Response Characteristics of Photosynthetic Productivity to the Canopy Spatial Distribution Pattern of Larix kaempferi
Abstract
:1. Introduction
2. Materials and Methods
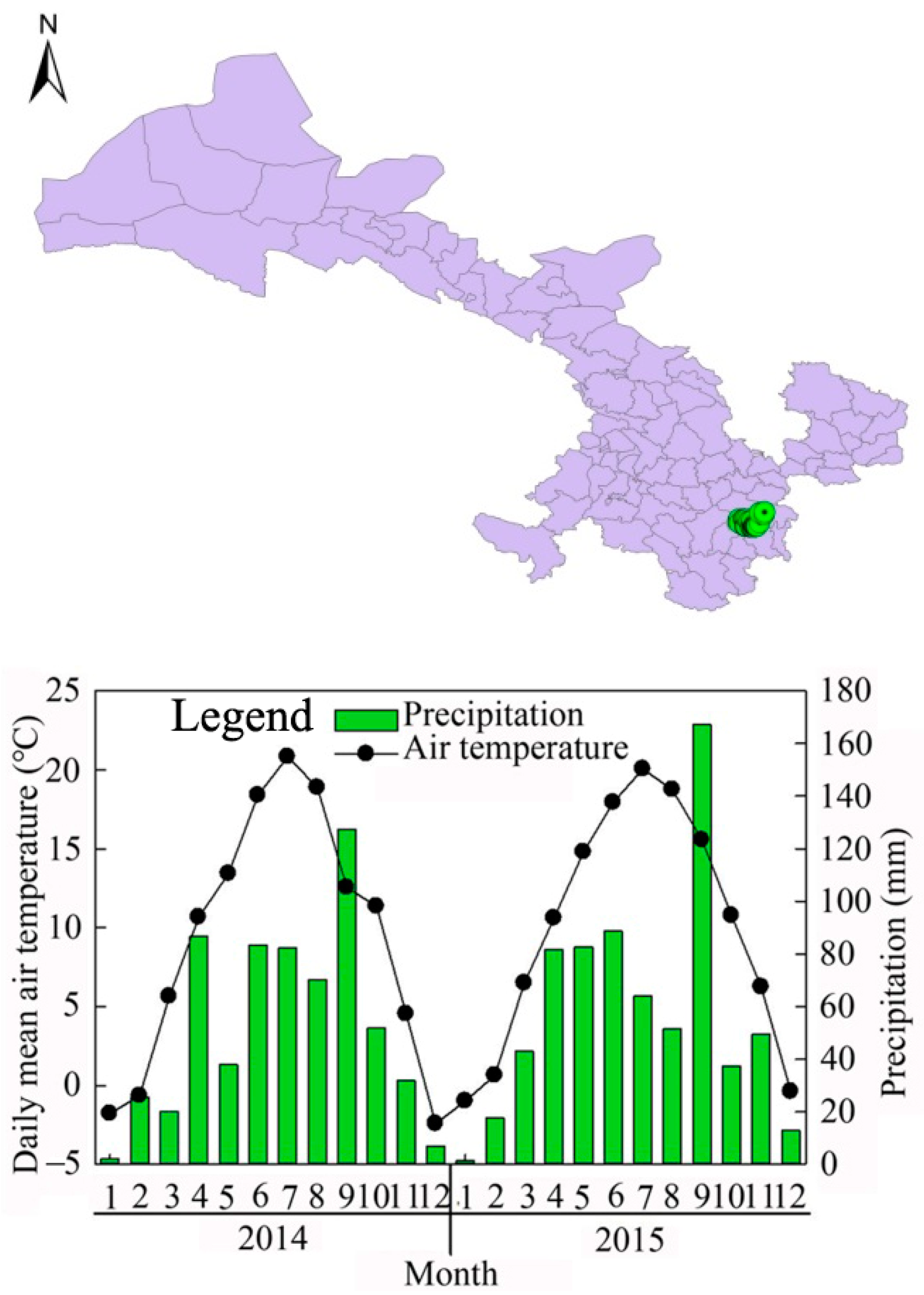
2.1. Site Description
2.2. Experimental Design and Sampling
2.3. Dendrometric and Photosynthetic Data Collection
2.4. Photosynthetic Light-Response Curve Measurement
2.5. Optimal LRC Model Selection
2.6. Leaf Area and Specific Leaf Area Determination
2.7. Canopy Photosynthesis Productivity Estimation
2.8. Data Analysis
3. Results
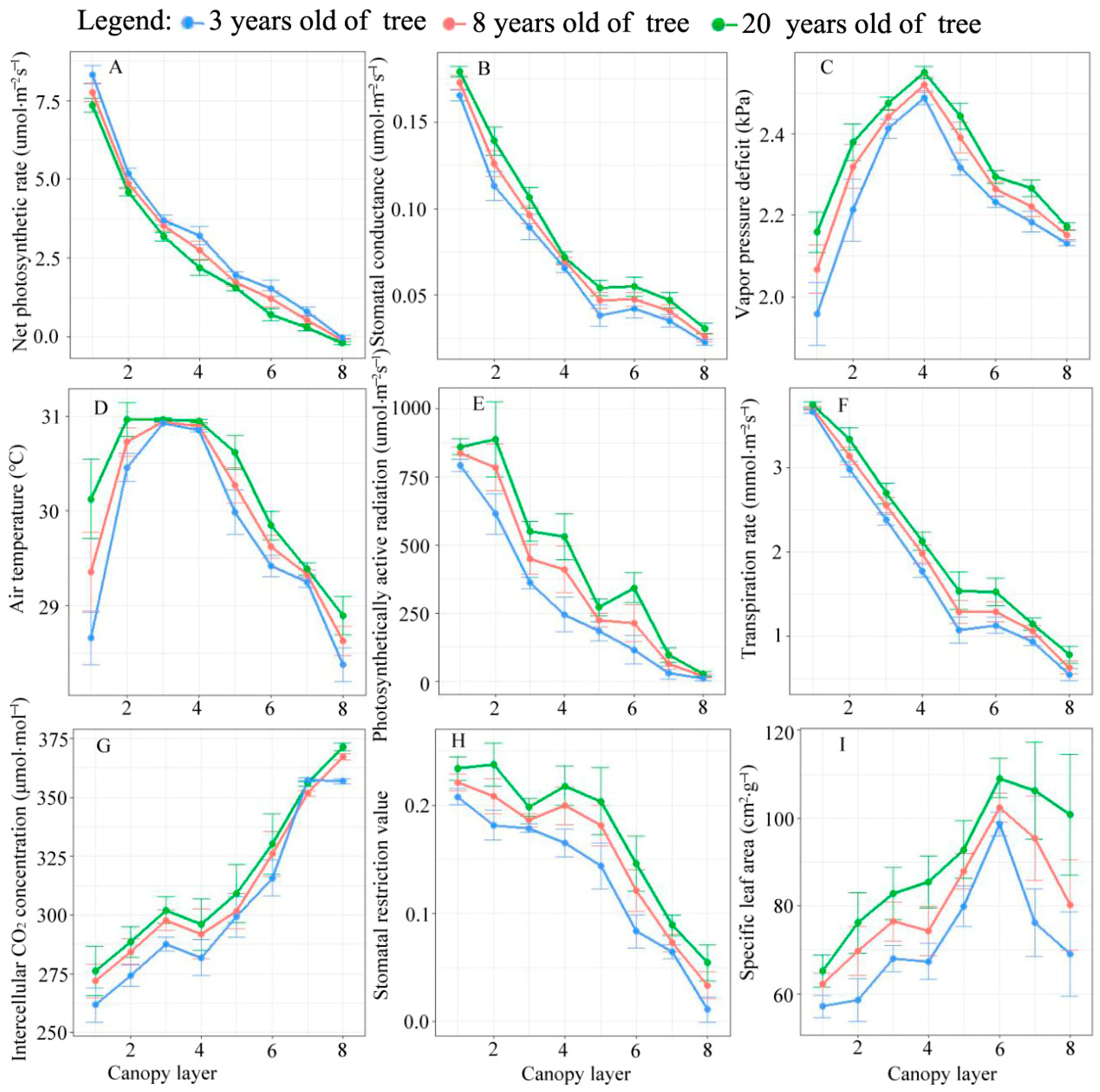
3.1. Distribution Pattern of Photosynthetic Parameters in Canopy
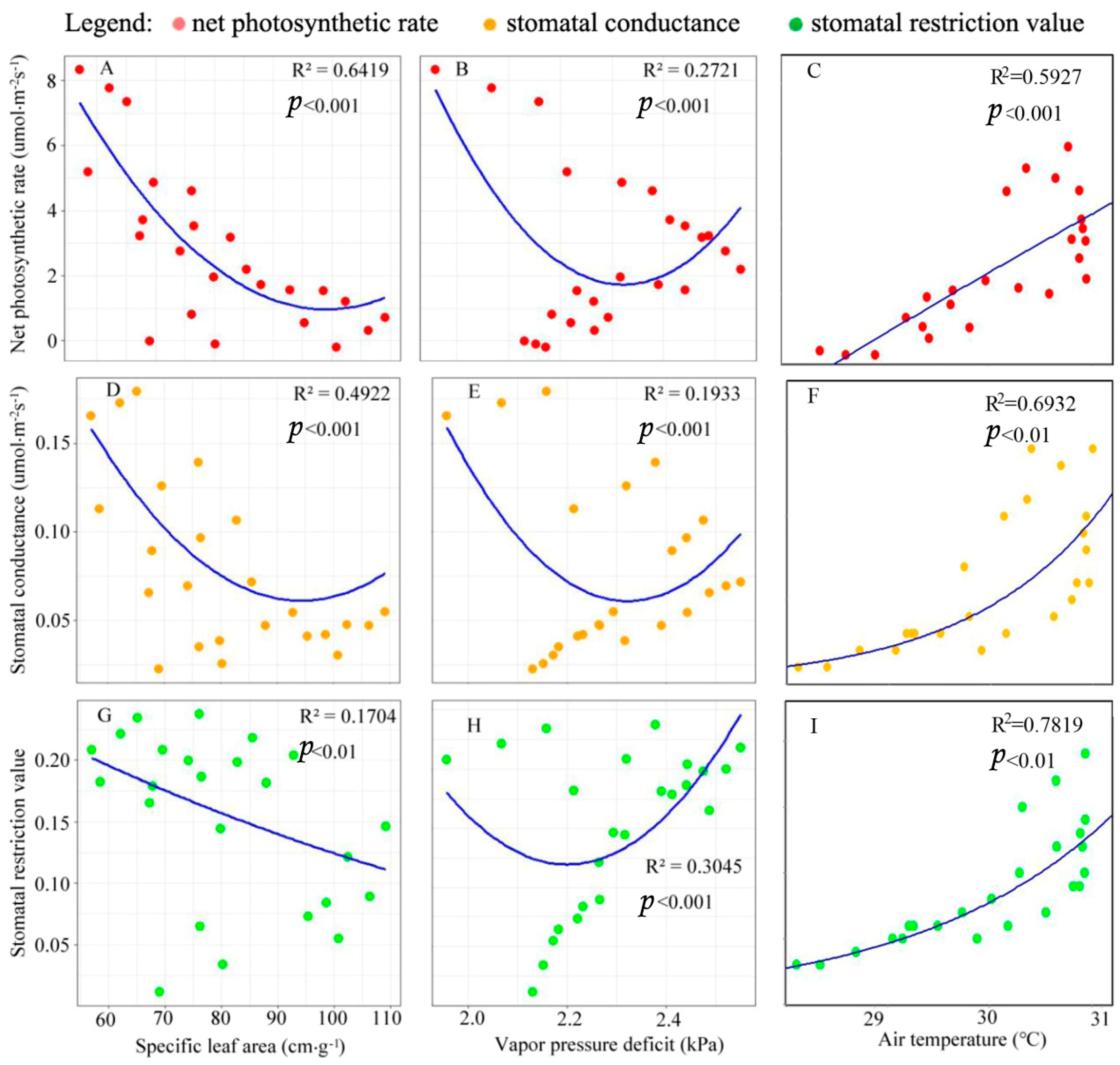
3.2. Relationships between Photosynthetic Parameters versus VPD, SLA, and Ta
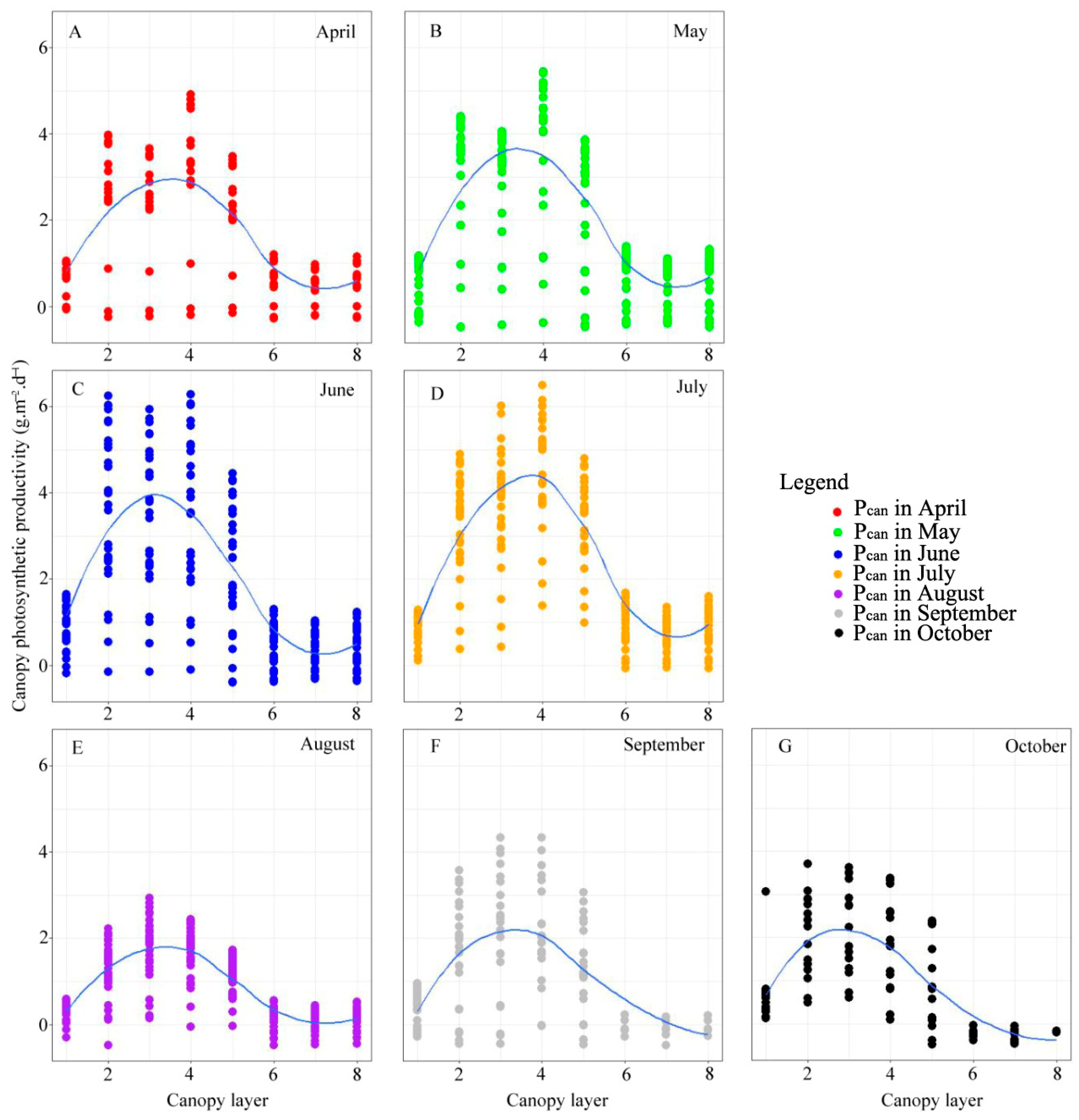
3.3. Vertical Profiles of Canopy Photosynthesis Productivity during the Growing Season
3.4. Effect of Leaf Area on Photosynthetic Productivity
3.5. Effect of Canopy Layer on Photosynthetic Productivity
4. Discussion
4.1. Response of Photosynthetic Productivity of Larch to Canopy Spatial Structure Pattern
4.2. Response of Physiological Parameters of Larch to Canopy Spatial Structure Pattern
4.3. Correlation between Canopy Spatial Distribution and Photosynthetic Productivity
4.4. Forestry Management Methods to Improve Forest Productivity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| LRC | photosynthetic light-response |
| L. kaempferi | Larix kaempferi (Lamb.) Carr |
| PAR | photosynthetically active radiation |
| NRH | Nonrectangle hyperbola model |
| MRH | Modified rectangle hyperbola model |
| MM | Modigliani Miller model |
| Pn | net photosynthesis rate |
| Pmax | the maximum Pn |
| AQY | apparent quantum yield |
| Rd | dark respiration |
| R2 | the coefficient of determination |
| ME | mean error |
| MRE | mean relative error |
| RMSE | mean squared error |
| RRMSE | relative mean squared error |
| SLA | specific leaf area |
| Wdry | dry weight |
| S | the conical projection area |
| Pcan | canopy photosynthesic productivity |
| Pday | daily canopy photosynthesic productivity |
| LA | the leaf area |
| Ta | air temperature |
| Ci | intercellular CO2 concentration |
| Ca | atmospheric CO2 concentration |
| Ls | stomatal limit value |
| gs | stomatal conductance |
| VPD | vapor pressure deficit |
| Tair | air temperature |
| NPP | net primary productivity |
References
- Badgley, G.; Field, C.B.; Berry, J.A. Canopy near-infrared reflectance and terrestrial photosynthesis. Sci. Adv. 2017, 3, e1602244. [Google Scholar] [CrossRef] [Green Version]
