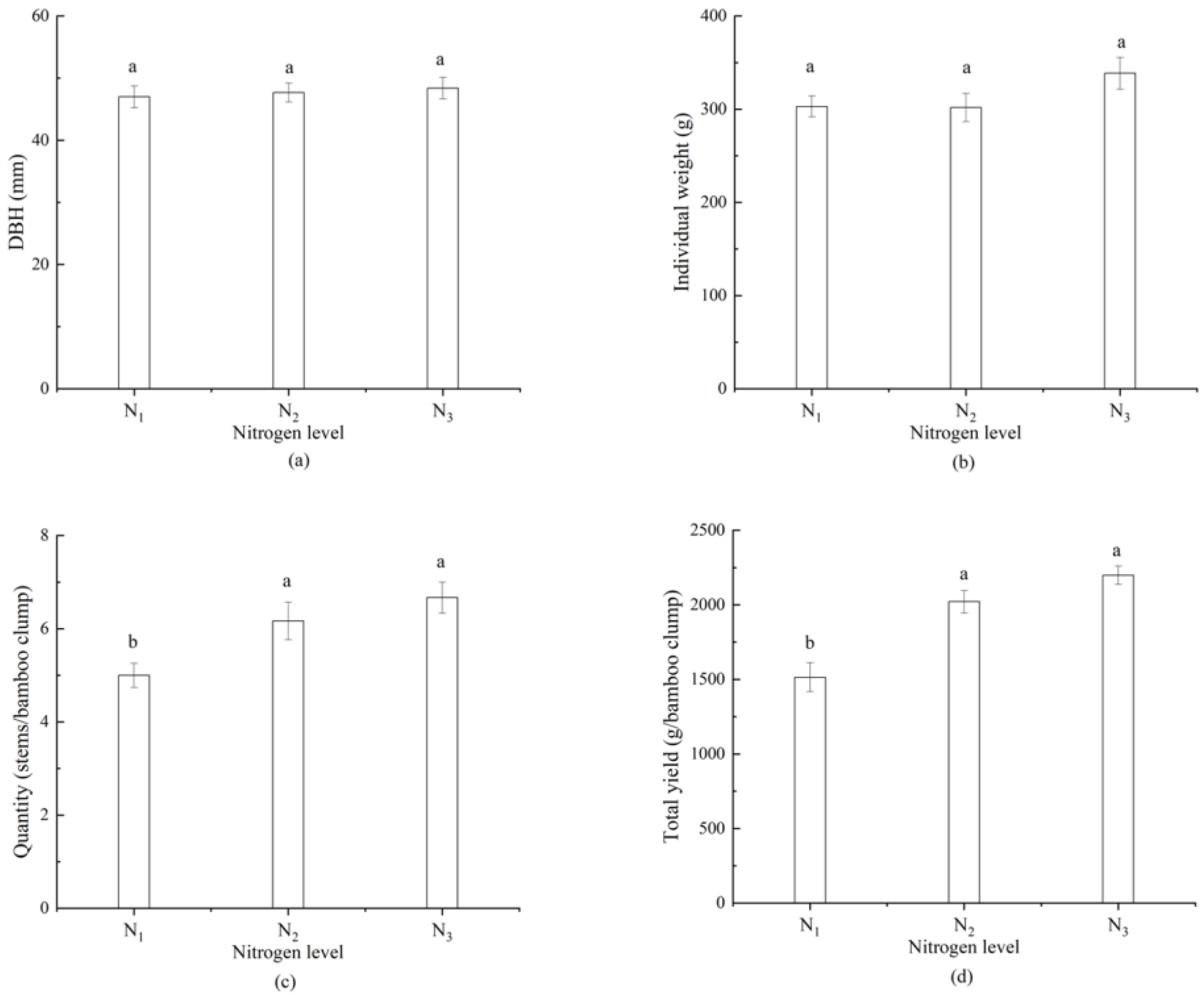
3.3. Response of Bamboo Shoots Yield and Nutrient Content to Nitrogen Application
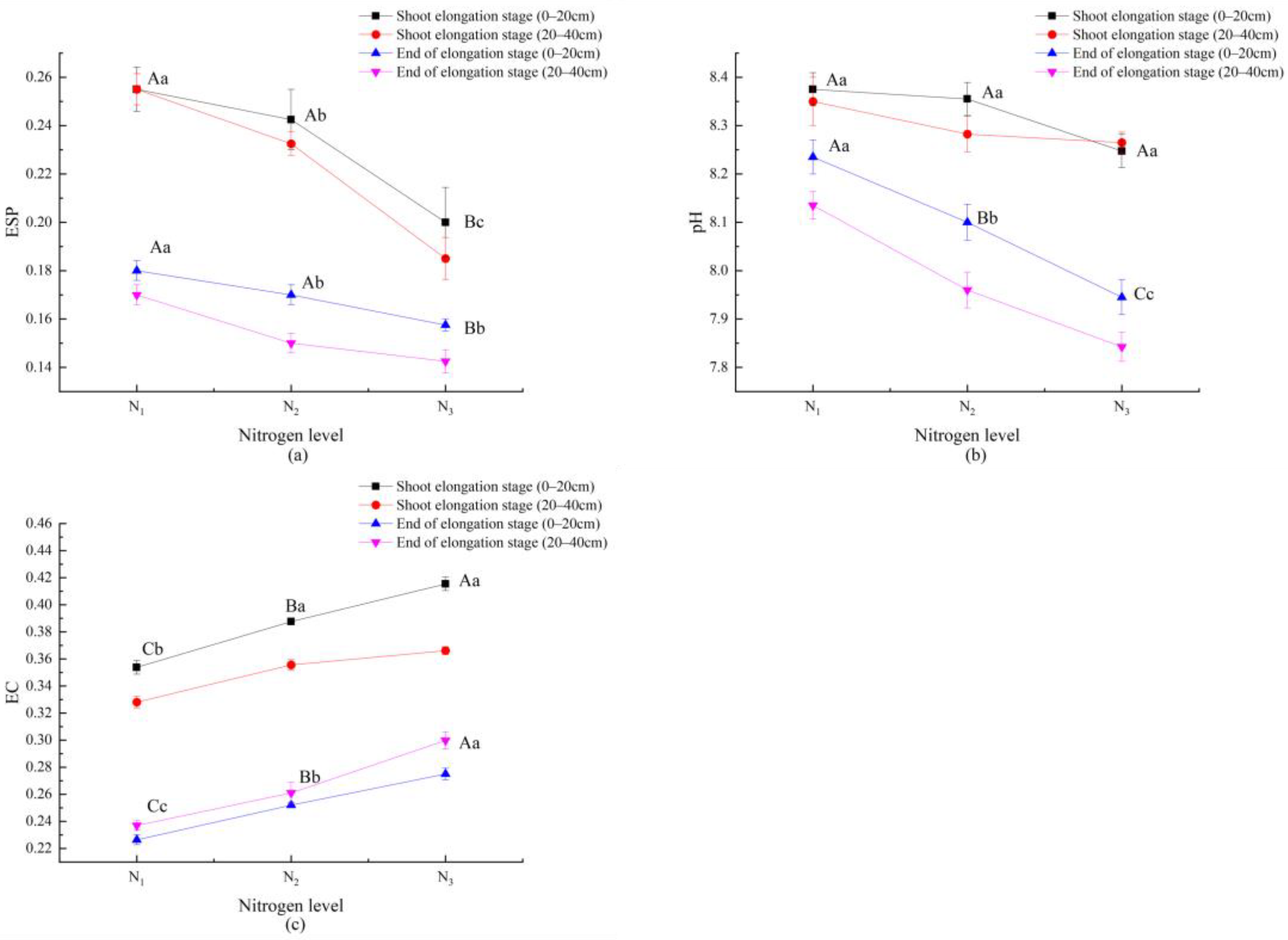
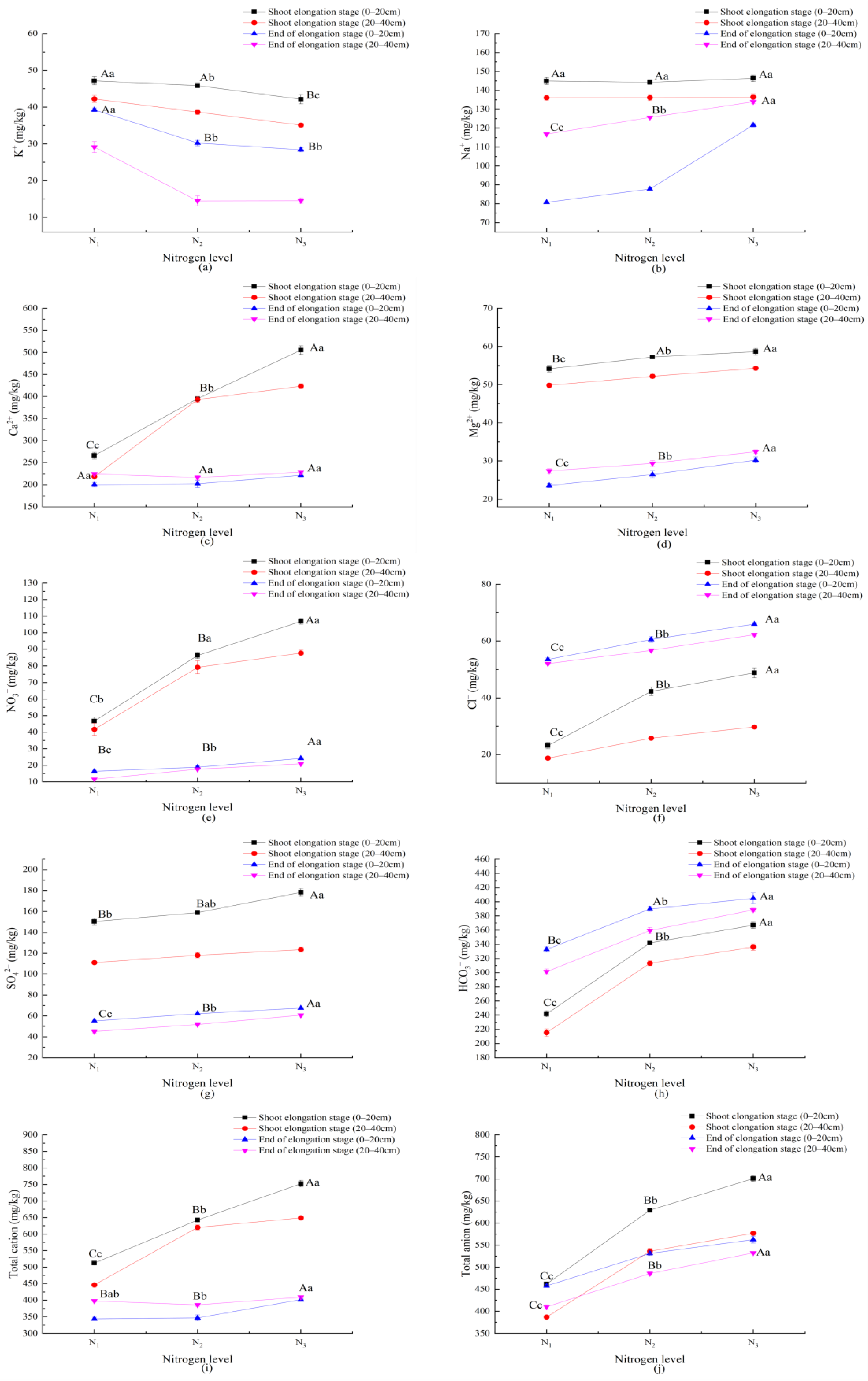
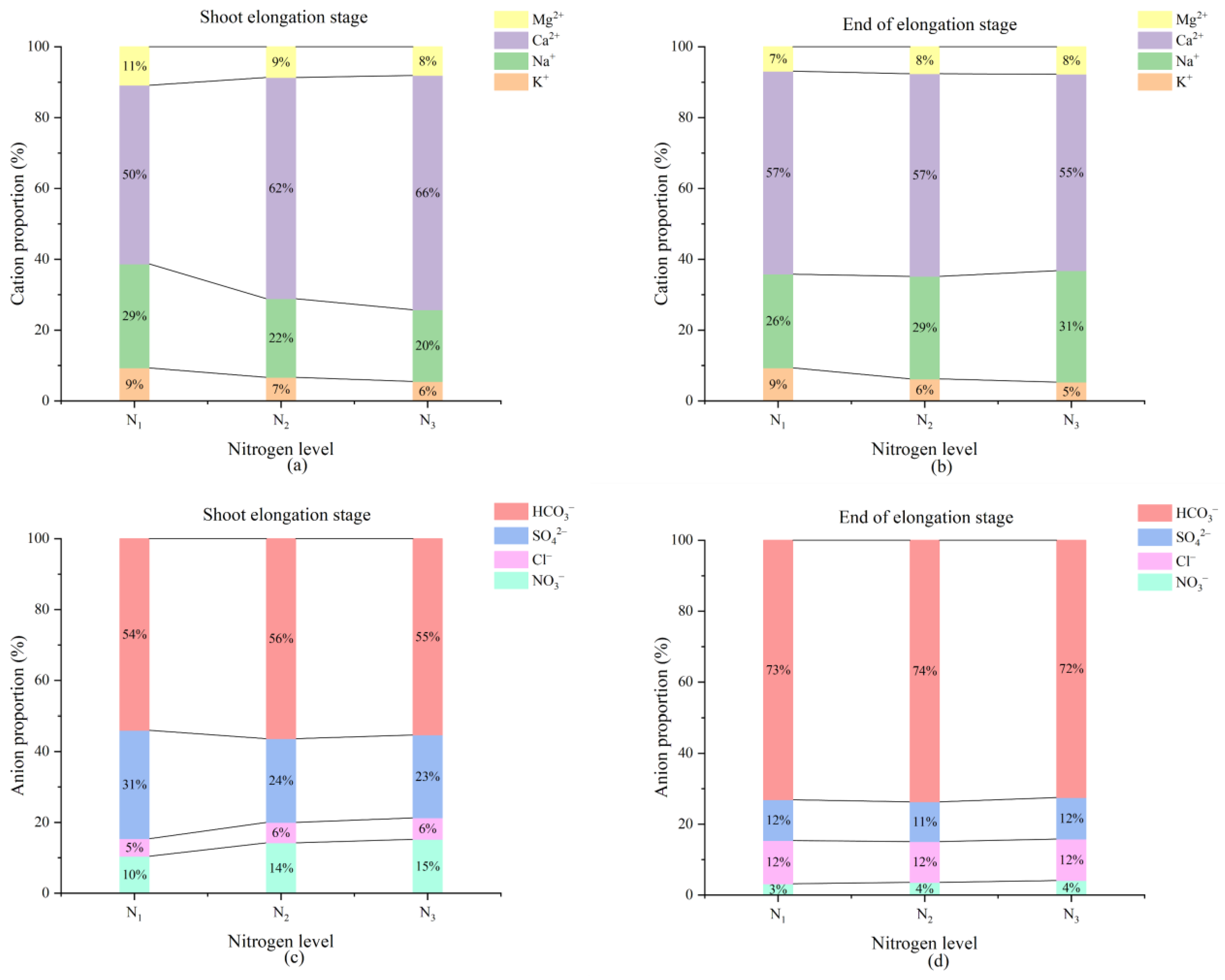
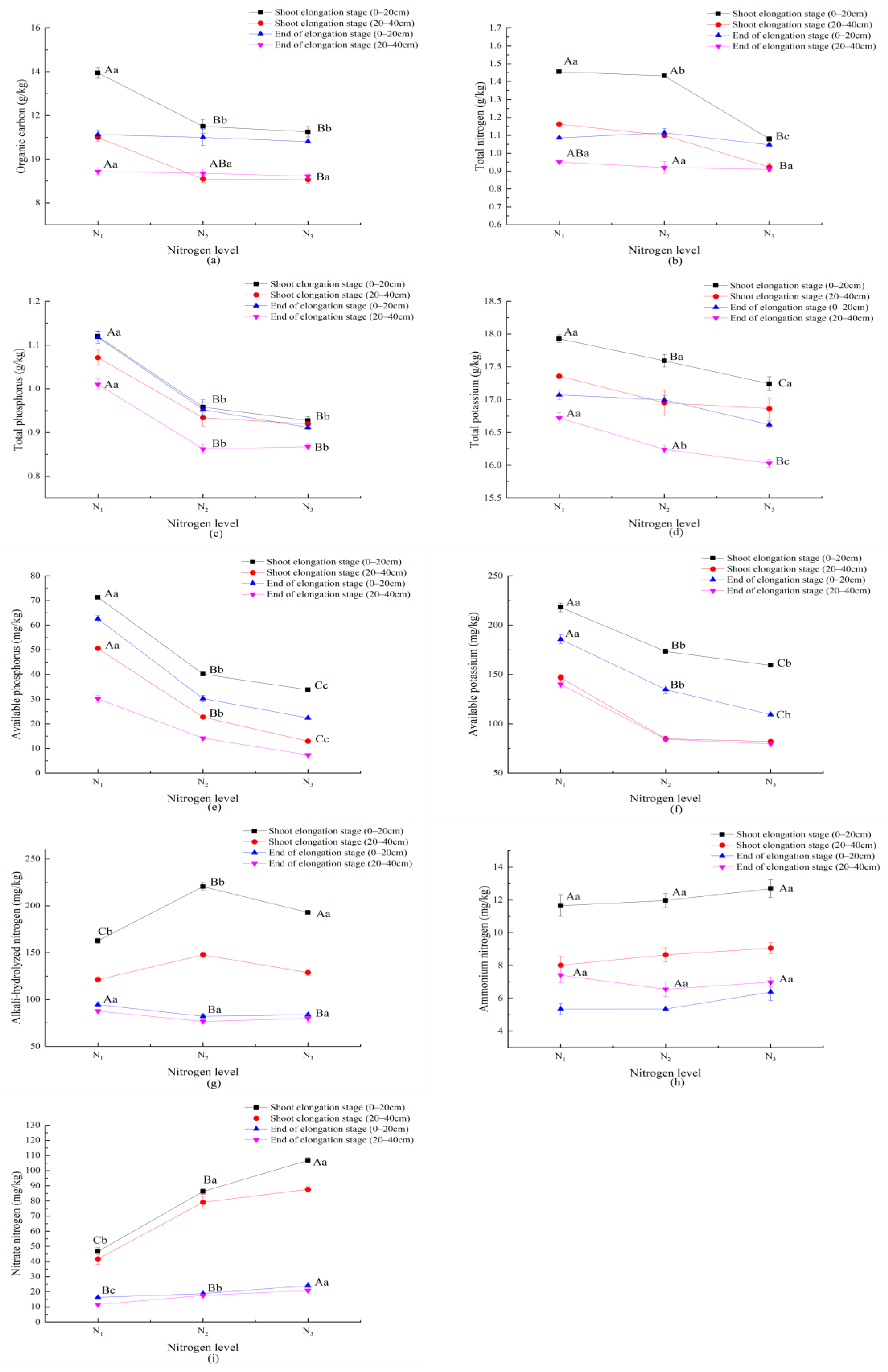
3.4. Response of Soil Properties to Nitrogen Application
4. Discussion
4.1. Effects of Planting Years on Soil Saline–Alkali Properties and Nutrient Changes
4.2. Effects of Nitrogen Application on Soil Saline–Alkali Properties, Soil Nutrients, and Bamboo Shoots Nutrients during the Growing Stage
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iizumi, T.; Furuya, J.; Shen, Z.; Kim, W.; Okada, M.; Fujimori, S.; Hasegawa, T.; Nishimori, M. Responses of crop yield growth to global temperature and socioeconomic changes. Sci. Rep. 2017, 7, 7800. [Google Scholar] [CrossRef] [PubMed]
- Trresen, K.; Fykse, H.; Rafoss, T.; Gerowitt, B. Autumn growth of three perennial weeds at high latitude benefits from climate change. Glob. Chang. Biol. 2019, 26, 2561–2572. [Google Scholar] [CrossRef]
- Xiao, G.; Zhang, Q.; Wang, J. Impact of global climate change on agro-ecosystem. Chin. J. Appl. Ecol. 2007, 18, 1877–1885. (In Chinese) [Google Scholar]
- Lobell, D.; Gourdji, S. The influence of climate change on global crop productivity. Plant Physiol. 2012, 160, 1686–1697. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Ort, D.R. How do we improve crop production in a warming world? Plant Physiol. 2010, 154, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Wng, X.; Cheng, J. Cold Resistance Assessment of Bambusoideae Beijing Botanical Garden. World Bam. Rat. 2012, 10, 1–8. (In Chinese) [Google Scholar]
