Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Plant Growth and Biomass Estimation
2.3. Physical–Chemical Characterization of Plant and Soil Material
2.4. Data Analysis
3. Results
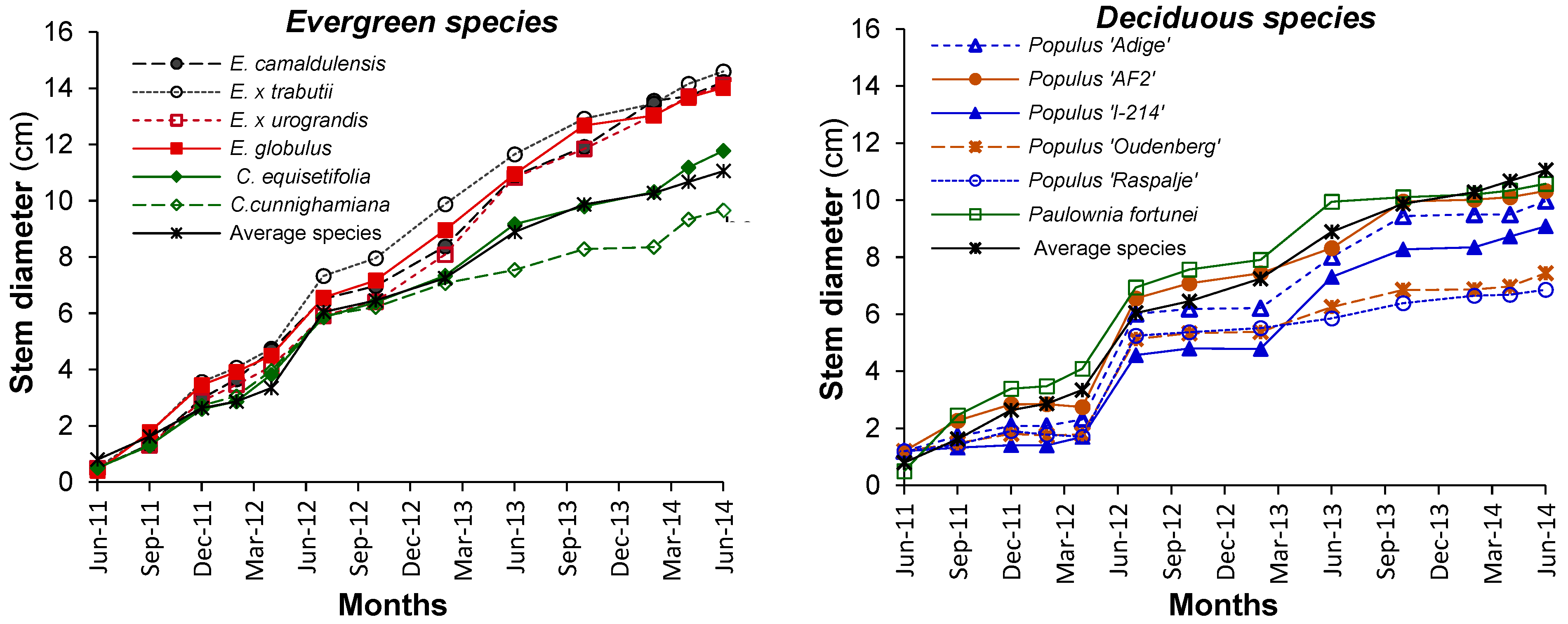
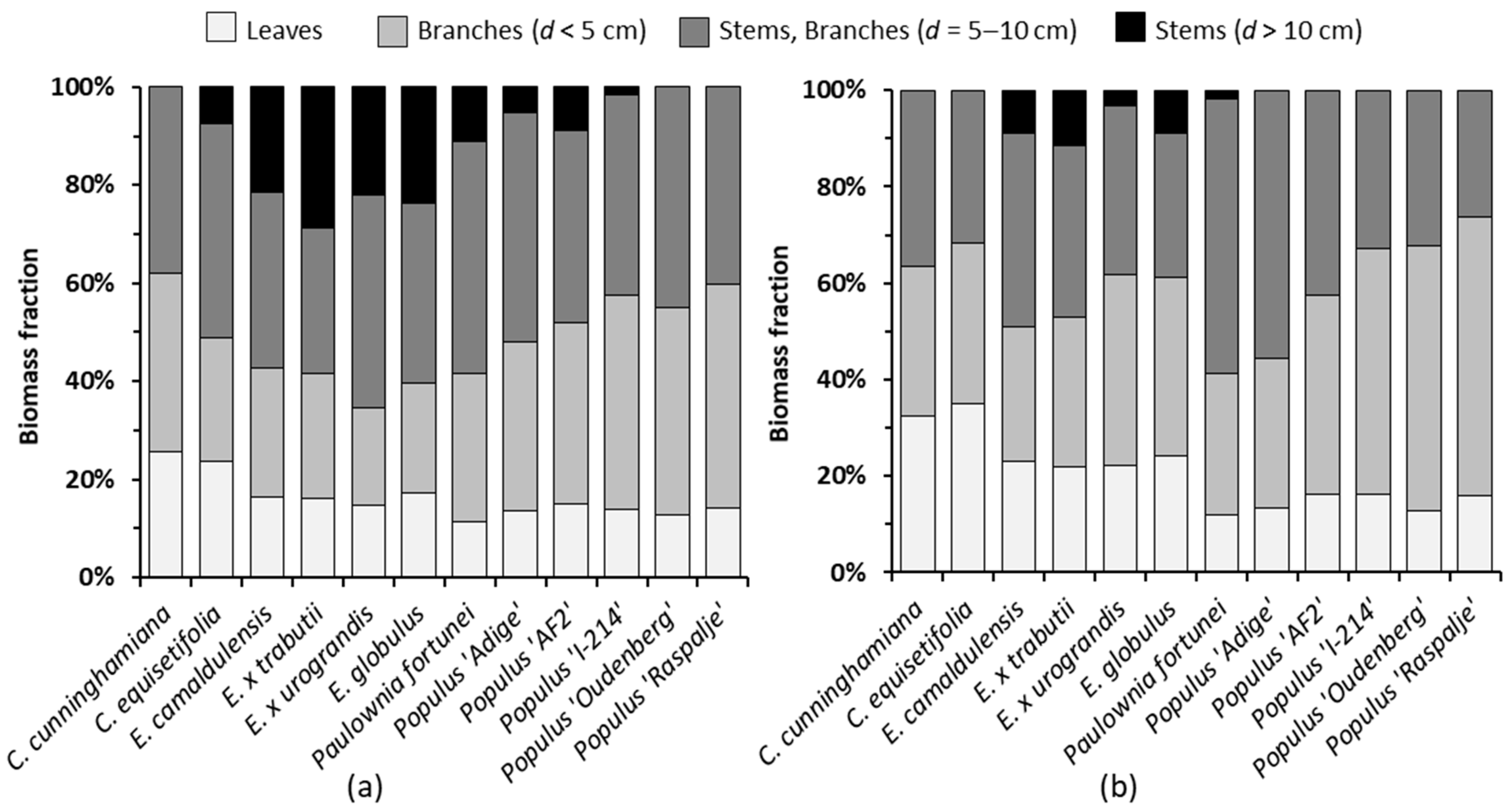
3.1. Growth and Biomass Partitioning
3.2. Physical–Chemical Characterization of Plant and Soil Material
- Taking into account the stoniness (18%) and bulk density (1.56 kg dm−3) of the soil, the amount of total N per hectare in the 0–30 cm layer at the beginning of the trial averaged: (0.0017 + 0.0014 kgof N/kgof soil) × (0.15 m × 10 000 m2) × (1.0 − 0.18) × 1.56 = 5948 kg of N;
- The amount of total N per hectare in the 0–30 cm soil layer seven years later (8th year) averaged: (0.0015 + 0.0011 kgof N/kgof soil) × (0.15 m × 10 000 m2) × (1.0 − 0.18) × 1.56 = 4989 kg of N;
- Therefore, after seven years of cultivation, the N content of the soil decreased by an average of 5948 − 4989 = 959 kg of N. That is, 137 kg ha−1 year−1 of N, but in a range from 27.4 kg ha−1 year−1 (Casuarina equisetifolia) to 274.1 kg ha−1 year−1 (E. × trabutii).