- Bar-Even, A. Daring metabolic designs for enhanced plant carbon fixation. Plant Sci. 2018, 273, 71–83. [Google Scholar] [CrossRef]
- Smith, W.K.; Bell, D.T.; Shepherd, K.A. Associations between leaf structure, orientation, and sunlight exposure in five Western Australian communities. Am. J. Bot. 1998, 85, 56–63. [Google Scholar] [CrossRef]
- Lowman, M.D.; Schowalter, T.D. Plant science in forest canopies—The first 30 years of advances and challenges (1980–2010). New Phytol. 2012, 194, 12–27. [Google Scholar] [CrossRef]
- Slot, M.; Winter, K. In situ temperature response of photosynthesis of 42 tree and liana species in the canopy of two Panamanian lowland tropical forests with contrasting rainfall regimes. New Phytol. 2017, 214, 1103–1117. [Google Scholar] [CrossRef] [Green Version]
- Meir, P.; Mencuccini, M.; Binks, O.; Da Costa, A.L.; Ferreira, L.; Rowland, L. Short-term effects of drought on tropical forest do not fully predict impacts of repeated or long-term drought: Gas exchange versus growth. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170311. [Google Scholar] [CrossRef] [Green Version]
- Kira, T.; Shinozaki, K.; Hozumi, K. Structure of forest canopies as related to their primary productivity1. Plant Cell Physiol. 1969, 10, 129–142. [Google Scholar] [CrossRef]
- Kurachi, N.; Hagihara, A.; Hozumi, K. Evaluation of the light interception by non-photosynthetic organs in a Larix leptolepis plantation. Ecol. Res. 1986, 1, 173–183. [Google Scholar] [CrossRef]
- Parker, G.G. Structure and microclimate of forest canopies. For. Canopies 1995, 73–106. [Google Scholar]
- Kurachi, N.; Hagihara, A.; Hozumi, K. Canopy photosynthetic production in a Japanese larch stand. I. Seasonal and vertical changes of leaf characteristics along the light gradient in a canopy. Ecol. Res. 1992, 7, 255–265. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, C.; Zuo, S.; Li, Z. Scaling up of biomass simulation for Eucalyptus plantations based on landsenses ecology. Int. J. Sustain. Dev. World Ecol. 2017, 242, 135–148. [Google Scholar] [CrossRef]
- Fien, E.K.; Fraver, S.; Teets, A.; Weiskittel, A.R.; Hollinger, D.Y. Drivers of individual tree growth and mortality in an uneven-aged, mixed-species conifer forest. For. Ecol. Manag. 2019, 449, 117446. [Google Scholar] [CrossRef]