- Xia, W.; Yin, Z.; Guan, F. Physiological response characteristics of Phyllostachys aureosulcata and its varieties after natural extreme low temperature. J. Beijing For. Univ. 2022, 44, 75–84. (In Chinese) [Google Scholar]
- Munir, A.; Mahtab, A.; Ahmed, H.N.; Adel, R.; Adel, A.; Meththika, V.; Jamal, E.; Abdulelah, A.F.; Mohammad, I.W. Aging Effects of Organic and Inorganic Fertilizers on Phosphorus Fractionation in a Calcareous Sandy Loam Soil. Pedosphere 2018, 28, 873–883. [Google Scholar]
- Zhang, Z.; Sun, D.; Tang, Y.; Zhu, R.; Li, X.; Gruda, N.; Dong, J.; Duan, Z. Plastic shed soil salinity in China: Current status and next steps. J. Clean. Prod. 2021, 296, 126453. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, Y.; Chen, Z.; Wang, X.; Huang, B. Comprehensive assessments of soil fertility and environmental quality in plastic greenhouse production systems. Geoderma 2021, 385, 114899. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Liang, W. Accumulation of Soil Soluble Salt in Vegetable Greenhouses Under Heavy Application of Fertilizers. Agric. J. 2013, 1, 123–127. [Google Scholar]
- Li, J.; Han, J. Transport Characteristics of Soil Salinity in Saline-alkali Land under Water Storage and Drainage Conditions. Asian Agric. Res. 2015, 7, 65–69,72. [Google Scholar]
- Li, T.; Yu, L.; Wu, Y.; Wan, G.; Li, J. Secondary Salinization of Greenhouse Vegetable Soils and Its Affecting Factors in Shandong Province, China. Acta Pedol. Sin. 2018, 55, 100–110. (In Chinese) [Google Scholar]
- Chen, B.; Yang, H.; Li, Y.; Li, X.; Zhou, J. Variation Characteristics of Soil Water-soluble Salts of Large Plastic House Vegetable Field for Different Cultivating Years. J. Soil Water Conserv. 2012, 26, 241–245. (In Chinese) [Google Scholar]
- Yang, S.; Huo, L.; Wang, C.; Jiang, W. Salt accumulation and ion composition changes in soil under solar greenhouses in Lanzhou Region. J. Agro Environ. Sci. 2016, 35, 1541–1549. (In Chinese) [Google Scholar]