- No significant differences among experimental units or between the two replicates of the test were found, so global mean values as a whole are shown;
- The mineral composition of the different biomass fractions differed significantly (0.001 ≤ p ≤ 0.016), with the only exception being the H content (p = 0.492). The leaves and bark presented the highest ash and mineral percentages. On the opposite side was the thick wood, with a tendency to higher values if the bark was preserved, but without differing significantly from the debarked one (Table 5), possibly due to the low percentage of bark in this fraction (11.0%–15.6% on average).
- The differences among taxa were not significant for most of the mineral elements analyzed (0.058 ≤ p ≤ 0.921), except for N (p < 0.001), Fe (p < 0.001) and C (p = 0.017). The Taxon × Biomass fraction interaction was not significant in any case (0.130 ≤ p ≤ 0.996). Table 6 shows the physical-mechanical and chemical properties of the wood (main stems and thick branches) for the different taxa. Paulownia fortunei wood stood out for its low percentage of minerals and ash but had the lowest wood density of all the taxa studied. However, Casuarina sp., Eucalyptus camaldulensis and Eucalyptus × trabutii, on the one hand, had the handicap of high mineral concentrations but, on the other hand, they had a high-density wood;
- As a rough estimate, during the studied period, and for all the taxa and the two rotations as a whole, the following can be noted:
- ○
- According to Figure 3, on average, the leaves, thin branches and thick wood represented 16.6, 36.3 and 47.1%, respectively, of the aboveground dry mass, which contained the N concentrations shown in Table 5. So, the average amount of N removed from the field plot with the harvested biomass was 227.6 kg ha−1 year−1 (from 63.6 kg ha−1 year−1 for Populus ‘Raspalje’ to 308.9 kg ha−1 year−1 for Eucalyptus × trabutii), of which an average of 40.1% (91.3 kg ha−1 year−1) corresponded to leaves;
- ○
- The litterfall contained an average of 11.4 kg ha−1 year−1 of N;
- ○
- The amount of N supplied by the fertilizer was 75 kg ha−1 year−1, which must be added to 18.5 kg ha−1 year−1 supplied by irrigation water (400 mm annually on average);
- ○
- The removed 227.6 kg ha−1 year−1 of N from the biomass is quite close to the N supplied by fertilization and irrigation water (93.5 kg ha−1 year−1); plus, the N removed from the soil litterfall set (137–11.4 = 125.6 kg ha−1 year−1); which amounts to 219.1 kg ha−1 year−1 of N on average;
- ○
- Other inputs and outputs of N have not been considered in this rough approximation (leaching, emission of N oxides, N supplied by rainwater, rock decomposition, other deeper layers of soil, etc.).
4. Discussion
5. Conclusions
- The biomass yield of short-rotation forest tree species (hybrid clones of Populus and Eucalyptus, monospecific clones of Eucalyptus and Paulownia fortunei, and Casuarina species) averaged 9–61 Mg ha−1 year−1 under a Mediterranean climate with mild winters, good availability of nutrients and water, and well-drained fertile soil;
- In terms of energy, the LHV of the woody biomass was equivalent to 38–241 MWh ha−1 year−1 (equivalent to the replacement of about 3400–22 000 L ha−1 year−1 of diesel);
- Plantation managers must take into account nutrient inputs and outputs in order to ensure the sustainability of the system and to prevent any loss in soil fertility and productivity;
- The biomass quality varied among the taxa studied, but they all have enough commercial quality to add value through their transformation into chips or pellets;
- It is recommended that the lowest quality biomass fractions for energy use (leaves and thinnest branches < 25 mm diameter) should not be removed from the cultivation land due to their contribution to the nutrient cycle;
- The use of N-fixing species, such as Casuarina sp., should be considered in future plantations, mainly in degraded soils.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. In Proceedings of the Conference of the Parties on Its Twenty-First Session, Paris, France, 30 November–13 December 2015; Available online: http://unfccc.int/resource/docs/2015/cop21/eng/10a01.pdf (accessed on 19 February 2023).
- European Union. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Use of Energy from Renewable Sources. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 15 January 2023).
- European Commission. Communication from The Commission to The European Parliament, The Council, The European Economic and Social Committee and The Committee of The Regions Energy Roadmap 2050/* COM/2011/0885 final */. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:52011DC0885 (accessed on 15 January 2023).
- Picchio, R.; Latterini, F.; Venanzi, R.; Stefanoni, W.; Suardi, A.; Tocci, D.; Pari, L. Pellet Production from Woody and Non-Woody Feedstocks: A Review on Biomass Quality Evaluation. Energies 2020, 13, 2937. [Google Scholar] [CrossRef]
- REN21, Renewables. Global Status Report, Renewable Energy Policy Network for the 21st Century, France. Available online: https://www.ren21.net/wp-content/uploads/2019/05/GSR2017_Full-Report_English.pdf (accessed on 20 February 2023).
- Kummamuru, B. WBA Global Bioenergy Statistics. World Bioenergy Association. 2017, p. 80. Available online: http://worldbioenergy.org/uploads/WBA%20GBS%202017_hq.pdf (accessed on 15 January 2023).
- IEA. World Energy Outlook International Energy Agency. Available online: https://iea.blob.core.windows.net/assets/4a50d774-5e8c-457e-bcc9513357f9b2fb/World_Energy_Outlook_2017.pdf (accessed on 15 January 2023).