- Denison, W.C.; Tracy, D.M.; Rhoades, F.M.; Sherwood, M. Direct, non-destructive measurement of biomass and structure in living old-growth Douglas-fir. In Proceedings from Research on Coniferous Forest Ecosystems—A Symposium, Bellingham, Washington; U.S. Department of Agriculture, Forest Service, Pacific Northwest Forest and Range Experiment Station: Portland, OR, USA, 1972; pp. 23–24. [Google Scholar]
- Ishii, H.T.; Tanabe, S.I.; Hiura, T. Exploring the relationships among canopy structure, stand productivity, and biodiversity of temperate forest ecosystems. For. Sci. 2004, 50, 342–355. [Google Scholar]
- Wang, N.; Palmroth, S.; Maier, C.A.; Domec, J.; Oren, R. Anatomical changes with needle length are correlated with leaf structural and physiological traits across five Pinus species. Plant Cell Environ. 2019, 42, 1690–1704. [Google Scholar] [CrossRef]
- Yang, X.; Tang, J.; Mustard, J.F.; Lee, J.-E.; Rossini, M.; Joiner, J.; Munger, J.W.; Kornfeld, A.; Richardson, A.D. Solar-induced chlorophyll fluorescence that correlates with canopy photosynthesis on diurnal and seasonal scales in a temperate deciduous forest. Geophys. Res. Lett. 2015, 42, 2977–2987. [Google Scholar] [CrossRef]
- Takala, T.L.; Mõttus, M. Spatial variation of canopy PRI with shadow fraction caused by leaf-level irradiation conditions. Remote Sens. Environ. 2016, 182, 99–112. [Google Scholar] [CrossRef]
- Atherton, J.; Olascoaga, B.; Alonso, L.; Porcar-Castell, A. Spatial Variation of Leaf Optical Properties in a Boreal Forest Is Influenced by Species and Light Environment. Front. Plant Sci. 2017, 8, 309. [Google Scholar] [CrossRef] [Green Version]
- Kurachi, N.; Hagihara, A.; Hozumi, K. Canopy photosynthetic production in a Japanese larch stand. I. Estimation of the canopy photosynthetic production. Ecol. Res. 1993, 8, 349–361. [Google Scholar] [CrossRef]
- Ryan, M.G.; Binkley, D.; Fownes, J.H. Age-Related Decline in Forest Productivity: Pattern and Process. Adv. Ecol. Res. 1997, 27, 213–262. [Google Scholar] [CrossRef]
- Oren, R.; Schulze, E.-D.; Matyssek, R.; Zimmermann, R. Estimating photosynthetic rate and annual carbon gain in conifers from specific leaf weight and leaf biomass. Oecologia 1986, 70, 187–193. [Google Scholar] [CrossRef]
- Bosc, A.; De Grandcourt, A.; Loustau, D. Variability of stem and branch maintenance respiration in a Pinus pinaster tree. Tree Physiol. 2003, 23, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, G.; Luo, H.; Zhang, Y.; Gou, L.; Yao, Y.; Lin, Y.; Zhang, W. Relationship between plant canopy characteristics and photosynthetic productivity in diverse cultivars of cotton (Gossypium hirsutum L.). Crop J. 2016, 4, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Cavaleri, M.A.; Coble, A.P.; Ryan, M.G.; Bauerle, W.L.; Loescher, H.W.; Oberbauer, S.F. Tropical rainforest carbon sink declines during El Niño as a result of reduced photosynthesis and increased respiration rates. New Phytol. 