Poplar Sawdust Biochar Altered Community Composition of Dominant Soil Fungi but Not Bacteria Depending on Pyrolysis Temperature
Abstract
1. Introduction
2. Materials and Methods
2.1. Biochar Pyrolysis and Properties
2.2. Experimental Design for Soil Sampling and Processing
2.3. Analysis of Soil Physical and Chemical Properties
2.4. Analysis of Soil Microbial Biomass
2.5. Analysis of Soil Microbial Communities
2.6. Data Processing and Statistical Analysis
3. Results
3.1. Soil Basic Properties
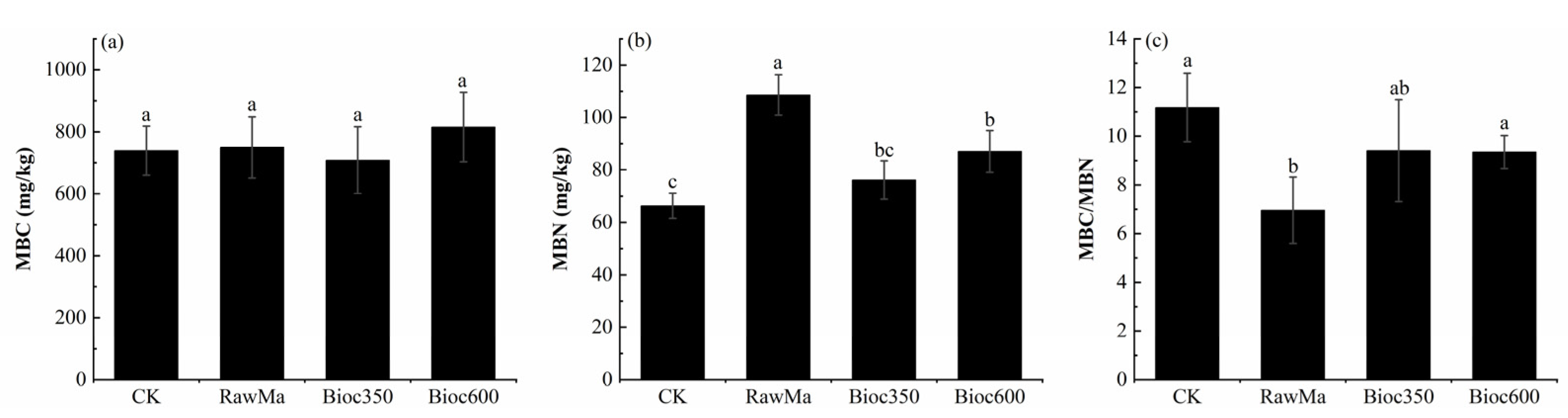
3.2. Soil Microbial Biomass
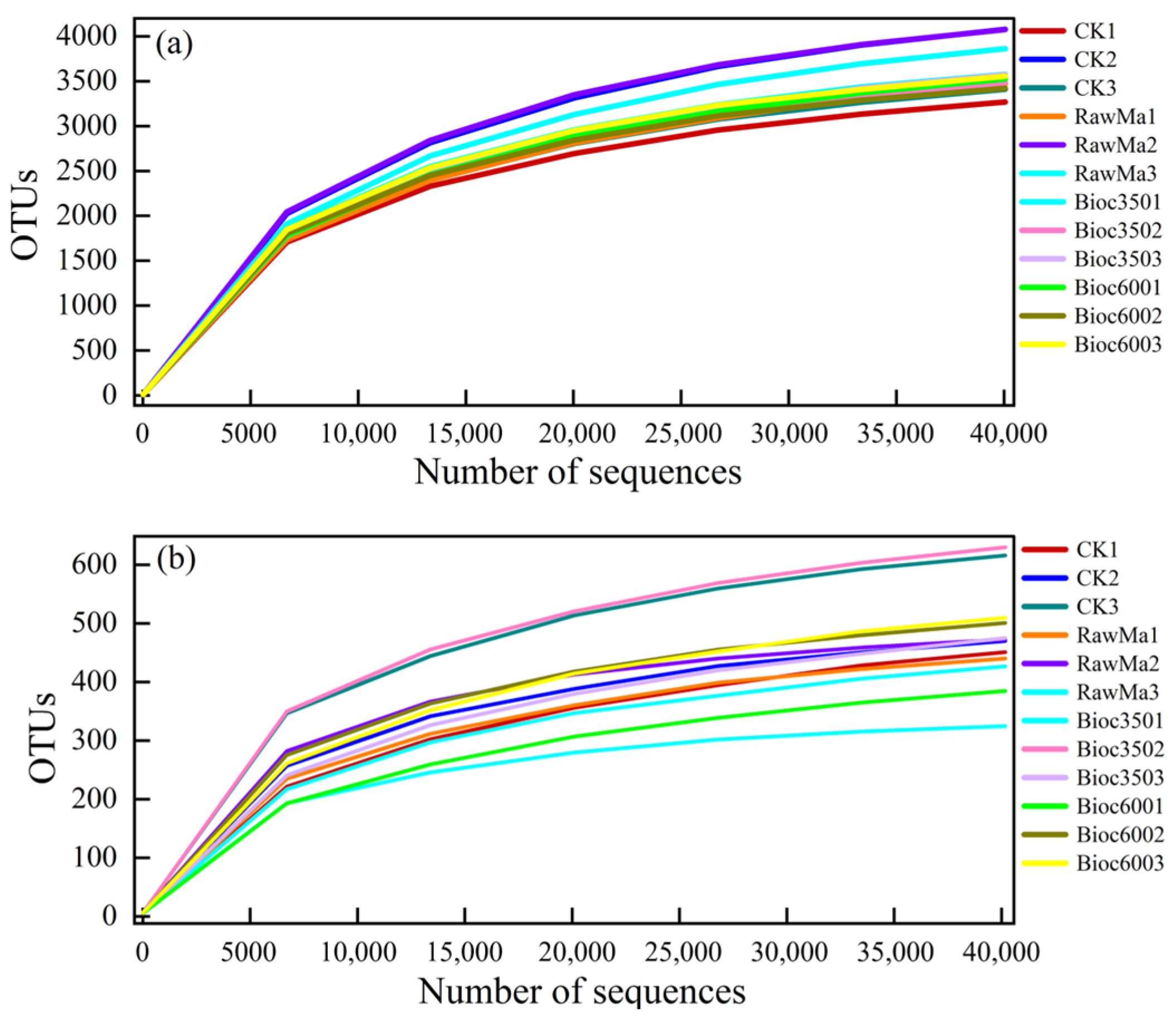
3.3. Sequence Data and Alpha Diversity of Soil Microorganisms
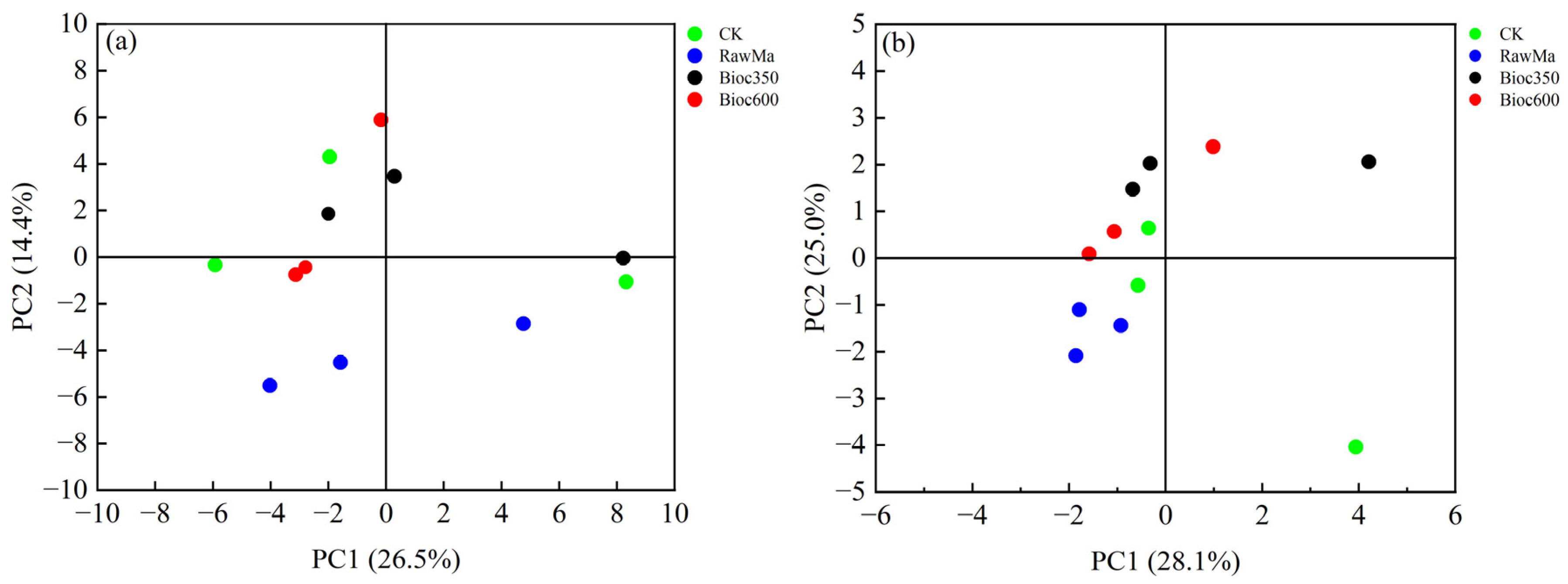
3.4. Bacterial and Fungal Community Structures
3.5. Bacterial and Fungal Community Composition
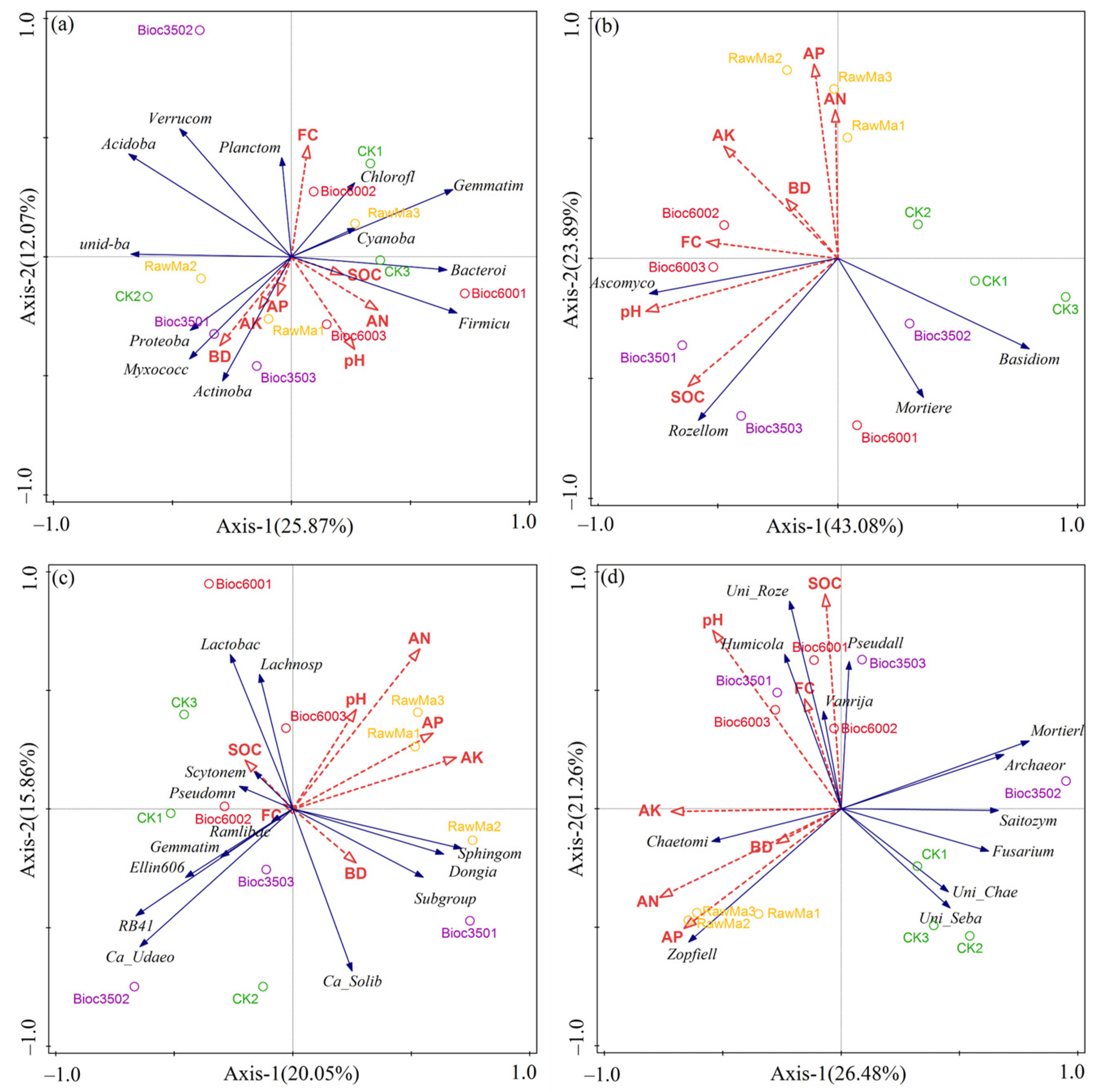
3.6. Linking Microbial Community Composition to Soil Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verheijen, F.G.; Jeffery, S.; Bastos, A.C.; Velde, M.; Diafas, I.B. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, Processes and Functions; EUR 24099 EN; European Commission: Luxembourg, JRC55799; 2010; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC55799 (accessed on 9 March 2023).
