Abstract
Pinus elliottii Engelm bark is a byproduct of Uruguay’s milling industry. As a circular economy strategy, it is burned in broilers for energy production. Aiming to increase the added value of the bark, this work analyzes the extraction of its tannins to use them in the development of formaldehyde-free adhesives, while evaluating whether it retains its calorific power for further energy production. The best extraction conditions (methanol at 65 °C for 2 h) were identified at a laboratory level after which they were scaled up to 50 L, which did not affect extraction yield. The Stiasny number remained above 65%, meaning the extractive was suitable for its use in adhesive formulations. The characterization of the extractives was completed with molecular weight distribution, FTIR-ATR, ABES and DSC. Finally, two formaldehyde-free adhesive formulations were developed using hexamine and glyoxal as hardeners. Their behaviors were compared through rheological analysis, DSC and ABES. It was determined that the adhesive formulations with hexamine at pHs of 8 and 10 are suitable for their use in the timber industry. It was noted that they react the best at a pressing temperature of 160 °C. After the extraction, the calorific power of the P. elliottii Engelm bark decreased by only 13%, thus remaining useful for energy production.
1. Introduction
Over the last few decades, the development of innovative technologies with sustainable patterns [1] has been encouraged to alleviate climate change produced by the ceaseless industrial overproduction of CO2 and economies based on fossil fuel consumption [2,3]. Many countries persistently search for strategies to promote circular economies. For example, biorefinery allows for the transformation of biomass (wood, grass, etc.) into products of added value, such as biofuels and biochemical products, while solving waste management issues [4,5,6,7,8]. Wood consumption in the milling industry creates copious amounts of waste (sawdust and bark) [9], which constitute a source of raw matter that could be used in biorefinery through the extraction of its biocomponents such as polyphenols and stilbenes and waxes, among others [5,10,11,12]. These compounds can be used in the timber industry with bioadhesives, bioplastics or preservatives of lower environmental impact [5,7]. Forestry is one of the main industries in Uruguay: cultivated forests of fast-growing species cover 1.48228 ha 14% of these species belong to the Pinus genus, among which are Pinus taeda L. and Pinus elliottii Engelm, [13]. Pine bark waste is generated by the debarking of wood logs during the primary stage of production, potentially amounting to 60,000 tons a year [14]. As of today, pine bark mixed with other lignocellulosic waste is burnt in boilers and used in combustion and gasification processes. Pine bark has a low calorific value of 18.8 MJ/kg [6]; however, pine bark also happens to have a high content of extractives (soluble in water or organic solvents), ash and phenolic compounds [5,11]. Furthermore, the bark of conifers is one of the richest sources of tannins [9,14], reaching up to 40% of their dry weight [15]. Due to their structural similarity with synthetic phenol [16,17] tannin extractives can be used in adhesive formulations instead of phenol and phenol–formaldehyde, allowing for the reduction of formaldehyde emissions in the environment [7,15,18]. This work studies the valorization of a byproduct—bark from Pinus elliottii Englem grown in Uruguay— for the first time by extracting its tannins to completely replace formaldehyde in adhesive formulations for the timber industry. Meanwhile, true to a circular economy philosophy and aiming to reduce waste, the calorific value of the bark was analyzed before and after extraction. In order to evaluate the impact of the methodology and conditions of extraction on the properties of the tannins which would in turn impact the adhesives, such variables were first studied at the laboratory level [19,20,21] and then scaled up to a pilot plant. The effect of conditions was evaluated through extraction yields and by the following characteristics of the extractives: total polyphenol content, condensed tannins content, Stiasny number, and molecular weight distribution through gel permeation chromatography (GPC). Chemical characteristics of the extractives were analyzed by Fourier transform infrared spectroscopy: attenuated total reflection (FTIR-ATR). The curing reaction by autocondensation of the extracts was studied with an automated bonding evaluation system (ABES) and through differential scanning calorimetry (DSC) [5,10,11,22,23]. Once extraction conditions were optimized, the extracts were used in adhesive formulations with glyoxal (Gly) and hexamethylenetetramine (Hex) as hardeners instead of formaldehyde. The effects of the hardeners on rheological properties and curing reaction of the adhesives were analyzed through DSC and ABES [11,15,16,24,25,26,27].
2. Materials and Methods
2.1. Pine Bark
Pinus elliottii Englem bark was gathered by hand at random from a pile of logs of trees aged 25 to 30 years from the company Arboreal in Rivera, Uruguay. The bark was air dried for four weeks and then milled in a Marconi Mod. MA 680 sawmill (São Paulo, Brazil) into particles below 1 mm and sieved through an American Society for Testing and Materials (ASTM) Nº 20 sieve (0.850 mm). Laboratory scale tests were carried out at the Forest Laboratory of the Sede Tacuarembó of the Universidad de la República in Uruguay. 1 L and 50 L scale tests as well as the adhesive formulations were carried out in the Unidad de Desarrollo Tecnológico of the Universidad de Concepción, Chile.