2017, 216, 136–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azuma, W.; Ishii, H.R.; Masaki, T. Height-related variations of leaf traits reflect strategies for maintaining photosynthetic and hydraulic homeostasis in mature and old Pinus densiflora trees. Oecologia 2019, 189, 317–328. [Google Scholar] [CrossRef]
- Legner, N.; Fleck, S.; Leuschner, C. Within-canopy variation in photosynthetic capacity, SLA and foliar N in temperate broad-leaved trees with contrasting shade tolerance. Trees 2014, 28, 263–280. [Google Scholar] [CrossRef]
- Cáceres, C.B.; Hernández, R.E.; Fortin, Y. Shrinkage variation in Japanese larch (Larix kaempferi (Lamb.) Carr.) progenies/provenances trials in Eastern Canada. Wood Mater. Sci. Eng. 2017, 13, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.W. Study on the ecological survey of Larix kaempferi. Shandong For. Sci. Technol. 1985, 1, 9–18. (In Chinese) [Google Scholar]
- Pâque, L.E. Improvement of Larch (Larix sp.) for Better Growth, Stem form and Wood Quality: Proceedings of an International Symposium; INRA: Orleans, France, 2002. [Google Scholar]
- Hoshi, H. Forest tree genetic resources conservation stands of Japanese larch (Larix kaempferi (Lamb.) Carr.). For. Tree Gen. Res. Inf. 2004, 1, 1–4. [Google Scholar]
- Bhusal, N.; Lee, M.; Han, A.R.; Han, A.; Kim, H.S. Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. For. Ecol. Manag. 2020, 465, 118099. [Google Scholar] [CrossRef]
- Mueller, P.; Jensen, K.; Megonigal, J.P. Plants mediate soil organic matter decomposition in response to sea level rise. Glob. Chang. Biol. 2016, 22, 404–414. [Google Scholar] [CrossRef]
- Xia, G.W.; Chen, D.S.; Sun, X.M.; Zhang, S.G. Spatial heterogeneity of photosynthetic and physiological parameters in Larix kaempferi crown. For. Res. 2018, 31, 130–137. (In Chinese) [Google Scholar]
- Monsi, M.; Saeki, T. The light factor in plant communities and its significance for dry matter production. Jpn. J. Bot. 1953, 14, 22–52. [Google Scholar]
- Bassman, J.H.; Zwier, J.C. Gas exchange characteristics of Populus trichocarpa, Populus deltoides and Populus trichocarpa × P. deltoides clones. Tree Physiol. 1991, 8, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Dong, L.H.; Li, F.R. Modeling net CO2 assimilation (AN) within the crown of young planted Larix olgensis trees. Can. J. For. Res. 2018, 48, 1085–1098. [Google Scholar] [CrossRef] [Green Version]