- Preston, C.M.; Schmidt, M.W. Black (pyrogenic) carbon: A synthesis of current knowledge and uncertainties with special consideration of boreal regions. Biogeosciences 2006, 3, 397–420. [Google Scholar] [CrossRef]
- Deluca, T.H.; Mackenzie, M.D.; Gundale, M.J.; Holben, W.E. Wildfire-produced charcoal directly influences nitrogen cycling in Ponderosa Pine forests. Soil Sci. Soc. Am. J. 2006, 70, 448–453. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic values of greenwaste biochar as a soil amendment. Aust. J. Soil Res. 2008, 45, 629–634. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Li, S.M.; Barreto, V.; Li, R.W.; Chen, G.; Hsieh, Y.P. Nitrogen retention of biochar derived from different feedstocks at variable pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Dai, X.; Boutton, T.W.; Glaser, B.; Ansley, R.J.; Zech, W. Black carbon in a temperate mixed-grass savanna. Soil Biol. Biochem. 2005, 37, 1879–1881. [Google Scholar] [CrossRef]
- Malev, O.; Contin, M.; Licen, S.; Barbieri, P.; De Nobili, M. Bioaccumulation of polycyclic aromatic hydrocarbons and survival of earthworms (Eisenia andrei) exposed to biochar amended soils. Environ. Sci. Pollut. Res. 2016, 23, 3491–3502. [Google Scholar] [CrossRef]
- Liang, F.; Li, G.T.; Lin, Q.M.; Zhao, X.R. Crop yield and soil properties in the first 3 years after biochar application to a calcareous soil. J. Integr. Agric. 2014, 13, 525–532. [Google Scholar] [CrossRef]
- Lim, T.J.; Spokas, K.A.; Feyereisen, G.; Feyereisen, G.; Novak, J.M. Predicting the impact of biochar additions on soil hydraulic properties. Chemosphere 2016, 142, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Amoakwah, E.; Frimpong, K.A.; Okae-Anti, D.; Arthur, E. Soil water retention, air flow and pore structure characteristics after corn cob biochar application to a tropical sandy loam. Geofis. Int. 2017, 307, 189–197. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Song, Y.F.; Wu, Z.; Yan, X.Y.; Gunina, A. Effects of six-year biochar amendment on soil aggregation, crop growth, and nitrogen and phosphorus use effciencies in a rice-wheat rotation. J. Clean Prod. 2020, 242, 118435.1–118435.10. [Google Scholar] [CrossRef]
- Teutscherova, N.; Vazquez, E.; Masaguer, A.; Navas, M.; Scow, K.M.; Schmidt, R.; Benito, M. Comparison of lime- and biochar-mediated pH changes in nitrification and ammonia oxidizers in degraded acid soil. Biol. Fertil. Soils 2017, 53, 1–11. [Google Scholar] [CrossRef]
- Andrade, C.A.; Bibar, M.P.; Coscione, A.R.; Pires, A.M.; Soares, A.G. Mineralization and effects of poultry litter biochar on soil cation exchange capacity. Pesqui. Agropecu. Bras. 2015, 50, 407–416. [Google Scholar] [CrossRef]
- Sanford, J.R.; Larson, R.A. Assessing nitrogen cycling in corncob biochar amended soil columns for application in agricultural treatment systems. Agron. -Basel 2020, 10, 979. [Google Scholar] [CrossRef]
- Edenborn, S.L.; Johnson, L.M.; Edenborn, H.M.; Albarran-Jack, M.R.; Demetrion, L.D. Amendment of a hardwood biochar with compost tea: Effects on plant growth, insect damage and the functional diversity of soil microbial communities. Biol. Agric. Hortic. 2018, 34, 88–106. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Deng, F.; Zou, X.; Ruan, H.; Chen, H.Y. Responses of soil microbial biomass, diversity and metabolic activity to biochar applications in managed poplar plantations on reclaimed coastal saline soil. Soil Use Manag. 2019, 34, 597–605. [Google Scholar] [CrossRef]
- Xu, N.; Tan, G.C.; Wang, H.Y.; Gai, X.P. Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur. J. Soil Biol. 2016, 74, 1–8. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Brau, L. Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar] [CrossRef]
- Xu, H.J.; Wang, X.H.; Li, H.; Yao, H.Y.; Su, J.Q.; Zhu, Y.G. Biochar Impacts Soil Microbial Community Composition and Nitrogen Cycling in an Acidic Soil Planted with Rape. Environ. Sci. Technol. 2014, 48, 9391–9399. [Google Scholar] [CrossRef]