- Antczak, A.; Swierkosz, R.; Szeniawski, M.; Marchwicka, M.; Akus-Szylberg, F.; Przybysz, P.; Zawadzki, J. The comparison of acid and enzymatic hydrolysis of pulp obtained from poplar wood (Populus sp.) by the Kraft method. Drewno 2019, 63, 53–66. [Google Scholar] [CrossRef]
- Titus, B.D.; Brown, K.; Helmisaari, H.-S.; Vanguelova, E.; Stupak, I.; Evans, A.; Clarke, N.; Guidi, C.; Bruckman, V.J.; Varnagiryte-Kabasinskiene, I.; et al. Sustainable forest biomass: A review of current residue harvesting guidelines. Energy Sustain. Soc. 2021, 11, 10. [Google Scholar] [CrossRef]
- Małachowska, E.; Lipkiewicz, A.; Niemczyk, M.; Dubowik, M.; Boruszewski, P.; Przybysz, P. Influences of fiber and pulp properties on papermaking ability of cellulosic pulps produced from alternative fibrous raw materials. J. Nat. Fibers 2019, 18, 1751–1761. [Google Scholar] [CrossRef]
- Christersson, L.; Verma, K. Short-rotation forestry—A complement to “conventional” forestry. Unasylva 2006, 57, 34–39. Available online: https://www.fao.org/3/A0532e/A0532e07.pdf (accessed on 27 January 2023).
- BIC. Strategic innovation and research agenda (SIRA) Bio-based and renewable industries for development and growth in europe. In A Public-Private Partnership on Bio-Based Industries; Biobased Industries Consortium, Ed.; Biobased Industries Consortium: Brussels, Belgium, 2013; Available online: https://ec.europa.eu/research/participants/data/ref/h2020/other/legal_basis/jtis/bbi/bbi-sira_en.pdf (accessed on 20 January 2023).
- Bogdanski, A.; Dubois, O.; Jamieson, C.; Krell, R. Making Integrated Food-Energy Systems Work for People and Climate; FAO: Rome, Italy, 2010; p. 116. Available online: http://www.fao.org/docrep/013/i2044e/i2044e.pdf (accessed on 27 January 2023).
- Riffell, S.; Verschuyl, J.; Miller, D.; Wigley, T.B. Biofuel harvests, coarse woody debris, and biodiversity—A meta-analysis. For. Ecol. Manag. 2011, 261, 878–887. [Google Scholar] [CrossRef]
- Bessaad, A.; Bilger, I.; Korboulewsky, N. Assessing Biomass Removal and Woody Debris in Whole-Tree Harvesting System: Are the Recommended Levels of Residues Ensured? Forests 2021, 12, 807. [Google Scholar] [CrossRef]
- Fernández, M.; Alaejos, J.; Andivia, E.; Vázquez-Piqué, J.; Ruiz, F.; López, F.; Tapias, R. Eucalyptus x urograndis biomass production for energy purposes exposed to a Mediterranean climate under different irrigation and fertilisation regimes. Biomass Bioenergy 2018, 111, 22–30. [Google Scholar] [CrossRef]
- Alesso, S.P.; Tapias, R.; Alaejos, J.; Fernández, M. Biomass Yield and Economic, Energy and Carbon Balances of Ulmus pumila L., Robinia pseudoacacia L. and Populus × euroamericana (Dode) Guinier Short-Rotation Coppices on Degraded Lands under Mediterranean Climate. Forests 2021, 12, 1337. [Google Scholar] [CrossRef]
- FAO. Combating land degradation for food security and provision of soil ecosystem services in Europe and Central Asia—International year of soil Ed. FAO–Food and agriculture organization of United nations. In Proceedings of the 39th Session of the European Commission on Agriculture, Budapest, Hungary, 22–23 September 2015; Available online: http://www.fao.org/europe/commissions/eca/eca-39/en/ (accessed on 27 January 2023).
- Fernández, M.; Alaejos, J.; Andivia, E.; Madejón, P.; Díaz, M.; Tapias, R. Short rotation coppice of leguminous tree Leucaena spp. improves soil fertility while producing high biomass yields in Mediterranean environment. Ind. Crop. Prod. 2020, 157, 112911. [Google Scholar] [CrossRef]
- Aravanopoulos, F.A. Breeding of fast growing forest tree species for biomass production in Greece. Biomass Bioenergy 2010, 34, 1531–1537. [Google Scholar] [CrossRef]
- Durán Zuazo, V.H.; Jiménez Bocanegra, J.A.; Perea Torres, F.; Rodríguez Pleguezuelo, C.R.; Francia Martínez, J.R. Biomass yield potential of Paulownia trees in a semi-arid Mediterranean environment (S Spain). Int. J. Renew. Energy Res. 2013, 3, 789–793. Available online: https://ijrer.org/ijrer/index.php/ijrer/article/view/844 (accessed on 20 January 2023).
- Navarro, A.; Stellacci, A.M.; Campi, P.; Vitti, C.; Modugno, F.; Mastrorilli, M. Feasibility of SRC species for growing in mediterranean conditions. Bioenerg. Res. 2016, 9, 208–223. [Google Scholar] [CrossRef]
- Bouvet, A.; Nguyen-The, N.; Melun, F. Nutrient concentration and allometric models for hybrid eucalyptus planted in France. Ann. For. Sci. 2013, 70, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, N.; del Rio, M.; Forrester, D.; Rodríguez-Soalleiro, R.; Pérez-Cruzado, C.; Canellas, I.; Sixto, H. Mixed short rotation plantations of Populus alba and Robinia pseudoacacia for biomass yield. For. Ecol. Manag. 2018, 410, 48–55. [Google Scholar] [CrossRef]
- Seserman, D.-M.; Pohle, I.; Veste, M.; Freese, D. Simulating Climate Change Impacts on Hybrid-Poplar and Black Locust Short Rotation Coppices. Forests 2018, 9, 419. [Google Scholar] [CrossRef] [Green Version]
- Sixto, H.; Cañellas, I.; van Arendonk, J.; Ciria, P.; Camps, F.; Sánchez, M.; Sánchez-González, M. Growth potential of different species and genotypes for biomass production in short rotation in Mediterranean environments. For. Ecol. Manag. 2015, 354, 291–299. [Google Scholar] [CrossRef]
- Oliveira, N.; Sixto, H.; Cañellas, I.; Rodríguez-Soalleiro, R.; Pérez-Cruzado, C. Productivity model and reference diagram for short rotation biomass crops of poplar grown in Mediterranean environments. Biomass Bioenergy 2015, 72, 309–320. [Google Scholar] [CrossRef]
- Parrotta, J. Productivity, nutrient cycling, and succession in single-and mixed-species plantations of Casuarina equisetifolia, Eucalyptus robusta, and Leucaena leucocephala in Puerto Rico. For. Ecol. Manag. 1999, 124, 45–77. [Google Scholar] [CrossRef]