- Thornley, J.H.M. Mathematical Models in Plant Physiology; Academic Press: London, UK; New York, NY, USA, 1976; pp. 108–110. [Google Scholar]
- Boonen, C.; Samson, R.; Janssens, K.; Pien, H.; Lemeur, R.; Berckmans, D. Scaling the spatial distribution of photosynthesis from leaf to canopy in a plant growth chamber. Ecol. Model. 2002, 156, 201–212. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017; Available online: http://www.R-project.org/ (accessed on 25 June 2021).
- Escalona, J.M.; Bota, J.; Medrano, H. Distribution of leaf photosynthesis and transpiration within grapevine canopies under different drought conditions. VITIS-J. Grapevine Res. 2015, 42, 57. [Google Scholar] [CrossRef]
- Walker, B.J.; Drewry, D.T.; Slattery, R.A.; VanLoocke, A.; Cho, Y.B.; Ort, D.R. Chlorophyll Can Be Reduced in Crop Canopies with Little Penalty to Photosynthesis. Plant Physiol. 2018, 176, 1215–1232. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, A.; Kondo, K.; Ikka, T.; Takai, T.; Tanabata, T.; Yamamoto, T. A novel QTL associated with rice canopy temperature difference affects stomatal conductance and leaf photosynthesis. Breed. Sci. 2018, 68, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Peters, J.; Morales, D. Gas exchange characteristics of Pinus canariensis needles in a forest stand on Tenerife, Canary Islands. Trees 2003, 17, 492–500. [Google Scholar] [CrossRef]
- Fuchs, M.; Stanghellini, C. The functional dependence of canopy conductance on water vapor pressure deficit revisited. Int. J. Biometeorol. 2018, 62, 1211–1220. [Google Scholar] [CrossRef]
- Shirke, P.A.; Pathre, U.V. Influence of leaf-to-air vapour pressure deficit (VPD) on the biochemistry and physiology of photosynthesis in Prosopis juliflora. J. Exp. Bot. 2004, 55, 2111–2120. [Google Scholar] [CrossRef]
- Shibuya, T.; Kano, K.; Endo, R.; Kitaya, Y. Effects of the interaction between vapor-pressure deficit and salinity on growth and photosynthesis of Cucumis sativus seedlings under different CO2 concentrations. Photosynthetica 2018, 56, 893–900. [Google Scholar] [CrossRef]