- Zhang, H.J.; Wang, S.J.; Zhang, J.X.; Tian, C.J.; Luo, S.S. Biochar application enhances microbial interactions in mega-aggregates of farmland black soil. Soil Tillage Res. 2021, 213, 105145. [Google Scholar] [CrossRef]
- Zheng, J.F.; Chen, J.H.; Pan, G.P.; Liu, X.Y.; Zhang, X.H.; Li, L.Q.; Sian, R.J.; Cheng, K. Biochar decreased microbial metabolic quotient and shifted community composition four years after a single incorporation in a slightly acid rice paddy from southwest China. Sci. Total Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wang, L.; Sun, Z.Y.; Wang, S.T.; Shen, C.H.; Tang, Y.Q.; Kida, K. Biochar addition reduces nitrogen loss and accelerates composting process by affecting the core microbial community during distilled grain waste composting. Bioresour. Technol. 2021, 337, 125492. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Lv, C.Q.; Fernandez-Garcia, V.; Huang, B.L.; Yao, J.M.; Ding, W. Biochar and PGPR amendments influence soil enzyme activities and nutrient concentrations in a eucalyptus seedling plantation. Biomass. Convers. Biorefinery 2021, 11, 1865–1874. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622, 1391–1399. [Google Scholar] [CrossRef]
- Li, Y.; Wu, J.S.; Shen, J.L.; Liu, S.L.; Wang, C.; Chen, D.; Huang, T.P.; Zhang, J.B. Soil microbial C:N ratio is a robust indicator of soil productivity for paddy fields. Sci. Rep. 2016, 6, 35266. Available online: https://www.nature.com/articles/srep35266 (accessed on 9 March 2023). [CrossRef]
- Lusiba, S.; Odhiambo, J.; Ogola, J. Effect of biochar and phosphorus fertilizer application on soil fertility: Soil physical and chemical properties. Arch. Agron. Soil Sci. 2016, 63, 477–490. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Xu, X.M.; Liu, T.M.; Wang, H.W.; Yang, Y.; Chen, X.R.; Zhu, S.Y. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmerianivea L. Gaud) fields in different areas in China. Sci. Rep. 2020, 10, 3264. Available online: https://www.nature.com/articles/s41598-020-58608-0 (accessed on 9 March 2023). [CrossRef] [PubMed]
- Simarani, K.; Halmi, M.F.; Abdullah, R. Short-term effects of biochar amendment on soil microbial community in humid tropics. J. Clean Prod. 2018, 64, 1847–1860. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, J.F.; Liu, C.M.; Gu, B.W.; Han, M.; Li, J.W.; Li, N.; Liu, N.; An, N.; Dai, J.; et al. Effects of long-term biochar and biochar-based fertilizer application on brown earth soil bacterial communities. Agric. Ecosyst. Environ. 2021, 309, 107285. [Google Scholar] [CrossRef]
- Wang, H.; Li, Q.; Tang, M.; Wang, E.P.; Chen, X.; Yu, Z.; Chen, C.B. Effects of rice husk biochar and biochar-based fertilizer application on soil enzyme activities and bacterial communities in continuously cropped ginseng. Allelopath. J. 2021, 53, 165–176. [Google Scholar] [CrossRef]
- Laghari, M.; Naidu, R.; Xiao, B.; Hu, Z.Q.; Mirjat, M.S.; Hu, M.; Kandhro, M.N.; Chen, Z.H.; Guo, D.B.; Jogi, Q.; et al. Recent developments in biochar as an effective tool for agricultural soil management: A review. J. Sci. Food Agric. 2016, 96, 4840–4849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Bolan, N.; Prevoteau, A.; Vithanage, M.; Biswas, J.K.; Yong, S.O.; Wang, H. Applications of biochar in redox-mediated reactions. Bioresour. Technlol. 2017, 246, 271–281. [Google Scholar] [CrossRef]
- Wang, T.T.; Liu, H.T.; Duan, C.H.; Xu, R.; Zhang, Z.Q.; She, D.; Zheng, J.Y. The eco-friendly biochar and valuable bio-oil from Caragana korshinskii: Pyrolysis preparation, characterization, and adsorption applications. Materials 2020, 13, 3391. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Pu, S.; Adhikari, S.; Ma, H.; Kim, D.H.; Bai, Y.C.; Hou, D.Y. Progress and future prospects in biochar composites: Application and reflection in the soil environment. Crit. Rev. Environ. Sci. Technol. 2020, 51, 219–271. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and chemical characterization of biochars derived from different agricultural residues. Biogeosciences 2014, 11, 6613–6621. [Google Scholar] [CrossRef]