- Madejón, P.; Alaejos, J.; García-Álbala, J.; Fernández, M.; Madejón, E. Three-year study of fast-growing trees in degraded soils amended with composts: Effects on soil fertility and productivity. J. Environ. Manag. 2016, 169, 18–26. [Google Scholar] [CrossRef]
- Giuntoli, J.; Agostini, A.; Edwards, R.; Marelli, L. Solid and gaseous bioenergy pathways: Input values and GHG emissions. In Calculated According to the Methodology Set in COM (2016) Version 2, EUR 27215 EN; Publications Office of the European Union: Luxembourg, 2017. [Google Scholar] [CrossRef]
- Vanguelova, E.; Pitman, R. Impacts of short rotation forestry on soil sustainability. In Short Rotation Forestry: Review of Growth and Environmental Impacts, Forest Research Monograph, Forest Research; McKay, H., Ed.; Forest Research Alice Holt Lodge: Farnham, Surrey, UK, 2011; p. 37. ISBN 978-0-85538-827-0. [Google Scholar]
- Augusto, L.; Achat, D.L.; Bakker, M.R.; Bernier, F.; Bert, D.; Danjon, F.; Khlifa, R.; Meredieu, C.; Trichet, P. Biomass and nutrients in tree root systems-sustainable harvesting of an intensively managed Pinus pinaster (Ait.) planted forest. GCB Bioenergy 2014, 7, 231–243. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Mixed biomass pellets for thermal energy production: A review of combustion models. Appl. Energy 2014, 127, 135–140. [Google Scholar] [CrossRef]
- Carone, M.T.; Pantaleo, A.; Pellerano, A. Influence of process parameters and biomass characteristics on the durability of pellets from the pruning residues of Olea europaea L. Biomass and Bioenergy 2011, 35, 402–410. [Google Scholar] [CrossRef]
- Stelte, W.; Sanadi, A.R.; Shang, L.; Holm, J.K.; Ahrenfeldt, J.; Henriksen, U.B. Recent developments in biomass palletization—A review. BioRes. 2012, 7, 4451–4490. [Google Scholar] [CrossRef]
- Nielsen, N.P.K.; Gardner, D.J.; Poulsen, T.; Felby, C. Importance of Temperature, Moisture Content, and Species for the Conversion Process of Wood Residues Into Fuel Pellets. Wood Fiber Sci. 2009, 41, 414–425. Available online: https://forestbioproducts.umaine.edu/wp-content/uploads/sites/202/2010/10/George-Marra-Award-Articlepdf_1273348264.pdf (accessed on 19 February 2023).
- Bajwa, D.S.; Peterson, T.; Sharma, N.; Shojaeiarani, J.; Bajwa, S.G. A review of densified solid biomass for energy production. Renew. Sustain. Energy Rev. 2018, 96, 296–305. [Google Scholar] [CrossRef]
- El-Juhany, L.I.; Aref, M.; El-Wakeel, A.O. Evaluation of above-ground biomass and stem volume of three Casuarina species grown in the central region of Saudi Arabia, Emir. J. Food Agric. 2002, 14, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Mason, P.; George, D. Biomass potential of tree species for biofuel production in Southeast Queensland. In Proceedings of the International Proceedings of Chemical, Biological and Environmental Engineering (IPCBEE) 2nd International Conference on Geological and Civil Engineering, Dubai, United Arab Emirates, 10–11 January 2015. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Bevilacqua, E.; Briggs, R.; Volk, T. Allometric biomass equations for tree species used in agroforestry systems in Uganda. Agrofor. Syst. 2013, 87, 781–795. [Google Scholar] [CrossRef]
- Kuyah, S.; Dietz, J.; Muthuri, C.; van Noordwijk, M.; Neufeldt, H. Allometry and partitioning of above- and below-ground biomass in farmed eucalyptus species dominant in Western Kenyan agricultural landscapes. Biomass Bioenergy 2013, 55, 276–284. [Google Scholar] [CrossRef]
- Hunter, I. Above ground biomass and nutrient uptake of three tree species (Eucalyptus camaldulensis, Eucalyptus grandis and Dalbergia sissoo) as affected by irrigation and fertiliser, at 3 years of age, in southern India. For. Ecol. Manag. 2001, 144, 189–199. [Google Scholar] [CrossRef]
- Ounban, W.; Puangchit, L.; Diloksumpun, S. Development of general biomass allometric equations for Tectona grandis Linn.f. and Eucalyptus camaldulensis Dehnh. plantations in Thailand. Agric. Nat. Resour. 2016, 50, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, G.; Scaramuzzi, G. Influenza della distanza d’impianto sulla densiti del legno in Eucalyptus globulus ed E. x trabutti. Cellul. E Carta 1982, 33, 44–52. [Google Scholar]
- Palanti, S.; Susco, D.; Feci, E. Natural durability of eucalypt from Italian plantations against fungi and cerambicid Trichoferus holosericeus Rossi. Eur. J. Wood Prod. 2010, 68, 59–62. [Google Scholar] [CrossRef]
- Giulietti, V.; Roncucci, N.; Nassi o Di Nasso, N.; Tozzini, C.; Ragaglini, C.; Guidi, W.; Taccini, F.; Bonari, E. Suitability of Eucalyptus short rotation coppice in the Mediterranean environment: Preliminary results. Asp. Appl. Biol. 2011, 111, 279–283. [Google Scholar]
- Viera, M.; Bonacina, D.M.; Schumacher, M.V.; Calil, F.N.; Caldeira, M.V.W.; Watzlawick, L.F. Biomass and nutrients in Eucalyptus urograndis stands in southeastern Mountain Range-RS. Ciências Agrárias 2012, 33, 2481–2490. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.M.; Balieiro, F.C.; Ataide, D.H.; Diniz, A.R.; Chaer, G.M. Dynamics of aboveground biomass accumulation in monospecific and mixed-species plantations of Eucalyptus and Acacia on a Brazilian sandy soil. For. Ecol. Manag. 2013, 363, 86–97. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Merino, A.; Rodríguez-Soalleiro, R. A management tool for estimating bioenergy production and carbon sequestration in Eucalyptus globulus and Eucalyptus nitens grown as short rotation woody crops in north-west Spain. Biomass Bioenergy 2011, 35, 2839–2851. [Google Scholar] [CrossRef]