- Qian, X. Characteristics of secondary salinization of vegetable soil in plastic film greenhouse. Soil Fertil. Sci. Chin. 2017, 2, 73–79. (In Chinese) [Google Scholar]
- Sun, H.; Wei, C.; Xu, W.; Yang, J.; Wang, X.; Qiu, Y. Characteristics of salt contents in soils under greenhouse conditions in China. Environ. Sci. Pollut. Res. Int. 2019, 26, 3882–3892. [Google Scholar] [CrossRef]
- Zhang, J.; Sui, S.; Li, Y.; Wei, M.; Zhao, L. Soil Salinity Changes in Greenhouse with Years and Their Effects on Soil Degradation. Soils 2019, 51, 1183–1187. (In Chinese) [Google Scholar]
- Wang, Y.; Li, S.; Qin, Y.; Lv, J. Study on dynamics of soil salinity and nutriation of greenhouses in different years. Agric. Res. Arid Areas 2011, 29, 161–164. (In Chinese) [Google Scholar]
- Dang, J.; Guo, W.; Guo, J.; Lv, J.; Wang, J. Study of the Regularity of the Salt Accumulation of Topsoil and NO3−-N Migration in Greenhouse Soil and Years of Vegetables Cultivation. Chin. Agric. Sci. Bull. 2004, 20, 189–191. (In Chinese) [Google Scholar]
- Fei, L.; Zhao, M.; Chen, X.; Shi, Y. Effects of Phosphorus Accumulation in Soil with the Utilization Ages of the Vegetable Greenhouses in the Suburb of Shenyang. Procedia Environ. Sci. 2011, 8, 16–20. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Shi, Y.; Niu, M. Available K and Total Organic C Accumulation in Soil with the Utilization Ages of the Vegetable Greenhouses in the Suburb of Shenyang. Procedia Environ. Sci. 2011, 8, 48–53. [Google Scholar] [CrossRef]
- Shi, W.; Yao, J.; Yan, F. Vegetable cultivation under greenhouse conditions leads to rapid accumulation of nutrients, acidification and salinity of soils and groundwater contamination in South-Eastern China. Nutr. Cycl. Agroecosyst. 2009, 83, 73–84. [Google Scholar] [CrossRef]