2.2. Extraction on Laboratory Scale
To optimize extraction conditions, the bark was macerated [28] at room temperature (20 °C) for 24 h and heated in a water bath with stirring at the temperature and time laid out in the experimental design. To 1 g of milled bark, the corresponding amount of solvent was added at a 1/20 (m/v) ratio [28,29,30]. Using the Taguchi L8 (23) experimental design [31,32], the independent variables were the following: solvent (ethanol 80% and methanol 80%), temperature (65 °C and 75 °C), and time (2 h and 4 h). Each test was carried out thrice. The response variables were the following: average condensed tannins (mg cyanidin/g dry bark), Stiasny number [21,28,33]. The conditions of the 8 performed tests are shown in Table 1.

Table 1.
Conditions of extraction tests in accordance with the Taguchi experimental design.
To prevent the solvent from evaporating, maceration was performed in lidded glass crystallizers wrapped in aluminum foil. The solutions were filtered through sintered glass and concentrated by evaporation in a rotary evaporator (Büchi, Germany). Finally, the solutions were separated into phases by adding diethyl ether (3 × 5 mL). The aqueous phase was brought to 10 mL by adding distilled water; meanwhile, the diethyl ether was evaporated from the organic phase, which was dissolved in 5 mL of methanol [28].
2.2.1. Total Polyphenol Content
Following the Folin–Ciocalteu method [34], to 0.5 mL of solution diluted 200 times, 2.5 mL of Folin–Ciocalteu reagent diluted 10 times was added. The mixture was stirred and left to rest for 8 min. 2 mL of sodium carbonate was added to the mixture to be placed in an oven at 45 °C for 15 min. Absorbance was determined at 765 nm with a Spectrum S20 spectrophotometer, model SP-UV 300SRB (Talat Khwan, Thailand). Polyphenol content was expressed as equivalent milligrams of gallic acid (AGE) over grams of dry bark (mg AGE/g bark) [10,29].
2.2.2. Condensed Tannin Content
The BuOH/HCl test was used [21,28]. To 0.5 mL of the aqueous extract (diluted 100 times), 5 mL of a ferrous acid solution (77 mg of FeSO4.7H2O in 50 mL of HCl/BuOH (2/3)) was added. The tubes were covered with glass spheres, placed in a water bath at 95 °C for 15 min under a hood, and the absorbance was read immediately at 530 nm with a Spectrum S20 spectrophotometer, model SP-UV 300SRB (Talat Khwan, Thailand). Condensed tannin content was expressed as equivalent milligrams of cyanidin (Cya) over grams of dry bark according to the following formula [21,29]:
where A = absorbance value read at the corresponding wavelength for each case; V = total volume of the reaction; D = corresponds to dilution factor; M = molecular weight of cyanidin (g mol−1); V2 = volume of the aqueous extract after extraction with diethyl ether (mL); l = length of the cuvette (cm−1); ϵ = molar extinction coefficient (34,700 L mol −1 cm−1); v = 0.5 mL; m = dry weight of the bark (g).
mg Cya/g bark = A ∗ V ∗ D ∗ M ∗ V2/l ∗ ϵ ∗ v ∗ m
2.2.3. Stiasny Number
The Stiasny number is a gravimetric method of quantifying condensed tannins that react with formaldehyde in an acidic medium [21,24,35]. This method is used to determine whether an extract is suitable for its use in an adhesive formulation. Hence, all extracts with a Stiasny number below 65 % were not considered for the development of the adhesives. To 0.1 g of previously dried samples of each tannin extract, 10 mL of water, 2 mL of formaldehyde (38%) and 1 mL of HCl (10 N) were added. The mixture was heated for 30 min at constant temperature. The precipitate was vacuum filtered through a previously weighted paper, washed with distilled water until all formaldehyde was removed, dried in an oven at 105 °C until constant weight [19] and finally dried in a desiccator for 30 min. The Stiasny number was calculated as the percentage of precipitate obtained from the initial extract, as shown below:
Stiasny number (%) = ((dry weight of the precipitate/∗dry weight of the initial extract) ∗ 100. ∗dry weight of the extract = ((weight 10 mL ∗ % of solids)/100)
2.3. Extraction on a 1 L (L) Scale
Extractions were carried out in a closed 1 L stainless steel reactor heated with electric resistance. Once the desired temperature was reached, the reactor was placed in a thermic oil bath for as long as each test required. 80 % methanol was used; extraction time was 2 h; bark/solvent ratio was 1/20 (m/v). Three temperatures for extraction were tested: 55 °C, 65 °C and 75 °C. Each extraction condition was performed thrice (9 in total). The solutions were filtered and methanol was evaporated from the extract in a rotary evaporator (Büchi, Germany). Total extraction yield (%) was expressed as the weight of the recovered extract (g) over the initial weight of the dry bark (g) [20].
Yield (%) = ((Dry weight of the extract/Dry weight of the initial sample) × 100)
The total polyphenol content and Stiasny number of the extracts were determined as detailed in Section 2.2.1 and Section 2.2.3, respectively. The extracts were characterized according to molecular weight distribution and FTIR.
2.3.1. Average Molecular Weight Distribution by GPC
Molecular weight distribution was recorded by GPC/RID-UV with a Shimadzu Prominence chromatograph (Kyoto, JAPAN) with column oven and UV-Visible detector. Phenogel columns stuffed with a crosslinked polystyrene–divinylbenzene (PSDVB) compound were used [10].