- Cunningham, S. Photosynthetic responses to vapour pressure deficit in temperate and tropical evergreen rainforest trees of Australia. Oecologia 2005, 142, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Zhang, G.; Zhu, X.G. Optimal crop canopy architecture to maximize canopy photosynthetic CO2 uptake under elevated CO2—A theoretical study using a mechanistic model of canopy photosynthesis. Funct. Plant Biol. 2013, 40, 108–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Ryu, Y. Seasonal changes in vertical canopy structure in a temperate broadleaved forest in Korea. Ecol. Res. 2015, 30, 821–831. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant carbon metabolism and climate change: Elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [Green Version]
- Šimpraga, M.; Verbeeck, H.; Bloemen, J.; Vanhaecke, L.; Demarcke, M.; Joó, E.; Pokorska, O.; Amelynck, C.; Schoon, N.; Dewulf, J.; et al. Vertical canopy gradient in photosynthesis and monoterpenoid emissions: An insight into the chemistry and physiology behind. Atmos. Environ. 2013, 80, 85–95. [Google Scholar] [CrossRef]
- Bhusal, N.; Bhusal, S.J.; Yoon, T.-M. Comparisons of physiological and anatomical characteristics between two cultivars in bi-leader apple trees (Malus × domestica Borkh.). Sci. Hortic. 2018, 231, 73–81. [Google Scholar] [CrossRef]
- Kenzo, T.; Inoue, Y.; Yoshimura, M.; Yamashita, M.; Tanaka-Oda, A.; Ichie, T. Height-related changes in leaf photosynthetic traits in diverse Bornean tropical rain forest trees. Oecologia 2015, 177, 191–202. [Google Scholar] [CrossRef]
- Kaiser, E.; Matsubara, S.; Harbinson, J.; Heuvelink, E.; Marcelis, L.F. Acclimation of photosynthesis to light flecks in tomato leaves: Interaction with progressive shading in a growing canopy. Physiol. Plant 2018, 162, 506–517. [Google Scholar] [CrossRef] [Green Version]
- Qu, L.Y.; Ji, D.H.; Shi, F.C.; Kaichiro, S.; Takayoshi, K. Growth and photosynthetic performance of seedlings of two larch species grown in shaded conditions. Eurasian J. For. Res. 2005, 8, 43–51. [Google Scholar]
- Elferjani, R.; DesRochers, A.; Tremblay, F. Plasticity of bud phenology and photosynthetic capacity in hybrid poplar plantations along a latitudinal gradient in northeastern Canada. Environ. Exp. Bot. 2016, 125, 67–76. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Xu, C.L.; Sun, X.M.; Zhang, S.G. Comparative analysis on photosynthetic productivity of Larix kaempferi, L. olgensis and their hybrids. J. Northwest For. Univ. 2012, 27, 129–133. (In Chinese) [Google Scholar]
- Wright, I.J.; Cooke, J.; Cernusak, L.A.; Hutley, L.B.; Scalon, M.C.; Tozer, W.C.; Lehmann, C.E. Stem diameter growth rates in a fire-prone savanna correlate with photosynthetic rate and branch-scale biomass allocation, but not specific leaf area. Austral Ecol. 2019, 44, 339–350. [Google Scholar] [CrossRef]