- Hang, S.; Wang, Y.; Jin, J.; Tang, J. Status of salinity, pH and nutrients in soils in main vegetable production regions in China. J. Plant Nutr. Fertil. 2011, 17, 906–918. (In Chinese) [Google Scholar]
- Chen, Y.; Liu, Y.; Ge, J.; Li, R.; Zhang, R.; Zhang, Y.; Huo, Z.; Xu, K.; Wei, H.; Dai, Q. Improved physiological and morphological traits of root synergistically enhanced salinity tolerance in rice under appropriate nitrogen application rate. Front. Plant Sci. 2022, 13, 982637. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, V.; Syvertsen, J.; Nieves, M.; Simón, I.; Martínez, V.; García-Sánchez, F. Additional nitrogen fertilization affects salt tolerance of lemon trees on different rootstocks. Sci. Hortic. 2009, 121, 298–305. [Google Scholar] [CrossRef]
- Sun, X.; Guo, Y.; Zeng, L.; Li, X.; Liu, X.; Li, J.; Cui, D. Combined Urea Humate and Wood Vinegar Treatment Enhances Wheat–Maize Rotation System Yields and Nitrogen Utilization Efficiency through Improving the Quality of Saline–Alkali Soils. J. Soil Sci. Plant Nut. 2021, 21, 1759–1770. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Shao, X.; Geng, Y.; Liu, J. Effects of combined nitrogen fertilizer and nano-carbon application on yield and nitrogen use of rice grown on saline-alkali soil. J. Food Agric. Environ. 2012, 10, 558–562. [Google Scholar]
- Meng, X.; Ran, C.; Liu, B.; Zhao, Z.; Bai, T.; Zhao, M.; Cheng, Z.; Chen, G.; Geng, Y. Effect of straw return with nitrogen fertilizer on photosynthetic characteristics and yield of rice in soda saline–alkali rice paddy fields. Cereal Res. Commun. 2022. Available online: https://link.springer.com/article/10.1007/s42976-022-00312-y (accessed on 22 April 2023).