2.3.2. FTIR Analysis
FTIR spectra were recorded with a Bruker Alpha T FTIR (BRUKER, Billerica, MA, USA) spectrometer equipped with diamond total attenuated reflectance (ATR). Spectral range was 4000–400 cm−1. Results were analyzed with OPUS 7.0 software [5,11].
2.4. Extraction on a 50 L (L) Scale
The extractions were carried out in a pilot plant in a 50 (L) stainless steel closed rotating reactor. 80% methanol was used; extraction time was 2 h; bark/solvent ratio was ½ (mass/volume). Extraction was carried out twice at 65 °C. The methodology detailed in 2.2.2 was performed on the resulting extracts. They were then dried in a spray dryer (BH, Germany) to be used and stored as powder. As was in the laboratory scale tests, total polyphenol content and the Stiasny number were quantified and FTIR spectrometry was performed.
2.5. Adhesive Formulation
Two formaldehyde-free adhesive formulations were prepared:
Formulation 1: 40% of dry tannin extract + 7% of hexamine (Hex), with pH of 8 and 10 [11].
Formulation 2: 40% of dry tannin extract + 12% of glyoxal (Gly), with pH of 4 and 6 [11,36].
2.5.1. Rheological Behavior and Useful Life
The useful life of the pine extracts and the adhesive formulations were evaluated. Analysis were carried out under the assumption that their rheological behavior obeyed a power law: σ = Kγn (1)
The consistency coefficient K (Pa sn) is the measured shear strength (σ) at an applied shear rate (γ) of 1.0 s−1. The dimensionless exponent n is the flow behavior index and indicates the correspondence with the Newtonian flow [11].
Rheological behavior was measured at 25 °C with a Fungilab Smart L (España) viscometer set on different times (60 and 300 min). The useful life of a 40% aqueous solution (by weight) prepared with different hardeners (hexamine and glyoxal) was also evaluated at pHs of 6, 8 and 10 [11].
2.5.2. Differential Scanning Calorimetry (DSC)
DSC analysis was performed by a NETZSCH DSC (204 F1 Phoenix, Selb, Germany). 7 ± 3 mg samples were pressure sealed. The chosen temperature range was between 25 °C and 250 °C with a heat rate of 10 °C min−1, using indium calibration standards (purity > 99.999%) [5,11].
2.5.3. Automated Bonding Evaluation System (ABES)
ABES (Adhesive Evaluation Systems, Corvallis, OR, USA) was used to measure maximum resistance of the wood-adhesive system under defined conditions of temperature and time. From 0.7 mm Fagus sylvatica L. sheets conditioned for a week in a chamber at 20 °C and 53% relative humidity, 117 mm × 20 mm strips were cut. Using 2 mg of adhesive or plain water, the strips were glued along the direction of the fiber with a 100 mm2 overlap. Measurements were taken at 4 temperatures: 90, 120, 140 and 160 °C, and 6 times: 10, 15, 30, 60, 90 and 120 s. Measurements were taken twice per data point, and the results were averaged [5,11,22].
2.6. Energy Content and Ash Composition of Pinus elliottii Engelm Bark
The higher heating value (HHV) of pine bark before and after extraction was measured in accordance with the UNE-EN 14918 standard [37] using an XRY-1A calorimeter bomb (China). Ash content was determined in accordance with the ASTM E1755-01 standard [38]. 1 gr of material previously dried at 105 °C and milled was placed in a melting pot and weighted on an analytic scale. The dried sample was placed in the muffle, and the temperature was raised to 250 °C at 5 °C/min to prevent flames. The temperature was then raised to 575 °C and maintained for 2 h. The melting pot was then placed in a desiccator and weighed once it cooled down. This process was repeated every 30 min until weight was constant within 0.2 mg. Ash percentage was calculated with the following equation in accordance with the ASTM D1102-84 standard [39]: % ash = (Mc/Ms) × 100. Where Mc = ash sample (gr) and Ms = dry sample (gr).
Percentage of volatile matter was determined in accordance with the ASTM E872-82 standard [40]. After weighing an empty melting pot, the precision scale was tared and 1 gr of the sample, previously dried at 105 °C, was added to the melting pot to be weighed again. The muffle was lit up, and once it reached 950 °C, the melting pot with the dry material was placed for exactly 7 min, and then left to cool down and weighed. Weight loss percentage was calculated with the following equation:
% mass loss = (initial bark weight − melting pot weight)/(initial bark weight − weight) ∗ 100.
2.7. Statistical Analysis
The results obtained with the Taguchi experimental design were analyzed with ANOVA, set at a significance level of p < 0.05, and the LSD Fissher test was applied. Tests at a 1 L scale were carried out thrice, and the mean difference was analyzed by the Tukey test. Pilot plant tests were carried out twice. Results were expressed as the mean values with their respective standard deviations.
3. Results and Discussion
3.1. Extraction on a Laboratory Scale
Optimization of Conditions
Table 2 shows results through the response variables (condensed tannin content, Stiasny number and total yield) in accordance with the Taguchi experimental design, which evaluated temperature, time and solvent at two levels each.