- Muthuri, C.W.; Ong, C.K.; Black, C.R.; Ngumi, V.W.; Mati, B.M. Tree and crop productivity in Grevillea, Alnus and Paulownia-based agroforestry systems in semi-arid Kenya. For. Ecol. Manag. 2005, 212, 23–39. [Google Scholar] [CrossRef]
- Cañellas, I.; Huelin, P.; Hernández, M.J.; Ciria, P.; Calvo, R.; Gea-Izquierdo, G.; Sixto, H. The effect of density on short rotation Populus sp. plantations in the Mediterranean area. Biomass Bioenergy 2012, 46, 645–652. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Sanchez-Ron, D.; Rodríguez-Soalleiro, R.; Hernández, M.J.; Sánchez-Martín, M.; Cañellas, I.; Sixto, H. Biomass production assessment from Populus spp. short-rotation irrigated crops in Spain. GCB Bioenergy 2014, 6, 312–326. [Google Scholar] [CrossRef]
- Paris, P.; Mareschi, L.; Sabatti, M.; Pisanelli, A.; Ecosse, A.; Nardin, F.; Scarascia-Mugnozza, G. Comparing hybrid Populus clones for SRF across northern Italy after two biennial rotations: Survival, growth and yield. Biomass Bioenergy 2011, 35, 1524–1532. [Google Scholar] [CrossRef]
- Pannacci, E.; Bartolini, S.; Covarelli, G. Evaluation of four poplar clones in a short rotation forestry in central Italy. Ital. J. Agron. 2009, 4, 191–198. [Google Scholar] [CrossRef]
- Sabatti, M.; Fabbrini, F.; Harfouche, A.; Beritognolo, I.; Mareschi, L.; Carlini, M.; Paris, P.; Scarascia-Mugnozza, G. Evaluation of biomass production potential and heating value of hybrid poplar genotypes in a short-rotation culture in Italy. Ind. Crops Prod. 2014, 61, 62–73. [Google Scholar] [CrossRef]
- Verlinden, M.; Broeckx, L.; Bulcke, J.V.D.; Van Acker, J.; Ceulemans, R. Comparative study of biomass determinants of 12 poplar (Populus) genotypes in a high-density short-rotation culture. For. Ecol. Manag. 2013, 307, 101–111. [Google Scholar] [CrossRef]
- Dillen, S.; Djomo, S.; Al Afas, N.; Vanbeveren, S.; Ceulemans, R.J. Biomass yield and energy balance of a short-rotation poplar coppice with multiple clones on degraded land during 16 years. Biomass Bioenergy 2013, 56, 157–165. [Google Scholar] [CrossRef] [Green Version]
- Laureysens, I.; Bogaert, J.; Blust, R.; Ceulemans, R. Biomass production of 17 poplar clones in a short-rotation coppice culture on a waste disposal site and its relation to soil characteristics. For. Ecol. Manag. 2004, 187, 295–309. [Google Scholar] [CrossRef]
- Kauter, D.; Lewandowski, I.; Claupein, W. Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use—A review of the physiological basis and management influences. Biomass Bioenergy 2003, 24, 411–427. [Google Scholar] [CrossRef]
- He, X.H.; Critchley, C. Frankia Nodulation, Mycorrhization and Interactions Between Frankia and Mycorrhizal Fungi in Casuarina Plants. In Mycorrhiza; Varma, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 767–781. [Google Scholar] [CrossRef]
- Resquin, F.; Navarro-Cerrillo, R.M.; Carrasco-Letelier, L.; Rachid Casnati, C. Influence of contrasting stocking densities on the dynamics of above-ground biomass and wood density of Eucalyptus benthamii, Eucalyptus dunnii, and Eucalyptus grandis for bioenergy in Uruguay. For. Ecol. Manag. 2019, 438, 63–74. [Google Scholar] [CrossRef]
- Eufrade-Junior, H.J.; Rebessi de Sousa, J.M.; Guerra, S.P.S. Efeito da adubação mineral e densidade de plantio nas características dendrométricas de plantações de eucalipto de rápido crescimento. Ci. Fl. Santa Maria 2021, 31, 350–366. [Google Scholar] [CrossRef]
- Nardini, C.; Schwerz, F.; Dourado Neto, D.; Eloy, E.; Elli, E.F.; Sgarbossa, J.; Tibolla, L.B.; Schmidt, D.; Caron, B.O. Biomass and radiation use efficiency in Eucalyptus plantations as affected by spacing of planting. Sci. For. 2020, 48, e3413. [Google Scholar] [CrossRef]
- Ramalho, F.M.G.; Pimenta, E.M.; Goulart, C.P.; De Almeida, M.N.F.; Vidaurre, G.B.; Hein, P.R.G. Effect of stand density on longitudinal variation of wood and bark growth in fast-growing Eucalyptus plantations. iForest 2019, 12, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Chan, N.; Takeda, S.; Suzuki, R.; Yamamoto, S. Establishment of allometric models and estimation of biomass recovery of swidden cultivation fallows in mixed deciduous forests of the Bago Mountains, Myanmar. For. Ecol. Manage. 2013, 304, 427–436. [Google Scholar] [CrossRef]
- Fehrmann, L.; Kleinn, C. General considerations about the use of allometric equations for biomass estimation on the example of Norway spruce in central Europe. For. Ecol. Manag. 2006, 236, 412–421. [Google Scholar] [CrossRef]
- Henry, M.; Picard, N.; Trotta, C.; Manlay, R.J.; Valentini, R.; Bernoux, M.; Saint-André, L. Estimating tree biomass of sub-Saharan African forests: A review of available allometric equations. Silva Fenn. 2011, 45, 477–569. [Google Scholar] [CrossRef] [Green Version]
- Magar, L.B.; Khadka, S.; Joshi, J.R.R.; Pokharel, U.; Rana, N.; Thapa, P.; Sharma, K.R.S.R.; Khadka, U.; Marasini, B.P.; Parajuli, N. Total Biomass Carbon Sequestration Ability Under the Changing Climatic Condition by Paulownia tomentosa. Steud. Int. J. Appl. Sci. Biotechnol. 2018, 6, 220–226. [Google Scholar] [CrossRef]
- Fatemi, F.R.; Yanai, R.D.; Hamburg, S.P.; Vadeboncoeur, M.A.; Arthur, M.A.; Briggs, R.D.; Levine, C.R. Allometric equations for young northern hardwoods: The importance of age-specific equations for estimating aboveground biomass. Can. J. For. Res. 2011, 41, 881–891. [Google Scholar] [CrossRef]
- Senelwa, K.; Sims, R.E.H. Tree biomass equations for short rotation eucalypts grown in New Zealand. Biomass Bioenergy 1997, 13, 133–140. [Google Scholar] [CrossRef]
- UNE-EN ISO 17225-2. Solid biofuels-Fuel Specifications and Classes-Part 2: Graded Wood Pellets (ISO 17225-2:2021). ISO: Geneva, Switzerland, 2021. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0067970 (accessed on 15 January 2023).