- Wei, Q.; Yin, R.; Huang, J.; Vogler, A.P.; Li, Y.; Miao, X.; Kardol, P. The diversity of soil mesofauna declines after bamboo invasion in subtropical China. Sci. Total Environ. 2021, 789, 147982. [Google Scholar] [CrossRef]
- Ouyang, M.; Tian, D.; Pan, J.; Chen, G.; Su, H.; Yan, Z.; Yang, Q.; Ji, C.; Tang, Z.; Fang, J. Moso bamboo (Phyllostachys edulis) invasion increases forest soil pH in subtropical China. Catena 2022, 215, 106339. [Google Scholar] [CrossRef]
- Xu, Q.; Liang, C.; Chen, J.; Li, Y.; Qin, H.; Fuhrmann, J. Rapid bamboo invasion (expansion) and its effects on biodiversity and soil processes. Glob. Ecol. Conserv. 2020, 21, e00787. [Google Scholar] [CrossRef]
- Li, W.; Sheng, H.; Liu, Y.; Chen, W. Responses of soil bacterial compositions to concentrations of nitrogen forms in the process of Moso bamboo invasion. Ecol. Res. 2019, 34, 743–752. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Y.; Qin, H.; Liang, C.; Shao, S.; Fuhrmann, J.; Chen, J.; Xu, Q. Moso bamboo invasion has contrasting effects on soil bacterial and fungal abundances, co-occurrence networks and their associations with enzyme activities in three broadleaved forests across subtropical China. For. Ecol. Manag. 2021, 498, 119549. [Google Scholar] [CrossRef]
- Wang, H.; Tian, G.; Chiu, C. Invasion of moso bamboo into a Japanese cedar plantation affects the chemical composition and humification of soil organic matter. Sci. Rep. 2016, 6, 32211. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Ren, J.; Zhang, S.; Wang, Y.; Fang, Y. Forest and grass composite patterns improve the soil quality in the coastal saline-alkali land of the Yellow River Delta, China. Geoderma 2019, 349, 25–35. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, X.; Chen, Q.; Rong, J.; He, T.; Zheng, Y. Research Progress on Response of Bamboo Plants to Salt Stress and Cultivation and Conservation. J. Anhui Agric. Sci. 2020, 48, 6–10. [Google Scholar]
- Li, K. Study of Photosynthetic Physiological Response of Bambusa. oldhami Leaves under Sea Salt Stress. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2013. [Google Scholar]
- Jin, C.; Wang, Y. A Study on the Technique for Seabeach Cultivation of Dendrocalamopsis oldhami. For. Res. 1997, 10, 42–45. (In Chinese) [Google Scholar]
- Yin, Z.; Fan, S.; Xia, W.; Zhou, Y.; Zhou, X.; Zhang, X.; Li, C.H.; Guan, F. Response of growth, metabolism and yield of Dendrocalamopsis oldhami to long-day photoperiod and fertilizer compensation. J. For. Res. 2023, 34, 151–166. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, Y.; Zhang, M.; Jiang, X.; Yan, X.; Huang, L.; Guan, F. Interactive effects of light intensity and nitrogen supply on leaf carbon and nitrogen metabolism of Dendrocalamopsis oldhami. Chin. J. Ecol. 2020, 39, 3979–3988. (In Chinese) [Google Scholar]
- Lu, Y. Study on Height Growth Control and Provenance Adaptation of Dendrocalamopsis oldhami in Facility Cultivation. Master’s Thesis, Chinese Academy of Forestry, Beijing, China, 2019. [Google Scholar]
- Zhu, Y. Dendrocalamopsis oldhami Forest Management; Xiamen University Press: Xiamen, China, 2017; pp. 88, 192. [Google Scholar]
- Singh, D.; Shi, L.; Adams, J.M. Bacterial diversity in the mountains of South-West China: Climate dominates over soil parameters. J. Microbiol. 2013, 51, 439–447. [Google Scholar]
- Wang, X.; Cui, S.; Wang, H.; Li, Z. Response of cucumber photosynthetic system to long-term high temperature and CO2 enrichment stress in solar greenhouse. Modern Agric. 2020, 5, 18–21. (In Chinese) [Google Scholar]
- Li, F.; Zhang, H.; Zhang, Z.; Wang, Q.; Li, W.; Gao, L.; Ding, S. Introduction of Hungarian grape variety KM183 in Beijing greenhouse. Shanxi Fruit Tree 2015, 6, 19–20. (In Chinese) [Google Scholar]
- Wu, R.; Sun, H.; Xue, J.; Yan, D.; Liu, Y.; Gui, D.; Wang, X.; Yang, J. Acceleration of soil salinity accumulation and soil degradation due to greenhouse cultivation: A survey of farmers’ practices in China. Environ. Monit. Assess. 2020, 192, 399. [Google Scholar] [CrossRef]
- Chang, L.; Han, Z.; Zhang, D.; Zhu, X. Variations of total salt and salt ion compositions in vegetable greenhouse soil in suburbs of Qinhuangdao City. Chin. J. Soil Sci. 2010, 39, 1126–1130. (In Chinese) [Google Scholar]
- Huang, S.; Gao, W.; Tang, J.; Li, C. Total salt content and ion composition in tillage layer of soils in the main vegetable production regions of China. J. Plant Nutr. Fertil. 2016, 22, 965–977. (In Chinese) [Google Scholar]
- Yang, H.; Gu, F.; Du, T. Study on the Accumulation Characteristics of the Soil Nitrate Nitrogen and Salinity in Greenhouses of Different Cultivation Years. Chin. Agr. Sci. Bull. 2014, 30, 240–247. (In Chinese) [Google Scholar]