Table 2.
Average yield of condensed tannin content (mg cyanidin/g dry bark), Stiasny number and total yield (%) of the extractions (eight combinations of time, solvent and temperature).
ANOVA testing proved that, with a value of p < 0.05, temperature is the most consequential factor in extraction. The literature also points at temperature as a very important variable when choosing tannin extraction conditions [19,21,28].
Only extracts with a Stiasny number of 65% or above are considered for adhesive formulations for the timber industry [41]. Hence, extraction conditions of tests 4, 5, 7 and 8 were not considered for this study.
Extracting with methanol as the solvent at 65 °C for 2 h produced a significantly better result (for p < 0.05) than all other conditions; the Stiasny number was 91% and total yield 19.51%. This indicates a strong reactivity of the tannins extracted under those conditions to formaldehyde. While keeping the solvent and temperature the same, a longer extraction time (4 h) improved the extraction yield (20.81%), but the resulting Stiasny number was lower (88%). These results were higher than those obtained by Yazaki and Collins [41] who used P. elliottii Engelm (14.2%) extracted with water at 100 °C and a pH of 8.3. The same authors reported a Stiasny number of 54.4%.
At a higher temperature and longer time (75 °C for 4 h), the Stiasny number decreased significantly regardless of the solvent (methanol or ethanol). This could be explained by the condensation–degradation reactions of the condensed tannins, which affect their reactivity to formaldehyde. After 2 h of extraction, tannins start to react by autocondensation.
Extraction with methanol rather than ethanol produces phenolic compounds (anthocyanins, phenolic acids, catechins, flavanones, flavanols and procyanidins) [28] which contribute to a higher Stiasny number.
The lowest response variable values were obtained by extracting with ethanol at 65 °C for 2 and 4 h.
Extracting with ethanol at a higher temperature (75 °C) for 4 h produces a significant increase in condensed tannin yield (mg cyanidin/g dry bark). In contrast, Naima et al. [28] report a condensed tannin yield of 6.62 mg cyanidin/g dry bark from Acacia mollissima when extracting with methanol at 60 °C for 2 h; when extracting at 80 °C, the yield went down to 9.41 mg cyanidin/g dry bark. Regarding the extraction yield, the best results were obtained with methanol at 65 °C and 2 h. Methanol at 75 °C did not produce significantly better results than ethanol at the same temperature.
The statistical analysis shows that the interactions between variables (solvent, temperature and time) are negligible and indicates that temperature is the main factor that affects condensed tannin content, accounting for significant differences between 65 °C and 75 °C.
3.2. Extraction on a 1 L (L) Scale
From the laboratory scale tests, it was determined that 80% methanol is the better solvent and that 2 h of extraction produces the best results; these conditions were applied at a 1 L scale. Since statistically, temperature turned out to be the most important factor—it was tested at 3 levels: 55 °C, 65 °C and 75 °C. The total extraction yield, Stiasny number and total polyphenols were evaluated, and the FTIR spectra at the three temperatures were compared. The results are shown in Table 3.

Table 3.
Average extraction yield, Stiasny number and total polyphenols of P. elliottii Engelm for the different extraction conditions.
Extraction yield did not vary significantly with temperature differences (for p < 0.05). The values are similar to those in the literature for other species. [41] reported the following values: Pinus sylvestris (10.5%), Picea abies (13.8%), P. ellliottii (14.2%), Pinus pinaster (24.9%), and Pinus radiata (32.9%); [21] reported a 29.9% extraction yield for Pinus halepensis.
Compared to other authors, the Stiasny number of the extracts obtained at 65 °C was close to or slightly above those reported in the literature: 82.5% by Saad et al. [21] for P. halepensis (extracted at 70 °C); 89.9% to 96.3% for Acacia mollissima by [30]; 91 % for Acacia mangium by [24]; and 48.97% and 53.98% for Pinus pinaster by [20].
Polyphenol content also varied; extraction at 65 °C produced the best result (55.67 mg GAE/g dry bark). Compared to other potentially useful pine species for adhesive formulations, this result lies between what is reported for Pinus pinaster (54.23 mg GAE/g dry bark) [20] and P. halepensis (64.5 mg GAE/g dry bark) [21].
The Stiasny number obtained at 65 °C indicates better reactivity to formaldehyde, coinciding with a higher polyphenol content. As this extract proved to be the best suited for adhesive formulations, its extraction conditions were used in the 50 L tests.