- McFarquhar, M. Modeling Group-level repeated measurements of neuroimaging data using the univariate general linear model. Front. Neurosci. 2019, 13, 352. [Google Scholar] [CrossRef] [Green Version]
- Whitehead, D.; Beadle, C.L. Physiological regulation of productivity and water use in Eucalyptus: A review. For. Ecol. Manag. 2004, 193, 113–140. [Google Scholar] [CrossRef]
- Guo, L.B.; Sims, R.E.H.; Horne, D.J. Biomass production and nutrient cycling in Eucalyptus short rotation energy forests in New Zealand. I: Biomass and nutrient accumulation. Bioresour. Technol. 2002, 85, 273–283. [Google Scholar] [CrossRef]
- Sims, R.E.H.; Senelwa, K.; Maiava, T.; Bullock, B.T. Eucalyptus species for biomass energy in New Zealand. Part II: Coppice performance. Biomass Bioenergy 1999, 17, 333–343. [Google Scholar] [CrossRef]
- Goel, V.L.; Behl, H.M. Growth and productivity assessment of Casuarina glauca Sieb. ex. Spreng on sodic soil sites. Bioresour. Technol. 2005, 96, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Benetka, V.; Bartakova, I.; Mottl, J. Productivity of Populus nigra L. ssp. nigra under short-rotation culture in marginal areas. Biomass Bioenergy 2002, 23, 327–336. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Chandrasekaran, K.; Geetha, M.; Kalaiselvi, R. Growth response of Casuarina equisetifolia Forst. rooted stem cuttings to Frankia in nursery and field conditions. J. Biosci. 2013, 38, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, D.; Diem, H.G.; Dommerges, Y.R.; Ganry, F. Assessment of N fixation by Casuarina equisetifolia inoculated with Frankia ORS021001 using 15N methods. Soil Bid. Biochem. 1985, 17, 375–379. [Google Scholar] [CrossRef]
- Fernández, M.; García-Albalá, J.; Andivia, E.; Alaejos, J.; Tapias, R.; Menéndez, J.; Martín, R.T. Sickle bush (Dichrostachys cinerea L.) field performance and physical–chemical property assessment for energy purposes. Biomass Bioenergy 2015, 81, 483–489. [Google Scholar] [CrossRef]
- Coble, A.P.; Autio, A.; Cavaleri, M.A.; Binkley, D.; Ryan, M.G. Converging patterns of vertical variability in leaf morphology and nitrogen across seven Eucalyptus plantations in Brazil and Hawaii, USA. Trees 2014, 28, 1–15. [Google Scholar] [CrossRef]
- Pérez, C.; Frangi, J.; Goya, J.; Luy, A.; Arturi, M. Contenido de nutrientes en las raíces finas y el mantillo de rodales de Eucalyptus grandis de diferente edad en la Mesopotamia Argentina. Bosque 2013, 34, 303–310. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Krzyżaniak, M.; Szczukowski, S.; Tworkowski, J.; Bieniek, A. Dendromass derived from agricultural land as energy feedstock. Pol. J. Environ. Stud. 2013, 22, 511–520. [Google Scholar]
- Berdón, J.B.; Calvo, A.J.M.; Barroso, L.R.; Alcobendas, A.I.P.; Cortés, J.G. Study of Paulownia’s Biomass Production in Mérida (Badajoz), Southwestern Spain. Environ. Ecol. Res. 2017, 5, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Carmona, R.; Nuñez, T.; Alonso, M.F. Biomass yield and quality of an energy dedicated crop of poplar (Populus spp.) clones in the Mediterranean zone of Chile. Biomass Bioenergy 2015, 74, 96–102. [Google Scholar] [CrossRef]
- Ali, E.; Cramer, W.; Carnicer, J.; Georgopoulou, E.; Hilmi, N.J.M.; Le Cozannet, G.; Lionello, P. Cross-Chapter Paper 4: Mediterranean Region. In Climate Change 2022: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Pörtner, H.-O., Roberts, D.C., Tignor, M., Poloczanska, E.S., Mintenbeck, K., Alegría, A., Craig, M., Langsdorf, S., Löschke, S., Möller, V., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; pp. 2233–2272. [Google Scholar] [CrossRef]
- Carignato, A.; Vázuez-Piqué, J.; Tapias, R.; Ruiz, F.; Fernández, M. Variability and Plasticity in Cuticular Transpiration and Leaf Permeability Allow Differentiation of Eucalyptus Clones at an Early Age. Forests 2020, 11, 9. [Google Scholar] [CrossRef] [Green Version]
- UNE-EN ISO 17225-4:2021. Solid biofuels—Fuel specifications and classes—Part 4: Graded wood chips (ISO 17225-4:2021). ISO: Geneva, Switzerland, 2021. Available online: https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?c=N0067999 (accessed on 10 March 2023).
- Morhart, C.; Sheppard, J.; Spiecker, H. Above Ground Leafless Woody Biomass and Nutrient Content within Different Compartments of a P. maximowicii × P. trichocarpa Poplar Clone. Forests 2013, 4, 471–487. [Google Scholar] [CrossRef] [Green Version]
- Stolarski, M.J.; Stachowicz, P.; Dudziec, P. Wood pellet quality depending on dendromass species. Renew. Energy 2022, 199, 498–508. [Google Scholar] [CrossRef]
- Nunes, L.; Rodrigues, A.; Loureiro, L.; Sá, L.; Matias, J. Energy Recovery from Invasive Species: Creation of Value Chains to Promote Control and Eradication. Recycling 2021, 6, 21. [Google Scholar] [CrossRef]
- Vasiliki, K.; Ioannis, B. Utilization perspectives of wood and bark of the invasive species of Ailanthus and Acacia in the production of pellets. In Proceedings of the International Forestry and Environment Symposium, Trabzon, Turkey, 7–10 November 2017; Available online: http://users.auth.gr/jbarb/Publications/IFES2017-209_Utilization.pdf (accessed on 10 March 2023).
- Kumar, R.; Pandey, K.K.; Chandrashekar, N.; Mohan, S. Study of age and height wise variability on calorific value and other fuel properties of Eucalyptus hybrid, Acacia auriculaeformis and Casuarina equisetifolia. Biomass Bioenergy 2011, 35, 1339–1344. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. Heating values of wood pellets from different species. Biomass Bioenergy 2011, 35, 2634–2639. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. The explained variation by lignin and extractive contents on higher heating value of wood. Biomass Bioenergy 2011, 35, 1663–1667. [Google Scholar] [CrossRef]
- Holm, J.K.; Henriksen, U.B.; Hustad, J.E.; Sørensen, L.H. Toward an Understanding of Controlling Parameters in Softwood and Hardwood Pellets Production. Energy Fuels 2006, 20, 2686–2694. [Google Scholar] [CrossRef]
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. A study of bonding and failure mechanisms in fuel pellets from different biomass resources. Biomass Bioenergy 2011, 35, 910–918. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Agencia Andaluza de la energía. La bioenergía en Andalucía. 2020. Available online: https://www.agenciaandaluzadelaenergia.es/sites/default/files/Documentos/3_2_0068_20_LA_BIOENERGIA_EN_ANDALUCIA.PDF (accessed on 2 May 2023).
- World Bioenergy Association. Global Bioenergy Statistics 2021. Available online: https://www.worldbioenergy.org/uploads/211214%20WBA%20GBS%202021.pdf (accessed on 2 May 2023).