- Campos, P.; Miller, A.Z.; Prats, S.A.; Knicker, H.; Hagemann, N.; De la Rosa, J.M. Biochar amendment increases bacterial diversity and vegetation cover in trace element-polluted soils: A long-term field experiment. Soil Biol. Biochem. 2020, 150, 108014. [Google Scholar] [CrossRef]
- Luo, S.S.; Wang, S.J.; Tian, L.; Li, S.Q.; Li, X.J.; Shen, Y.F.; Tian, C.J. Long-term biochar application influences soil microbial community and its potential roles in semiarid farmland. Appl. Soil Ecol. 2017, 117, 10–15. [Google Scholar] [CrossRef]
- Xu, W.; Wu, Z.H.; Zou, Y.Y. Development and application of high-Valued wood products made of fast-growing Poplar. Adv. Mater. Process. 2012, 311–313, 117–121. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.G.; Pommerening, B.; Chaussod, R.; Brookes, P.C. Measurement of soil microbial biomass by fumigation-extraction: An automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Brookes, P.C. Ninhydrin-reactive nitrogen measurements of microbial biomass in 0.5 M K2SO4 soil extracts. Soil Biol. Biochem. 1990, 22, 1023–1027. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Subramanian, S.; Faith, J.J.; Gevers, D.; Gordon, J.I.; Knight, R.; Mills, D.A.; Caporaso, J.G. Quality-filtering vastly improves diversity estimates from Illumina amplicon sequencing. Nat. Methods 2013, 10, 57–59. Available online: https://www.nature.com/articles/nmeth.2276 (accessed on 9 March 2023). [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. Available online: https://www.nature.com/articles/nmeth.f.303 (accessed on 9 March 2023). [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Haas, B.J.; Gevers, D.; Earl, A.M.; Feldgarden, M.; Ward, D.V.; Giannoukos, G.; Ciulla, D.; Tabbaa, D.; Highlander, S.K.; Sodergren, E. Chimeric 16S rRNA sequence formation and detection in Sanger and 454–pyrosequenced PCR amplicons. Genome Res. 2011, 21, 494–504. Available online: https://genome.cshlp.org/content/21/3/494 (accessed on 9 March 2023). [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. Available online: https://www.nature.com/articles/nmeth.2604 (accessed on 9 March 2023). [CrossRef]
- Wang, Q.; Garrity, M.G.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucl. Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Stewart, C.E.; Sun, C.C.; Zheng, J.Y. Effects of biochar addition on evaporation in the five typical Loess Plateau soils. Catena 2018, 162, 29–39. [Google Scholar] [CrossRef]
- Liang, J.F.; Li, Q.W.; Gao, J.Q.; Feng, J.G.; Zhang, X.Y.; Hao, Y.J.; Yu, F.H. Biochar-compost addition benefits Phragmites australis growth and soil property in coastal wetlands. Sci. Total Environ. 2021, 769, 145166. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Sun, X.; Ding, J.; Jiang, Z.W.; Xu, J.Z. Effects of biochar addition on the NEE and soil organic carbon content of paddy fields under water-saving irrigation. Environ. Sci. Pollut. Res. 2019, 26, 8303–8311. [Google Scholar] [CrossRef]
- Fei, Y.H.; Chen, Y.X.; Liu, C.S.; Xiao, T.F. Biochar addition enhances phenanthrene fixation in sediment. Bull. Environ. Contam. Toxicol. 2019, 103, 163–168. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Chen, C.R.; Gray, E.M.; Boyd, S.E. Effect of feedstock and pyrolysis temperature on properties of biochar governing end use efficacy. Biomass. Bioenergy 2017, 105, 136–146. [Google Scholar] [CrossRef]
- Dai, Z.M.; Xiong, X.Q.; Zhu, H.; Xu, H.J.; Leng, P.; Li, J.H.; Tang, C.; Xu, J.M. Association of biochar properties with changes in soil bacterial, fungal and fauna communities and nutrient cycling processes. Biochar 2021, 3, 239–254. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, X.; Luo, X.X.; Wang, Z.Y.; Xing, B.S. Biochar-induced negative carbon mineralization priming efects in a coastal wetland soil: Roles of soil aggregation and microbial modulation. Sci. Total Environ. 2018, 610, 951–960. [Google Scholar] [CrossRef]