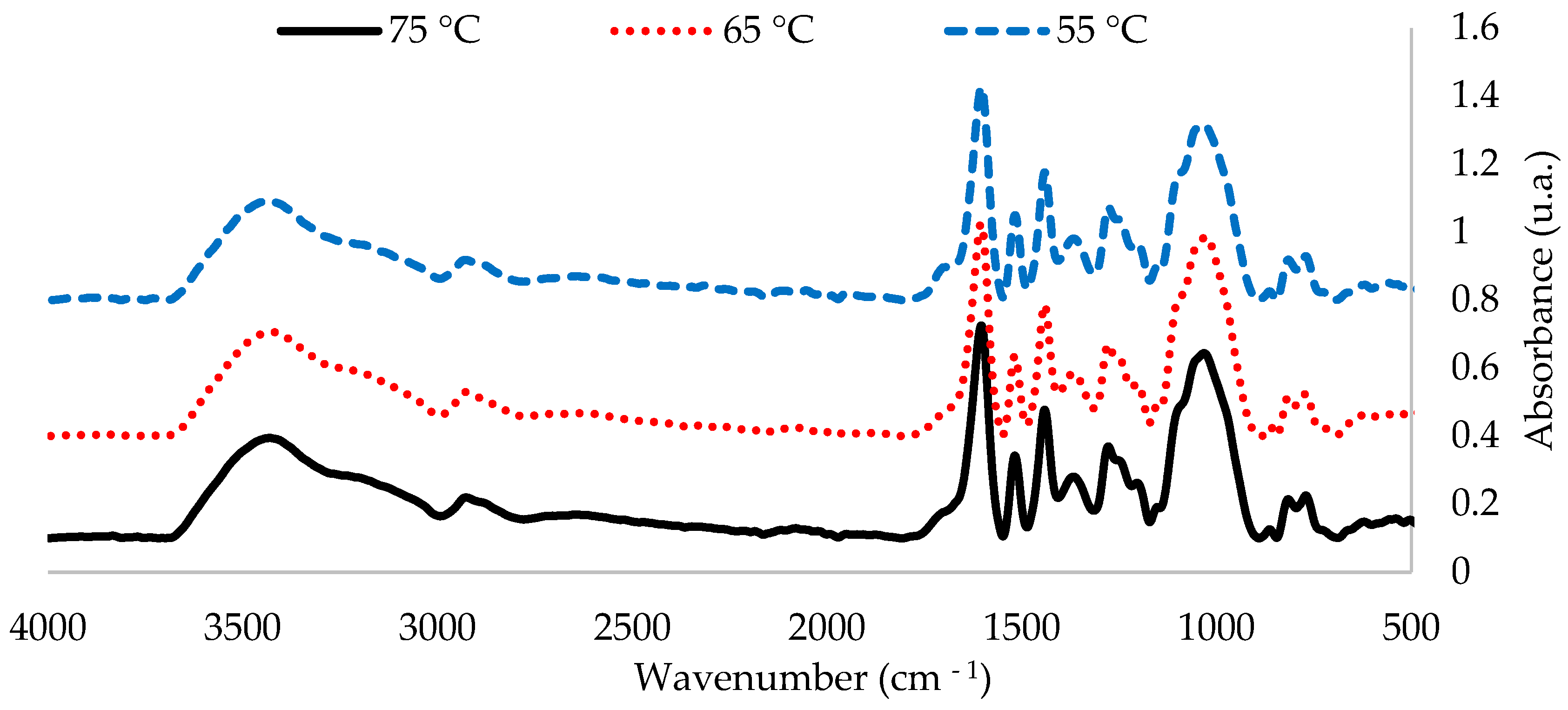
Figure 1 shows FTIR-ATR spectra of the extracts between 4000 and 500 cm−1 and Figure 2 shows the fingerprint region, obtained at the three tested temperatures. In order to better visualize the differences between spectra, the intensity values of the bands were normalized considering the vibration band of the polyphenol aromatic ring at 1600 cm−1 at a pH of 4, between the 500–4000 cm−1 range. The three spectra of the temperatures were analogous.
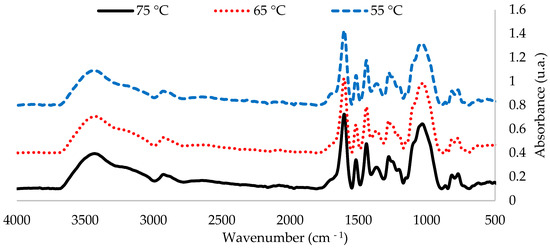
Figure 1.
FTIR spectra of the 1 L extractions at three tested temperatures (55 °C, 65 °C and 75 °C) for P. elliottii Engelm bark (4000–500 cm−1).