- Grieve, C.M.; Poss, J.; Suarez, D.; Dierig, D. Lesquerella growth and selenium uptake affected by saline irrigation water composition. Ind. Crops Prod. 2001, 13, 57–65. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Y.; Xu, A.; Zhang, L.; Zhou, J. Effects of the application of different nitrogen fertilizers on the ion compositions in solution of the greenhouse soil. J. Plant Nutr. Fertil. 2008, 14, 907–913. (In Chinese) [Google Scholar]
- Fan, Q.; Zhang, Y.; Chen, C. Effects of Protected Field Vegetable Cultivation on Soil Salinity Accumulating and pH. J. Soil Water Conserv. 2009, 23, 103–106. (In Chinese) [Google Scholar]
- Li, C.; Ji, G. Interactions of Cl−, SO42− and H2PO4− anions with soils as inferred from conductivity dispersion. Acta Pedol. Sin. 1999, 36, 54–60. (In Chinese) [Google Scholar]
- Wang, H.; Zhou, J.; Chen, X.; Li, S.; Du, C.; Dong, C. Interaction of NPK Fertilizers During Their Transformation in Soils: I. Dynamic Changes of Soil pH. Pedosphere 2003, 13, 257–262. [Google Scholar]
- Zhang, J.; Sui, S.; Wei, M. Effect of Different K+/Mg2+ Ration Nutrient Solutions on Soil Salinity. Agric. Sci. Technol. 2017, 18, 175–177. [Google Scholar]
- Song, Q.; Yang, Q.; Liu, J.; Yu, D.; Fang, K.; Xu, P.; He, Y. Effects of Phyllostachys edulis expansion on soil nitrogen mineralization and its availability in evergreen broadleaf forest. Chin. J. Appl. Ecol. 2013, 24, 338–344. (In Chinese) [Google Scholar]
- Zhang, W.; Fan, S.; Su, W.; Liu, G.; Zhou, J. A Dynamic Study on Trace Element of Rhizosphere Soil during Bamboo Forming Stage of Phyllostachys edulis. For. Res. 2010, 23, 586–591. (In Chinese) [Google Scholar]
- Kawakami, E.; Ataka, M.; Kume, T.; Shimono, K.; Harada, M.; Hishi, T.; Katayama, A. Root exudation in a sloping Moso bamboo forest in relation to fine root biomass and traits. PLoS ONE 2022, 17, e0266131. [Google Scholar] [CrossRef]
- Cui, C.; Zhang, J.; Guo, X. Study on the influence of Phyllostachy edulis invasion in broad-leaved forest on the soil physical properties. South Chin. For. Sci. 2020, 48, 1–6. (In Chinese) [Google Scholar]
- Chen, Q.; Cao, X.; Li, Y.; Sun, Q.; Dai, L.; Li, J.; Guo, Z.; Zhang, L.; Ci, L. Functional carbon nanodots improve soil quality and tomato tolerance in saline-alkali soils. Sci. Total Environ. 2022, 830, 154817. [Google Scholar] [CrossRef]
- Zhao, J.; Dong, Y.; Xie, X.; Li, X.; Zhang, X.; Shen, X. Effect of annual variation in soil pH on available soil nutrients in pear orchards. Acta Ecol. Sin. 2011, 31, 212–216. [Google Scholar] [CrossRef]
- Ingeborg, C.; Nicholas, C.; Andis, L.; Iveta, V.K.; Karsten, R.R. Nutrient release capability in Nordic and Baltic forest soils determined by dilute nitric acid extraction—Relationships with indicators for soil quality, pH and sustainable forest management. Ecol. Indic. 2019, 96, 540–547. [Google Scholar]
- Bai, S.; Conant, R.T.; Zhou, G.; Wang, Y.; Wang, N.; Li, Y.; Zhang, K. Effects of moso bamboo encroachment into native, broad-leaved forests on soil carbon and nitrogen pools. Sci. Rep. 2016, 6, 31480. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, Y.; Conant, R.T.; Zhou, G.; Xu, Y.; Wang, N.; Fang, F.; Chen, J. Can native clonal moso bamboo encroach on adjacent natural forest without human intervention? Sci. Rep. 2016, 6, 31504. [Google Scholar] [CrossRef]
- Tian, H.; Chen, G.; Chi, Z.; Melillo, J.M.; Hall, C. Pattern and variation of C:N:P ratios in China’s soils: A synthesis of observational data. Biogeochemistry 2010, 98, 139–151. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Shi, W.; Kronzucker, H.J.; Hou, L.; Yang, H.; Song, Q.; Liu, J.; Shi, J.; Yang, Q.; et al. Coordination of nitrogen uptake and assimilation favours the growth and competitiveness of moso bamboo over native tree species in high-NH4+ environments. J. Plant Physiol. 2021, 266, 153508. [Google Scholar] [CrossRef] [PubMed]
- Yanai, J.; Robinson, D.; Young, I.M.; Kyuma, K.; Kosaki, T. Effects of the chemical form of inorganic nitrogen fertilizers on the dynamics of the soil solution composition and on nutrient uptake by wheat. Plant Soil 1998, 202, 263–270. [Google Scholar] [CrossRef]
- Li, X. Soil Chemistry; Higher Education Press: Beijing, China, 2001; pp. 168–180. [Google Scholar]
- Yuan, X.; Yang, J.; Wang, Z.; Yuan, S.; Yang, Z.; Chen, Y.; Yang, Y. The effects of warming and nitrogen addition on soil nutrients in the soil solutions from a subtropical Cunninghamia lanceolata plantation. Acta Ecol. Sin. 2018, 38, 2323–2332. (In Chinese) [Google Scholar]
- Sun, B.; Hu, Z.; Lv, J.; Zhou, L.; Xu, C. The leaching solution chemistry of a broad_leaved forest red soil under simulated N deposition in Southern China. Acta Ecol. Sin. 2006, 26, 1872–1881. (In Chinese) [Google Scholar]
- Dong, Y.; Yang, J.; Zhao, X.; Yang, S.; Mulder, J.; Drsch, P.; Zhang, G. Nitrate leaching and N accumulation in a typical subtropical red soil with N fertilization. Geoderma 2022, 407, 115559. [Google Scholar] [CrossRef]