- Agegnehu, G.; Bass, A.M.; Nelson, P.N.; Bird, M.I. Benefits of biochar, compost and biochar-compost for soil quality, maize yield and greenhouse gas emissions in a tropical agricultural soil. Sci. Total Environ. 2016, 543, 295–306. [Google Scholar] [CrossRef]
- Ameloot, N.; De Neve, S.; Jegajeevagan, K.; Yildiz, G.; Buchan, D.; Funkuin, Y.N.; Prins, W.; Bouckaert, L.; Sleutel, S. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biol. Biochem. 2013, 57, 401–410. [Google Scholar] [CrossRef]
- Farrell, M.; Kuhn, T.K.; Macdonald, L.M.; Maddern, T.M.; Murphy, D.V.; Hall, P.A.; Singh, B.P.; Baumann, K.; Krull, E.S.; Baldock, J.A. Microbial utilisation of biochar-derived carbon. Sci. Total Environ. 2013, 465, 288–297. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal responses to biochar in soil—Concepts and mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Andrew, S.P.; Jenkinson, D.S.; Lynch, J.M.; Goss, M.J.; Tinker, P.B. The turnover of organic carbon and nitrogen in soil: Discussion. Proc. R. Soc. B-Biol. Sci. 1990, 329, 361–368. [Google Scholar] [CrossRef]
- Liu, L.Y.; Tan, Z.X.; Gong, H.B.; Huang, Q.Y. Migration and transformation mechanisms of nutrient elements (N, P, K) within biochar in straw–biochar–soil–plant systems: A Review. ACS Sustain. Chem. Eng. 2019, 7, 22–32. [Google Scholar] [CrossRef]
- Prapagdee, S.; Tawinteung, N. Effects of biochar on enhanced nutrient use efficiency of green bean, Vigna radiata L. Environ. Sci. Pollut. Res. 2017, 24, 9460–9467. [Google Scholar] [CrossRef] [PubMed]
- Deng, H. A review of diversity-stability relationship of soil microbial community: What do we not know? J. Environ. Sci. 2012, 24, 1027–1035. [Google Scholar] [CrossRef]
- Li, H.B.; Dong, X.L.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.S.; Ma, L.N. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Li, X.N.; Wang, T.; Chang, S.X.; Jiang, X.; Song, Y. Biochar increases soil microbial biomass but has variable effects on microbial diversity: A meta-analysis. Sci. Total Environ. 2020, 749, 141593. [Google Scholar] [CrossRef]
- Miranda, N.D.; Pimenta, A.S.; da Silva, G.G.; Oliveira, E.M.; de Carvalho, M.A. Biochar as soil conditioner in the succession of upland rice and cowpea fertilized with nitrogen. Rev. Caatinga 2017, 30, 313–323. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Fitter, A. Are microorganisms more effective than plants at competing for nitrogen? Trends Plant Sci. 2000, 5, 304–308. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.T.; Yang, X.D.; Zhang, J.J.; Lin, Z.A.; Zhao, B.Q. Microbial community structure and functional metabolic diversity are associated with organic carbon availability in an agricultural soil. J. Integr. Agric. 2015, 14, 2500–2511. [Google Scholar] [CrossRef]
- Lei, Z.F.; Song, X.Z.; Zhang, Z.T.; Song, X.Z.; Zhang, Z.T.; Ying, Y.Q.; Peng, C.H. Biochar amendment decreases soil microbial biomass and increases bacterial diversity in Moso bamboo (Phyllostachys edulis) plantations under simulated nitrogen deposition. Environ. Res. Lett. 2018, 13, 044029. [Google Scholar] [CrossRef]
- Fierer, N.; Jackson, R.B. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of Soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Xiong, J.B.; Zhang, H.Y.; Feng, Y.Z.; Lin, X.G.; Li, X.Y.; Liang, W.J.; Chu, H.Y. Soil pH drives the spatial distribution of bacterial communities along elevation on Changbai Mountain. Soil Biol. Biochem. 2013, 57, 204–211. [Google Scholar] [CrossRef]
- Li, G.; Chen, W.J.; Xu, S.Q.; Xiong, S.G.; Zhao, J.Y.; Liu, D.L.; Ding, G.C.; Li, J.; Wei, Y.Q. Role of fungal communities and their interaction with bacterial communities on carbon and nitrogen component transformation in composting with different phosphate additives. Environ. Sci. Pollut. Res. 2023, 30, 44112–44120. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Ghodszad, L.; Reyhaniyabar, A.; Maghsoodi, M.R.; Lajayer, B.A.; Chang, S.X. Biochar affects the fate of phosphorus in soil and water: A critical review. Chemosphere 2021, 283, 131176. [Google Scholar] [CrossRef]