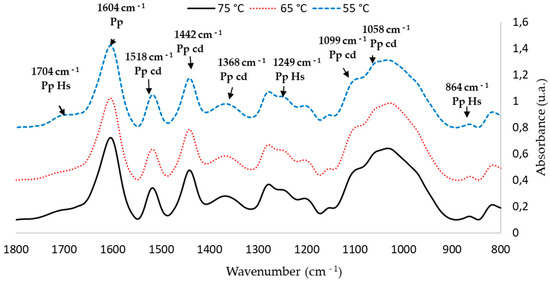
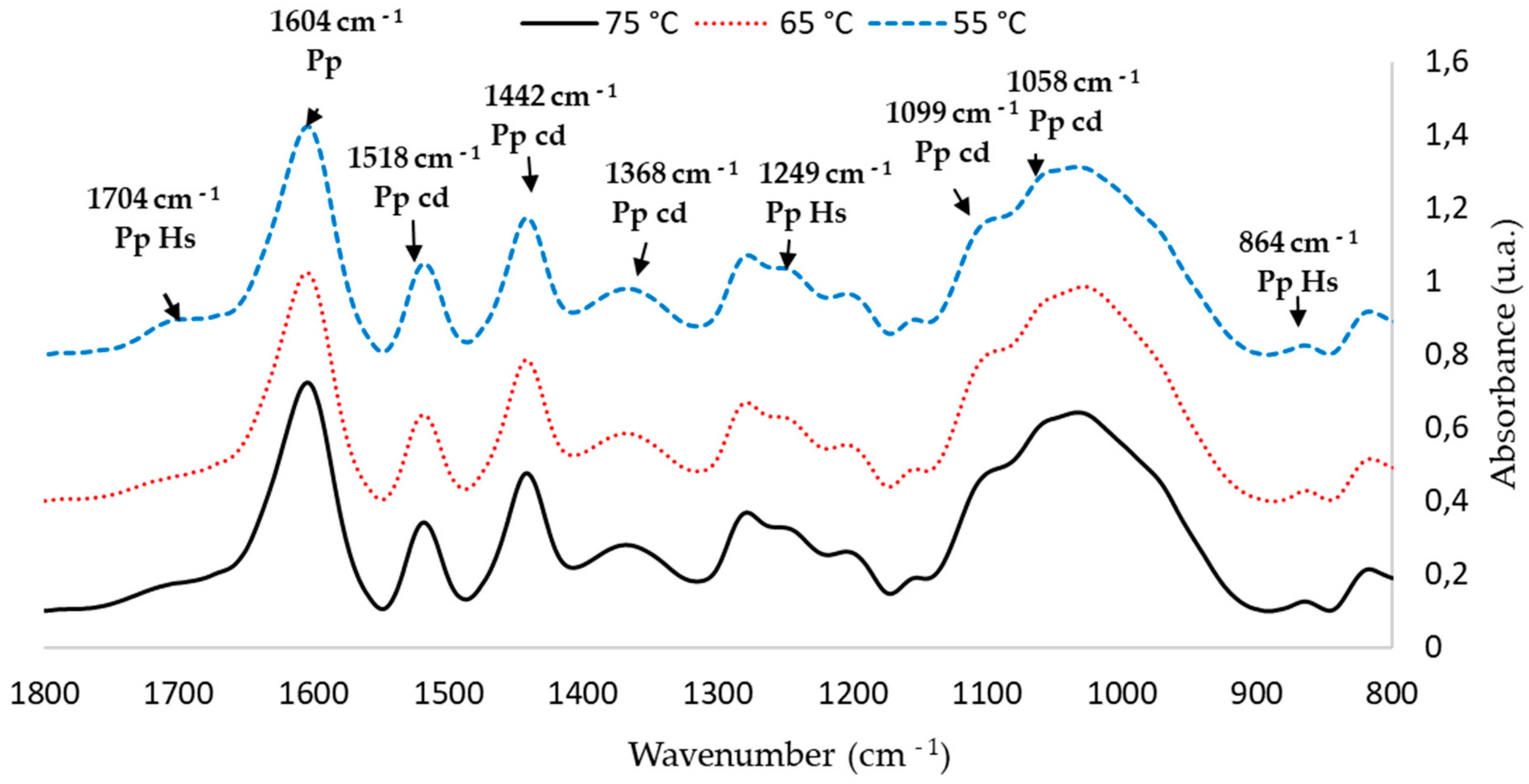
Figure 2.
FTIR spectra with main peaks of the 1 L extractions at three temperatures (55 °C, 65 °C and 75 °C) for P. elliotti Engelm bark (1800–800 cm−1).
Table 4 shows the characterization of the main peaks of the P. elliottii Engelm bark extract; intensity values were normalized with the vibration of the aromatic polyphenol ring at 1600 cm−1. Band assignment was performed based on previous studies [11,42].

Table 4.
FTIR peak assignment of P. elliottii Engelm bark extract.
FTIR-ATR spectra of the extracts show a band pattern characteristic of condensed polyphenol presence. Regardless of extraction conditions, all spectra of the P. elliottii Engelm bark extracts show a band distribution pattern similar to that produced by maritime pine bark extract [5]. This pattern indicates the presence of phloroglucinol structures as part of condensed tannins of the extracts with bands at 1604, 1518, 1442,1368, 1249, 1099, 1058 and 865 cm−1.
FTIR-ATR also indicated the presence of hydrolysable tannins, which are composed mainly of gallic acid and ellagic acid linked to sugars. Their most relevant structural characteristic is the presence of carboxylic acids, which produce vibration bands at 710–1720 cm−1 and at 1240–1250 cm−1. When analyzing these areas, it can be observed that extracting at a lower temperature led to an increased production of such tannins in the extract, which explains its lower Stiasny number.
In short, from the FTIR analysis, it can be ascertained that extracting at 65 °C produces the best outcome. Spectra show that the bands related to the vibration of groups C-O-C at 1105 cm−1 and C-O at 1058 cm−1, characteristic of condensed tannins, were the most intense in the extract obtained at 65 °C. These optimized conditions (80% methanol, 65 °C and 2 h of extraction) were used in the next stage.