- Zhu, X.; Miao, P.; Wang, P.; Zhang, S.; Chen, Z.; Zhou, J. Variations and influencing factors of nitrate accumulation in the deep soil profiles of apple orchards on the Loess Plateau. Agric. Ecosyst. Environ. 2022, 335, 108005. [Google Scholar] [CrossRef]
- Xu, L.; Xing, A.; Du, E.; Shen, H.; Yan, Z.; Jiang, L.; Tian, D.; Hu, H.; Fang, J. Effects of nitrogen addition on leaf nutrient stoichiometry in an old-growth boreal forest. Ecosphere 2021, 12, e03335. [Google Scholar] [CrossRef]
- Bell, C.; Acosta, M.; McIntyre, N.; Cox, S.; Tissue, D.; Zak, J. Linking microbial community structure and function to seasonal differences in soil moisture and temperature in a Chihuahuan desert grassland. Microb. Ecol. 2009, 58, 827–842. [Google Scholar] [CrossRef]
- Bremer, E.; Kuikman, P. Influence of competition for nitrogen in soil on net mineralization of nitrogen. Plant Soil 1997, 190, 119–126. [Google Scholar] [CrossRef]
- Puri, G.; Ashman, M. Relationship between soil microbial biomass and gross N mineralisation. Soil Biol. Biochem. 1998, 30, 251–256. [Google Scholar] [CrossRef]
- Fan, J.; Wang, J.; Hu, X.; Chen, F. Seasonal dynamics of soil nitrogen availability and phosphorus fractions under urban forest remnants of different vegetation communities in Southern China. Urban For. Urban Green. 2014, 13, 576–585. [Google Scholar] [CrossRef]
- Wang, H.; Hu, G.; Xu, W.; Boutton, T.; Zhuge, Y.; Bai, E. Effects of nitrogen addition on soil organic carbon mineralization after maize stalk addition. Eur. J. Soil Biol. 2018, 89, 33–38. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Christie, P.; Li, J. Environmental implications of low nitrogen use efficiency in excessively fertilized hot pepper (Capsicum frutescens L.) cropping systems. Agric. Ecosyst. Environ. 2005, 111, 70–80. [Google Scholar] [CrossRef]
- Anthony, D. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints upon Nitrogen Absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. [Google Scholar]
- Fageria, N.; Baligar, V. Enhancing Nitrogen Use Efficiency in Crop Plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Grunes, D.; Viets, F.; Shih, S. Proportionate Uptake of Soil and Fertilizer Phosphorus by Plants as Affected by Nitrogen Fertilization: I. Growth Chamber Experiment. Soil Sci. Soc. Am. J. 1958, 22, 43–48. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef]
- Devenport, J.; Provost, J. Cranberry tissue nutrient levels as impacted by three levels of nitrogen fertilizer and their relationship to fruit yield and quality. J. Plant Nutr. 1994, 17, 1625–1634. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.; Kronzucker, H. The nitrogen-potassium intersection: Membranes, metabolism, and mechanism. Plant Cell Environ. 2017, 40, 2029–2041. [Google Scholar] [CrossRef]
- Reid, J.; Trolove, S.; Tan, Y.; Johnstone, P. Nitrogen or potassium preconditioning affects uptake of both nitrate and potassium in young wheat (Triticum aestivum). Ann. Appl. Biol. 2016, 168, 66–80. [Google Scholar] [CrossRef]
- Huan, H.; Zhou, J.; Wang, H.; Gao, Y.; Duan, Z. Effects of N and P Application on Available Nutrients and Water Soluble Salt Ions in the Greenhouse Soil. Chin. J. Trop. Crops 2009, 30, 939–946. (In Chinese) [Google Scholar]
- Piotrowska, D.; Długosz, J.; Frąc, M.; Gryta, A.; Breza, B. Enzymatic activity and functional diversity of soil microorganisms along the soil profile—A matter of soil depth and soil-forming processes. Geoderma 2022, 416, 115779. [Google Scholar] [CrossRef]
- Li, Y.; Duan, X.; Li, Y.; Li, Y.; Zhang, L. Interactive effects of land use and soil erosion on soil organic carbon in the dry-hot valley region of southern China. Catena 2021, 201, 105187. [Google Scholar] [CrossRef]

| Average Temperature (°C) | Maximum Temperature (°C) | Minimum Temperature (°C) | Soil Temperature (°C) | Soil Humidity (%) | ||||
|---|---|---|---|---|---|---|---|---|
| 0−15 cm | 15−30 cm | 30−45 cm | 0−15 cm | 15−30 cm | 30−45 cm | |||
| 23.86 | 41.70 | 1.20 | 22.79 | 22.14 | 21.26 | 60.48 | 63.64 | 69.35 |
| Year | Number of Transplanting | Number of Mother Bamboo Alive | Total Number of New Shoots Stems/m2 | Total Biomass of New Shoots g/m2 | Standing Culm Density Stems/m2 |
|---|---|---|---|---|---|
| 2017 | 309 | 114 | 0.21 | 21.41 | 0.29 |
| 2018 | - | - | 0.92 | 174.35 | 0.61 |
| 2019 | - | - | 0.99 | 274.96 | 0.69 |
| DBH mm | Sample Number | Minimum Value | Maximum Value | Individual Weight g | Sample Number | Minimum Value | Maximum Value | |
|---|---|---|---|---|---|---|---|---|
| Mother bamboo | 31.86 ± 0.58 | 114 | 17.39 | 49.11 | - | - | - | - |
| 2017 | 17.56 ± 0.38 | 247 | 5.43 | 34 | 104.01 ± 7.30 | 10 | 79.35 | 150.12 |
| 2018 | 35.40 ± 0.77 | 125 | 11.45 | 55.23 | 189.86 ± 10.31 | 20 | 116.74 | 285.7 |