- Gao, L.; Wang, R.; Shen, G.M.; Zhang, J.X.; Meng, G.X.; Zhang, J.G. Effects of biochar on nutrients and the microbial community structure of tobacco-planting soils. J. Soil Sci. Plant Nutr. 2017, 17, 884–896. [Google Scholar] [CrossRef]
- Akter, M.; Deroo, H.; Grave, E.D.; Alboom, T.E.; Kader, M.A.; Pierreux, S.; Begum, M.A.; Boeckx, P.; Sleutel, S. Link between paddy soil mineral nitrogen release and iron and manganese reduction examined in a rice pot growth experiment. Geoderma 2018, 326, 9–21. [Google Scholar] [CrossRef]
- Yin, X.L.; Penuelas, J.; Xu, X.P.; Sardans, J.; Fang, Y.Y.; Wiesmeier, M.; Chen, Y.Y.; Chen, X.X.; Wang, W.Q. Effects of addition of nitrogen-enriched biochar on bacteria and fungi community structure and C, N, P, and Fe stoichiometry in subtropical paddy soils. Eur. J. Soil Biol. 2021, 106, 103351. [Google Scholar] [CrossRef]
- Nobaharan, K.; Abtahi, A.; Lajayer, B.M.; Hullebusch, E.D. Effects of biochar dose on cadmium accumulation in spinach and its fractionation in a calcareous soil. Arab. J. Geosci. 2022, 15, 336. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lin, B.F.; Huang, Y.P.; Liu, Y.B.; Wu, Y.H.; Qu, R.; Tang, C.L. Pyrolysis temperature affects the physiochemical characteristics of lanthanum-modified biochar derived from orange peels: Insights into the mechanisms of tetracycline ad-sorption by spectroscopic analysis and theoretical calculations. Sci. Total Environ. 2023, 862, 160860. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef]




| Material | Pyrolysis Temperature (°C) | SSA (m2/g) | TPO (cm3/g) | pH | OC (g/kg) | TN (g/kg) | C/N Ratio |
|---|---|---|---|---|---|---|---|
| Poplar sawdust | – | 1.81 | 0.003 | 6.84 ± 0.18 c | 45.5 ± 0.06 c | 0.17 ± 0.00 b | 267.8 ± 10.8 b |
| Biochar | 350 | 2.18 | 0.003 | 7.43 ± 0.01 b | 74.1 ± 0.32 b | 0.19 ± 0.01 b | 385.4 ± 21.0 a |
| 600 | 147.15 | 0.08 | 9.82 ± 0.31 a | 87.0 ± 0.43 a | 0.41 ± 0.04 a | 211.2 ± 12.5 c |
| Treatment | Bacterial 16S | Fungal ITS | ||||||
|---|---|---|---|---|---|---|---|---|
| OTUs | Shannon | Chao 1 | ACE | OTUs | Shannon | Chao 1 | ACE | |
| CK | 3426 ± 266 a | 9.74 ± 0.20 a | 3899 ± 441 a | 3951 ± 414 a | 512 ± 52 a | 4.20 ± 0.35 a | 607 ± 53 a | 619 ± 55 a |
| RawMa | 3569 ± 343 a | 10.12 ± 0.16 a | 3966 ± 194 a | 4227 ± 227 a | 413 ± 45 a | 4.32 ± 0.24 a | 444 ± 57 a | 454 ± 56 a |
| Bioc350 | 3661 ± 196 a | 9.94 ± 0.08 a | 4152 ± 110 a | 4228 ± 154 a | 511 ± 61 a | 4.07 ± 0.42 a | 613 ± 67 a | 613 ± 67 a |
| Bioc600 | 3645 ± 152 a | 9.91 ± 0.05 a | 4187 ± 658 a | 4174 ± 386 a | 465 ± 40 a | 3.71 ± 0.16 a | 560 ± 37 a | 559 ± 43 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Y.; Tian, Y.; Yang, R.; Li, W.; Liu, C.; Li, T. Poplar Sawdust Biochar Altered Community Composition of Dominant Soil Fungi but Not Bacteria Depending on Pyrolysis Temperature. Forests 2023, 14, 1114. https://doi.org/10.3390/f14061114
Jin Y, Tian Y, Yang R, Li W, Liu C, Li T. Poplar Sawdust Biochar Altered Community Composition of Dominant Soil Fungi but Not Bacteria Depending on Pyrolysis Temperature. Forests. 2023; 14(6):1114. https://doi.org/10.3390/f14061114
Chicago/Turabian StyleJin, Yuanyuan, Ye Tian, Rui Yang, Wenhao Li, Chengyu Liu, and Tong Li. 2023. "Poplar Sawdust Biochar Altered Community Composition of Dominant Soil Fungi but Not Bacteria Depending on Pyrolysis Temperature" Forests 14, no. 6: 1114. https://doi.org/10.3390/f14061114
APA StyleJin, Y., Tian, Y., Yang, R., Li, W., Liu, C., & Li, T. (2023). Poplar Sawdust Biochar Altered Community Composition of Dominant Soil Fungi but Not Bacteria Depending on Pyrolysis Temperature. Forests, 14(6), 1114. https://doi.org/10.3390/f14061114