Molecular Weight Distribution of the Extract Obtained at 65 °C
Usually, an extract of low molecular weight leads to an adhesive of low molecular weight, which would be weaker and less resistant to traction [16]. Therefore, the molecular weight of the extracts was determined. However, it must be noted that not only extraction conditions have an effect on molecular weight; other factors such as age of the trees and gathering and storage conditions, among others, must be taken into account [43].
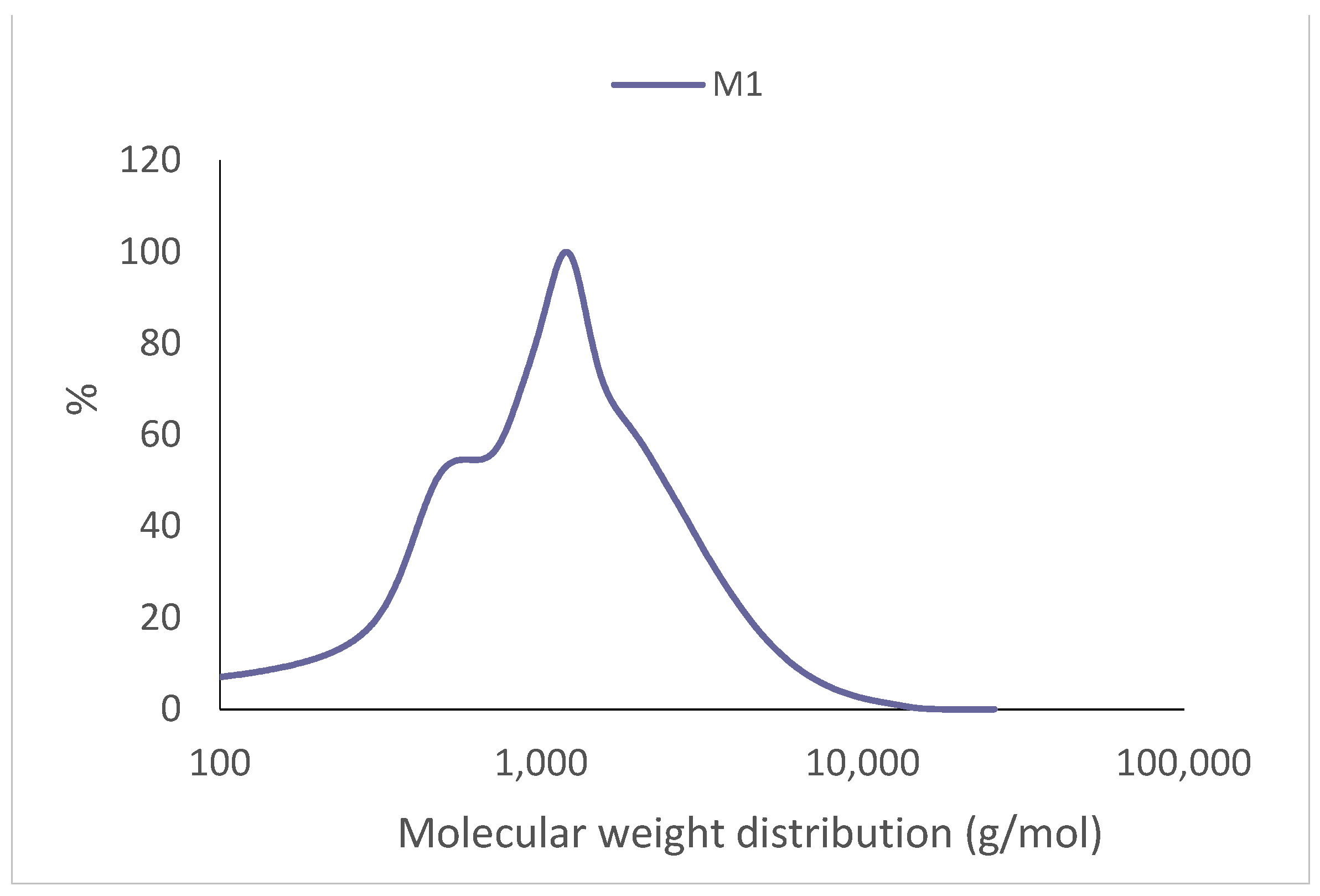
The molecular weight of the extract from the 1 L extraction was Mn = 436 g/mol, while the molecular weight was Mw = 1588 g/mol. Both values are below those reported in the literature for P. radiata [39], which obtained values of Mn = 1200 g/mol and Mw = 380 g/mol extracting with deionized water at 100 °C for 1 h; [29] and values of Mn = 1003 g/mol and Mw = 1689 g/mol extracting with an ethanol/water 1:20 (w/v) solution at 120 °C for 120 min. Figure 3 shows the molecular weight distributions of the P. elliottii. Engelm tannin extracts.
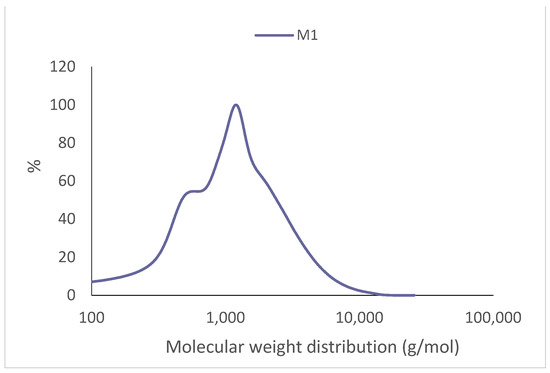
Figure 3.