| 2019 | 47.96 ± 1.19 | 20 | 34.64 | 55.34 | 277.27 ± 9.83 | 15 | 226.21 | 347.79 |
| Year | K+ mg/kg | Na+ mg/kg | Ca2+ mg/kg | Mg2+ mg/kg | Total Cation mg/kg |
| 2017 | 26.099 ± 0.632c | 90.540 ± 0.655c | 247.010 ± 3.196a | 40.128 ± 0.574a | 403.778 ± 4.277a |
| 2018 | 30.012 ± 0.699b | 93.911 ± 1.182b | 225.221 ± 2.909b | 34.679 ± 0.939b | 383.823 ± 5.548b |
| 2019 | 34.207 ± 0.717a | 98.790 ± 0.294a | 212.365 ± 1.904c | 25.485 ± 0.522c | 370.848 ± 2.964b |
| Year | NO3− mg/kg | Cl− mg/kg | SO42− mg/kg | HCO3− mg/kg | Total Anion mg/kg |
| 2017 | 18.623 ± 0.328a | 15.208 ± 1.022c | 123.100 ± 3.315c | 363.763 ± 5.664a | 520.695 ± 8.336a |
| 2018 | 19.355 ± 0.279a | 30.513 ± 0.475b | 135.300 ± 4.439b | 344.130 ± 4.806b | 529.300 ±9.574a |
| 2019 | 19.868 ± 0.566a | 52.750 ± 0.457a | 149.695 ± 1.185a | 317.037 ± 1.238c | 539.345 ±2.834a |
| Year | ESP | pH | EC | ||
| 2017 | 0.297 ± 0.004a | 8.263 ± 0.009a | 0.270 ± 0.005a | ||
| 2018 | 0.248 ± 0.006b | 8.228 ± 0.009ab | 0.240 ± 0.004b | ||
| 2019 | 0.175 ± 0.005c | 8.190 ± 0.027b | 0.233 ± 0.003b |
| Year | Organic Carbon g/kg | Total Nitrogen g/kg | C/N | Total Phosphorus g/kg | Total Potassium g/kg |
| 2017 | 9.645 ± 0.191a | 1.132 ± 0.005a | 8.527 ± 0.201b | 1.104 ± 0.021a | 17.140 ± 0.053a |
| 2018 | 10.188 ± 0.378a | 1.078 ± 0.006b | 9.452 ± 0.359a | 1.136 ± 0.016a | 17.223 ± 0.050a |
| 2019 | 10.280 ± 0.275a | 1.020 ± 0.007c | 10.092 ± 0.227a | 1.095 ± 0.014a | 17.035 ± 0.010a |
| Year | Available Phosphorus mg/kg | Available Potassium mg/kg | Alkali-Hydrolyzed Nitrogen mg/kg | Ammonium Nitrogen mg/kg | Nitrate Nitrogen mg/kg |
| 2017 | 28.133 ± 1.049c | 146.463 ± 1.134b | 59.038 ± 1.112c | 7.447 ± 0.296a | 18.623 ± 0.328a |
| 2018 | 32.300 ± 0.667b | 152.037 ± 3.227b | 70.013 ± 2.210b | 7.198 ± 0.353a | 19.355 ± 0.279a |
| 2019 | 46.363 ± 1.343a | 163.003 ± 3.819a | 91.168 ± 1.766a | 6.388 ± 0.362a | 19.868 ± 0.566a |
| Nitrogen Level | Nitrogen Concentration g/kg | Phosphorus Concentration g/kg | Potassium Concentration g/kg |
| N1 | 27.405 ± 0.323b | 2.658 ± 0.062c | 42.761 ± 0.247a |
| N2 | 31.378 ± 0.388a | 3.466 ± 0.063b | 40.193 ± 0.236b |
| N3 | 30.917 ± 0.362a | 3.856 ± 0.076a | 39.584 ± 0.256b |
| Nitrogen Level | Nitrogen Accumulation g | Phosphorus Accumulation g | Potassium Accumulation g |
| N1 | 2.740 ± 0.045b | 0.296 ± 0.010b | 4.287 ± 0.097a |
| N2 | 3.391 ± 0.069a | 0.377 ± 0.013a | 4.365 ± 0.130a |
| N3 | 3.434 ± 0.049a | 0.386 ± 0.009a | 4.417 ± 0.113a |
| Effects | K+ | Na+ | Ca2+ | Mg2+ | Total Cation | NO3− | Cl− | SO42− |
| FS | 286.340 ** | 73.650 ** | 63.286 ** | 891.797 ** | 233.751 ** | 142.070 ** | 814.944 ** | 2077.260 ** |
| FN | 175.210 ** | 22.551 ** | 4.539 ns | 2.656 ns | 51.926 ** | 2.593 ns | 5.782 ns | 68.407 ** |
| FD | 5.344 * | 0 ns | 0.942 ns | 0.007 ns | 1.062 ns | 0.696 ns | 2.152 ns | 29.784 ** |
| Effects | HCO3− | Total Anion | ESP | pH | EC | Organic Carbon | Total Nitrogen | Total Phosphorus |
| FS | 304.920 ** | 39.905 ** | 290.909 ** | 248.029 ** | 2360.273 ** | 12.505 ** | 75.042 ** | 3.552 ns |
| FN | 47.718 ** | 279.072 ** | 85.364 ** | 85.590 ** | 366.903 ** | 91.366 ** | 143.025 ** | 39.445 ** |
| FD | 4.938 * | 70.430 ** | 8.909 ** | 16.116 ** | 21.569 ** | 9.860 ** | 2.255 ns | 0.716 ns |
| Effects | Available Potassium | Alkali-Hydrolyzed Nitrogen | Ammonium Nitrogen | Nitrate Nitrogen | ||||
| FS | 20.849 ** | 155.435 ** | 81.084 ** | 142.070 ** | ||||
| FN | 271.212 ** | 17.240 ** | 2.818 ** | 2.593 ns | ||||
| FD | 8.695 ** | 1.008 ns | 0.848 ** | 0.696 ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, Z.; Zhou, X.; Fu, D.; Zhang, X.; Liu, L.; Li, Z.; Guan, F. Soil Nutrient, Salinity, and Alkalinity Responses of Dendrocalamopsis oldhami in High-Latitude Greenhouses Depending on Planting Year and Nitrogen Application. Forests 2023, 14, 1113. https://doi.org/10.3390/f14061113
Yin Z, Zhou X, Fu D, Zhang X, Liu L, Li Z, Guan F. Soil Nutrient, Salinity, and Alkalinity Responses of Dendrocalamopsis oldhami in High-Latitude Greenhouses Depending on Planting Year and Nitrogen Application. Forests. 2023; 14(6):1113. https://doi.org/10.3390/f14061113
Chicago/Turabian StyleYin, Zixu, Xiao Zhou, Dawei Fu, Xuan Zhang, Liyang Liu, Zhen Li, and Fengying Guan. 2023. "Soil Nutrient, Salinity, and Alkalinity Responses of Dendrocalamopsis oldhami in High-Latitude Greenhouses Depending on Planting Year and Nitrogen Application" Forests 14, no. 6: 1113. https://doi.org/10.3390/f14061113
APA StyleYin, Z., Zhou, X., Fu, D., Zhang, X., Liu, L., Li, Z., & Guan, F. (2023). Soil Nutrient, Salinity, and Alkalinity Responses of Dendrocalamopsis oldhami in High-Latitude Greenhouses Depending on Planting Year and Nitrogen Application. Forests, 14(6), 1113. https://doi.org/10.3390/f14061113