- Xue, W.; Lindner, S.; Dubbert, M.; Otieno, D.; Ko, J.; Muraoka, H.; Werner, C.; Tenhunen, J. Supplement understanding of the relative importance of biophysical factors in determination of photosynthetic capacity and photosynthetic productivity in rice ecosystems. Agric. For. Meteorol. 2017, 232, 550–565. [Google Scholar] [CrossRef]
- Hirtreiter, J.N.; Potts, D.L. Canopy structure, photosynthetic capacity and nitrogen distribution in adjacent mixed and monospecific stands of Phragmites australis and Typha latifolia. Plant Ecol. 2012, 213, 821–829. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Summer pruning and reflective film enhance fruit quality in excessively tall spindle apple trees. Hortic. Environ. Biotechnol. 2017, 58, 560–567. [Google Scholar] [CrossRef]
- Cao, N.; Yu, H.Q.; Wang, S.B.; Yu, T.; Cao, M.J. Analysis on canopy structure and photosynthetic characteristics of high yield maize population. J. Maize Sci. 2006, 14, 94–97. (In Chinese) [Google Scholar] [CrossRef]
- Li, F.R.; Wang, Z.F.; Wang, B.S. Studies on the effective crown development of Larix olgensis (I)-determination of the effective crown. J. Northeast For. Univ. 1996, 24, 1–8. (In Chinese) [Google Scholar]


| Age (a) | Slope | Density (Trees·hm−2) | Height (m) | DBH (cm) | Canopy Width (m) | Canopy Length (m) |
|---|---|---|---|---|---|---|
| 3 | South | 4400 | 4.3 ± 0.12 * | 4.4 ± 0.37 * | 1.4 ± 0.03 * | 4.0 ± 0.16 * |
| 8 | Southwest | 3700 | 10.0 ± 0.18 * | 7.5 ± 0.03 * | 2.3 ± 0.04 * | 5.9 ± 0.21 * |
| 20 | West | 2160 | 16.1 ± 0.14 * | 13.5 ± 0.20 * | 2.8 ± 0.04 * | 6.4 ± 0.11 * |
| Stand Age/(a) | Soil Depth/(cm) | C/(mg·g−1) | N/(mg·g−1) | P/(mg·g−1) | C:N | C:P | N:P |
|---|---|---|---|---|---|---|---|
| 3 | 0~10 | 19.53 ± 4.73 | 1.28 ± 0.42 | 0.58 ± 0.09 * | 15.90 ± 1.79 * | 32.79 ± 3.84 * | 2.10 ± 0.40 * |
| 10~20 | 17.92 ± 4.79 * | 1.24 ± 0.39 * | 0.55 ± 0.09 * | 15.47 ± 0.78 * | 30.12 ± 3.46 * | 2.03 ± 0.33 * | |
| 20~40 | 14.37 ± 6.13 | 0.98 ± 0.47 * | 0.52 ± 0.12 * | 15.47 ± 1.11 * | 24.87 ± 5.45 * | 1.66 ± 0.48 * | |
| 8 | 0~10 | 18.16 ± 3.71 | 1.68 ± 0.42 | 0.6 ± 0.050 * | 11.16 ± 0.69 * | 29.48 ± 4.39 * | 2.72 ± 0.57 |
| 10~20 | 16.19 ± 3.87 * | 1.58 ± 0.46 * | 0.64 ± 0.09 * | 10.65 ± 0.70 * | 23.18 ± 2.41 * | 2.23 ± 0.38 * | |
| 20~40 | 14.25 ± 6.54 * | 1.18 ± 0.56 * | 0.57 ± 0.12 * | 13.03 ± 0.14 * | 22.49 ± 6.13 * | 1.81 ± 0.53 * | |
| 20 | 0~10 | 18.93 ± 3.98 * | 1.57 ± 0.49 * | 0.72 ± 0.10 | 12.67 ± 2.04 * | 25.74 ± 2.21 * | 2.10 ± 0.42 * |