- Fernández, M.; Tapias, R.; Camacho, V.; Alaejos, J. Quality of the Pellets Obtained with Wood and Cutting Residues of Stone Pine (Pinus pinea L.). Forests 2023, 14, 1011. [Google Scholar] [CrossRef]
- Bioenergy Europe. Policy Brief: Pellets. Bioenergy Europe Statistical Report 2021. Available online: https://bioenergyeurope.org/article/328-pellets.html (accessed on 2 May 2023).

| Variable | Soil Layer (Depth Range) | |||
|---|---|---|---|---|
| 0–15 cm | 15–30 cm | |||
| 1st Year | 8th Year | 1st Year | 8th Year | |
| pH (H2O, 1:2.5) | 8.32 (0.05) | 7.98 (0.04) * | 8.34 (0.05) | 8.05 (0.05) * |
| Organic Matter (%) | 1.22 (0.02) | 1.63 (0.06) | 0.70 (0.05) | 1.17 (0.06) |
| C/N ratio | 4.08 (0.08) | 6.81 (0.40) | 3.02 (0.04) | 6.14 (0.40) |
| N (%) | 0.17 (0.01) | 0.15 (0.01) * | 0.14 (0.01) | 0.11 (0.01) * |
| Available P (mg kg−1) (1) | 8.59 (0.12) | 5.40 (0.28) * | 7.85 (0.34) | 4.50 (0.29) * |
| Available K (meq/100 g) | 0.17 (0.01) | 0.16 (0.02) | 0.12 (0.01) | 0.12 (0.01) |
| Available Mg (meq/100 g) | 1.97 (0.05) | 1.51 (0.04) * | 1.81 (0.06) | 1.54 (0.04) * |
| Species | Hybrid | Seedling or ‘Clone Name’ | Cited |
|---|---|---|---|
| Casuarina cunninghamiana | Seedlings | [38,39] | |
| Casuarina equisetifolia | Seedlings | [28,40] | |
| Eucalyptus camaldulensis | ‘ENCE’ | [41,42,43] | |
| Eucalyptus × trabutii | E. botryoides × E. camaldulensis | ‘Biopoplar’ | [44,45,46] |
| Eucalyptus × urograndis | E. urophylla × E. grandis | ‘ENCE’ | [16,47,48] |
| Eucalyptus globulus | ‘ENCE’ | [29,44,46,49] | |
| Paulownia fortunei | ‘UHU’ | [17,21,50] | |
| Populus × euramericana | P. deltoides × P. nigra | ‘Adige’ | [17] |
| Populus × euramericana | P. deltoides × P. nigra | ‘I-214’ | [17,20,26,51,52,53] |
| Populus × euramericana | P. deltoides × P. nigra | ‘AF2’ | [17,53,54,55] |
| Populus × euramericana | P. deltoides × P. nigra | ‘Oudenberg’ | [26,56] |
| Populus × interamericana | P. trichocarpa × P. deltoides | ‘Raspalje’ | [17,57,58,59] |
| Taxon | a | b | R2 | n |
|---|---|---|---|---|
| Casuarina cunninghamiana | 0.031 | 2.586 | 0.985 | 12 |
| Casuarina equisetifolia | 0.026 | 2.695 | 0.992 | 12 |
| Eucalyptus camaldulensis ‘ENCE’ | 0.027 | 2.670 | 0.919 | 12 |
| Eucalyptus × trabutii ‘Biopoplar’ | 0.025 | 2.704 | 0.981 | 13 |
| Eucalyptus × urograndis ‘ENCE’ | 0.043 | 2.495 | 0.969 | 15 |
| Eucalyptus globulus ‘ENCE’ | 0.031 | 2.597 | 0.985 | 20 |
| Paulownia fortunei ‘UHU’ | 0.006 | 3.143 | 0.971 | 14 |
| Populus × euramericana ‘Adige’ | 0.033 | 2.704 | 0.979 | 18 |
| Populus × euramericana ‘AF2’ | 0.024 | 2.721 | 0.971 | 22 |
| Populus × euramericana ‘I-214’ | 0.044 | 2.448 | 0.971 | 15 |
| Populus × euramericana ‘Oudenberg’ | 0.036 | 2.719 | 0.986 | 18 |
| Populus × interamericana ‘Raspalje’ | 0.039 | 2.637 | 0.980 | 15 |
| Taxon | Diametric Class (% Bark) | ||||
|---|---|---|---|---|---|
| d < 25 mm | d = 25–50 mm | d = 50–75 mm | d = 75–100 mm | d > 100 mm | |
| Casuarina cunninghamiana | 30.0 (2.3) bc | 21.4 (1.9) b | 19.9 (1.6) b | 14.3 (1.3) ab | 12.5 (1.2) b |
| Casuarina equisetifolia | 32.3 (3.9) bc | 19.1 (3.4) ab | 17.3 (3.1) b | 13.2 (2.6) ab | 12.3 (1.7) ab |
| Eucalyptus camaldulensis | 25.5 (2.5) ab | 17.3 (1.7) ab | 16.2 (1.3) ab | 14.3 (1.5) ab | 12.1 (1.4) ab |
| Eucalyptus × trabutii | 31.3 (3.8) bc | 17.2 (1.9) ab | 16.9 (1.4) ab | 15.2 (1.3) b | 12.2 (1.3) ab |
| Eucalyptus × urograndis | 24.7 (1.7) a | 18.4 (2.0) ab | 19.3 (2.3) b | 13.8 (1.8) ab | 8.6 (1.6) ab |
| Eucalyptus globulus | 24.0 (1.8) a | 19.3 (1.9) ab | 16.1 (1.2) ab | 15.4 (1.3) b | 12.7 (1.2) b |
| Paulownia fortunei | 23.1 (2.0) a | 16.8 (2.6) ab | 13.4 (1.1) ab | 10.3 (1.4) a | 7.7 (1.5) a |
| Populus ‘Adige’ | 33.1 (1.5) c | 17.4 (1.8) ab | 13.6 (1.6) ab | 10.9 (1.4) a | 10.6 (1.3) ab |
| Populus ‘AF2’ | 33.4 (2.3) c | 18.3 (2.3) ab | 15.3 (1.8) ab | 10.2 (1.5) a | 10.1 (1.5) ab |
| Populus ‘I-214’ | 26.0 (4.6) ab | 19.0 (1.5) ab | 13.1 (1.6) ab | 12.7 (1.3) ab | 10.5 (1.3) ab |
| Populus ‘Oudenberg’ | 33.9 (3.3) c | 16.3 (1.4) a | 11.2 (1.5) a | 11.5 (1.2) ab | 10.6 (1.2) ab |
| Populus ‘Raspalje’ | 31.4 (2.7) bc | 16.6 (1.7) ab | 14.6 (2.3) ab | 11.5 (1.7) ab | 10.5 (1.6) ab |
| Total | 29.2 (1.1) d | 18.1 (0.9) c | 15.6 (0.8) bc | 12.8 (0.8) ab | 11.0 (0.9) a |