| 10~20 | 16.58 ± 3.71 * | 1.62 ± 0.53 | 0.64 ± 0.08 * | 10.50 ± 1.54 | 24.08 ± 2.50 * | 2.33 ± 0.49 * | |
| 20~40 | 14.91 ± 6.60 | 1.23 ± 0.58 | 0.60 ± 0.14 * | 13.50 ± 0.56 * | 22.29 ± 5.19 | 1.78 ± 0.46 * |
| Canopy Layer | Top | 2nd | 3rd | 4th | 5th | 6th | 7th | Bottom | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pn | −0.101 * | 0.5361 * | 1.2121 * | 1.7211 * | 2.7521 * | 3.5171 * | 4.8541 * | 7.7661 * | 2.7821 * |
| gs | 0.0261 * | 0.0411 * | 0.0481 * | 0.0471 * | 0.0701 * | 0.0961 * | 0.1261 * | 0.1731 * | 0.0781 * |
| Tr | 0.631 * | 1.067 * | 1.290 * | 1.295 * | 1.980 * | 2.555 * | 3.137 * | 3.698 * | 1.957 * |
| Ci | 367.200 * | 351.800 * | 326.000 * | 301.700 * | 291.900 * | 297.800 * | 284.500 * | 272.000 * | 289.200 * |
| Ls | 0.03400 * | 0.07300 * | 0.12100 * | 0.18100 * | 0.20000 * | 0.18600 * | 0.20800 * | 0.22100 * | 0.15300 * |
| SLA | 80.1990 * | 95.3980 * | 102.415 * | 87.9305 * | 74.2485 * | 76.5025 * | 69.6535 * | 62.1725 * | 81.0655 * |
| Ta | 28.6295 * | 29.335 * | 29.622 * | 30.271 * | 30.889 * | 30.941 * | 30.728 * | 29.355 * | 29.971 * |
| VPD | 2.1521 * | 2.2211 * | 2.2651 * | 2.3901 * | 2.5201 * | 2.4421 * | 2.3201 * | 2.0681 * | 2.2971 * |
| PAR | 18.485 * | 64.460 * | 213.167 * | 224.682 * | 410.627 * | 448.559 * | 784.654 * | 836.806 * | 375.180 * |
| Canopy Layer | Parameter | p-Value | Pseudo-R2 | ||
|---|---|---|---|---|---|
| a | b | c | |||
| 1 | 0.120 | 0.891 | 0.452 | 0.07 | 0.28 |
| 2 | 0.341 | 0.734 | 0.371 | 0.24 | 0.13 |
| 3 | 0.715 | 0.205 | 0.518 | <0.05 | 0.36 |
| 4 | 0.093 | 0.810 | 0.418 | 0.18 | 0.21 |
| 5 | 0.548 | 0.336 | 0.425 | <0.01 | 0.41 |
| 6 | 0.639 | 0.267 | 0.481 | <0.01 | 0.47 |
| 7 | 0.257 | 0.609 | 0.375 | 0.05 | 0.31 |
| 8 | 0.364 | 0.578 | 0.306 | 0.11 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Chen, D.; Xia, G.; Sun, X.; Zhang, S. Response Characteristics of Photosynthetic Productivity to the Canopy Spatial Distribution Pattern of Larix kaempferi. Forests 2023, 14, 1171. https://doi.org/10.3390/f14061171
Wu C, Chen D, Xia G, Sun X, Zhang S. Response Characteristics of Photosynthetic Productivity to the Canopy Spatial Distribution Pattern of Larix kaempferi. Forests. 2023; 14(6):1171. https://doi.org/10.3390/f14061171
Chicago/Turabian StyleWu, Chunyan, Dongsheng Chen, Guowei Xia, Xiaomei Sun, and Shougong Zhang. 2023. "Response Characteristics of Photosynthetic Productivity to the Canopy Spatial Distribution Pattern of Larix kaempferi" Forests 14, no. 6: 1171. https://doi.org/10.3390/f14061171