| Variable | Biomass Fraction | ||||
|---|---|---|---|---|---|
| Wood with Bark | Debarked Wood | Bark | Thin Branches | Leaves | |
| C (%) | 48.1 (0.3) ab | 49.9 (0.5) a | 48.2 (0.4) ab | 47.3 (0.4) b | 47.1 (0.4) b |
| H (%) | 6.81 (0.27) a | 6.32 (0.39) a | 6.02 (0.35) a | 7.02 (0.34) a | 6.79 (0.34) a |
| N (%) | 0.38 (0.04) a | 0.35 (0.06) a | 1.48 (0.06) c | 0.66 (0.57) b | 1.69 (0.05) d |
| P (%) | 0.06 (0.01) a | 0.03 (0.01) a | 0.11 (0.01) b | 0.10 (0.01) b | 0.15 (0.01) c |
| K (%) | 0.35 (0.04) a | 0.22 (0.03) a | 0.51 (0.04) b | 0.54 (0.04) b | 0.92 (0.05) c |
| Ca (%) | 0.47 (0.07) a | 0.35 (0.10) a | 1.31 (0.10) b | 1.16 (0.09) b | 2.34 (0.10) c |
| Mg (%) | 0.14 (0.01) a | 0.13 (0.02) a | 0.18 (0.02) a | 0.14 (0.02) a | 0.30 (0.02) b |
| S (%) | 0.03 (0.02) a | 0.02 (0.02) a | 0.07 (0.03) a | 0.06 (0.03) a | 0.21 (0.03) b |
| Cl (%) | 0.08 (0.03) a | 0.05 (0.04) a | 0.26 (0.04) b | 0.04 (0.04) a | 0.31 (0.04) b |
| Fe (mg kg−1) | 50.4 (5.2) a | 56.6 (6.8) a | 80.2 (6.8) ab | 103.9 (6.3) b | 531.4 (7.5) c |
| Mn (mg kg−1) | 30.9 (3.1) ab | 27.6 (4.12) a | 75.2 (4.1) c | 35.7 (3.8) ab | 45.6 (4.5) b |
| Zn (mg kg−1) | 33.5 (4.1) ab | 23.6 (5.4) a | 58.3 (5.4) c | 47.9 (4.9) bc | 99.6 (5.9) d |
| B (mg kg−1) | 8.3 (1.0) a | 8.9 (1.3) a | 16.6 (1.3) b | 13.1 (10.4) ab | 49.7 (1.4) c |
| Ash (%) | 1.52 (0.05) b | 1.07 (0.06) a | 6.59 (0.06) d | 2.65 (0.06) c | 5.28 (0.08) e |
| Populus ‘Adige’ ‘AF2’ | Populus ‘I-214’, ‘Oudenberg’ ‘Raspalje’ | Casuarina sp. | Paulownia fortunei | Eucalyptus globulus | Eucalyptus × urograndis | Eucalyptus camaldulensis E. × trabutii | |
|---|---|---|---|---|---|---|---|
| C (%) | 48.7 (0.6) ab | 49.5 (0.7) b | 48.9 (0.5) ab | 49.2 (0.5) b | 48.0 (0.6) ab | 48.4 (0.5) ab | 47.7 (0.7) a |
| N (%) | 0.47 (0.05) bc | 0.34 (0.06) ab | 0.52 (0.05) c | 0.30 (0.04) a | 0.31 (0.05) a | 0.25 (0.05) a | 0.27 (0.06) a |
| S (%) | 0.04 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.03 (0.01) | 0.02 (0.01) | 0.03 (0.01) | 0.04 (0.01) |
| Cl (%) | 0.04 (0.01) | 0.04 (0.01) | 0.10 (0.02) | 0.03 (0.01) | 0.10 (0.02) | 0.10 (0.02) | 0.10 (0.02) |
| Fe (mg kg−1) | 57.0 (8.0) b | 44.5 (9.2) ab | 61.9 (10.2) ab | 23.1 (9.7) a | 52.0 (15.1) b | 55.7 (14.4) b | 64.9 (13.6) b |
| Ash (%) | 1.20 (0.07) b | 1.00 (0.07) b | 2.15 (0.10) c | 0.76 (0.06) a | 1.30 (0.12) b | 1.17 (0.11) b | 2.23 (0.13) c |
| HHV (MJ kg−1) | 19.1 (0.3) | 19.3 (0.2) | 19.2 (0.3) | 19.5 (0.3) | 19.2 (0.3) | 19.5 (0.4) | 18.9 (0.3) |
| LHV (MJ kg−1) | 17.8 (0.3) | 18.0 (0.2) | 17.8 (0.3) | 18.1 (0.3) | 17.7 (0.3) | 18.0 (0.3) | 17.8 (0.4) |
| Bdp (kg m−3) (1) | 659 (14) | 667 (14) | 674 (15) | 632 (11) | 648 (10) | 690 (12) | 682 (10) |
| MDp (%) (1) | 97.6 (1.2) | 97.5 (0.9) | 96.8 (1.0) | 97.7 (0.8) | 97.0 (0.8) | 96.6 (1.2) | 96.5 (1.1) |
| Moisturep (%) (1) | 6.4 (0.4) | 6.7 (0.5) | 6.3 (0.6) | 6.1 (0.4) | 6.8 (0.4) | 6.9 (0.5) | 6.6 (0.6) |
| LHVp (MJ kg−1) (2) | 16.5 (0.3) | 16.6 (0.3) | 16.5 (0.2) | 16.9 (0.3) | 16.5 (0.3) | 16.6 (0.3) | 16.5 (0.2) |
| Wd (kg dm−3) | 0.42 (0.05) b | 0.36 (0.02) b | 0.65 (0.03) d | 0.27 (0.03) a | 0.54 (0.04) c | 0.55 (0.03) c | 0.61 (0.03) cd |
| LHVv (MJ dm−3) | 7.5 (0.2) b | 6.5 (0.1) b | 11.6 (0.2) d | 4.9 (0.1) a | 9.6 (0.2) c | 9.9 (0.2) c | 10.8 (0.3) cd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alaejos, J.; Tapias, R.; López, F.; Romero, D.; Ruiz, F.; Fernández, M. Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate. Forests 2023, 14, 1156. https://doi.org/10.3390/f14061156
Alaejos J, Tapias R, López F, Romero D, Ruiz F, Fernández M. Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate. Forests. 2023; 14(6):1156. https://doi.org/10.3390/f14061156
Chicago/Turabian StyleAlaejos, Joaquín, Raúl Tapias, Francisco López, David Romero, Federico Ruiz, and Manuel Fernández. 2023. "Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate" Forests 14, no. 6: 1156. https://doi.org/10.3390/f14061156
APA StyleAlaejos, J., Tapias, R., López, F., Romero, D., Ruiz, F., & Fernández, M. (2023). Biomass Production and Quality of Twelve Fast-Growing Tree Taxa in Short Rotation under Mediterranean Climate. Forests, 14(6), 1156. https://doi.org/10.3390/f14061156