Molecular weight distribution (g/mol) of the P. elliottii Engelm bark extracts.
The polydispersity index was of D = 3.64, higher than that of Castanea sativa extracted with water 70 °C (D = 2.48) [19] and P. radiata (D = 2.03) [29].
3.3. Extraction at 50 L
Table 5 shows the average extraction yield, total polyphenol content and Stiasny number of the 50 L extraction. The extraction yield was slightly higher than the one obtained from the 1 L extractions, but it is still below what is reported for P. elliottii Engelm (14.2%) [41].

Table 5.
Average extraction yield, total polyphenols and Stiasny number of P. elliottii Engelm bark extracted with 50 L of methanol at 65 °C for 2 h.
The total polyphenol content was slightly below the one obtained with the 1 L extraction: from 55.67 mg GAE/g dry bark to 40.77 mg GAE/g dry bark.
Although the Stiasny number of the 50 L extractives was lower than in 3.2 (72.8%), it was still above 65% and, therefore, still suitable for adhesive formulations [20,24,30,41].
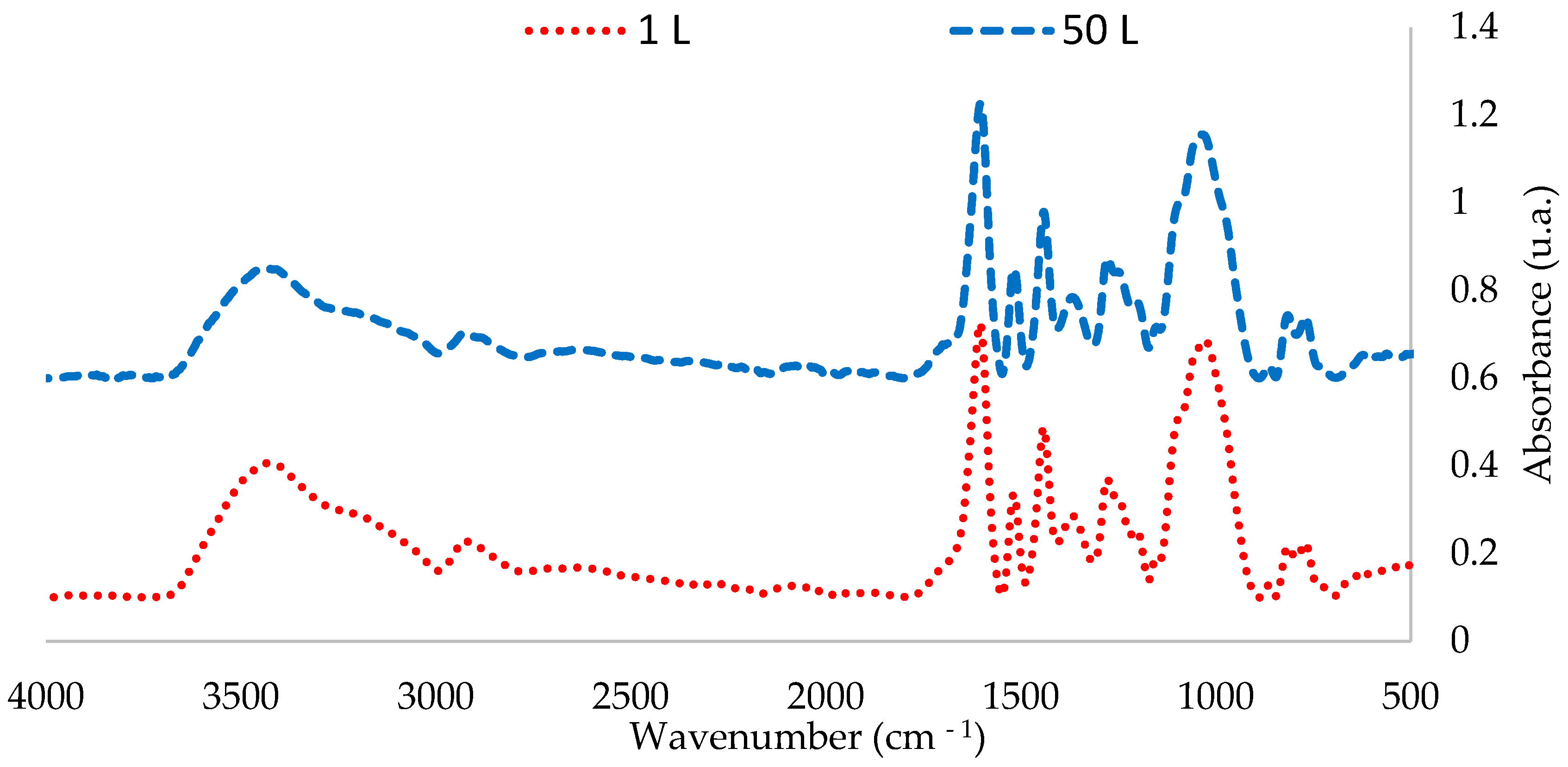
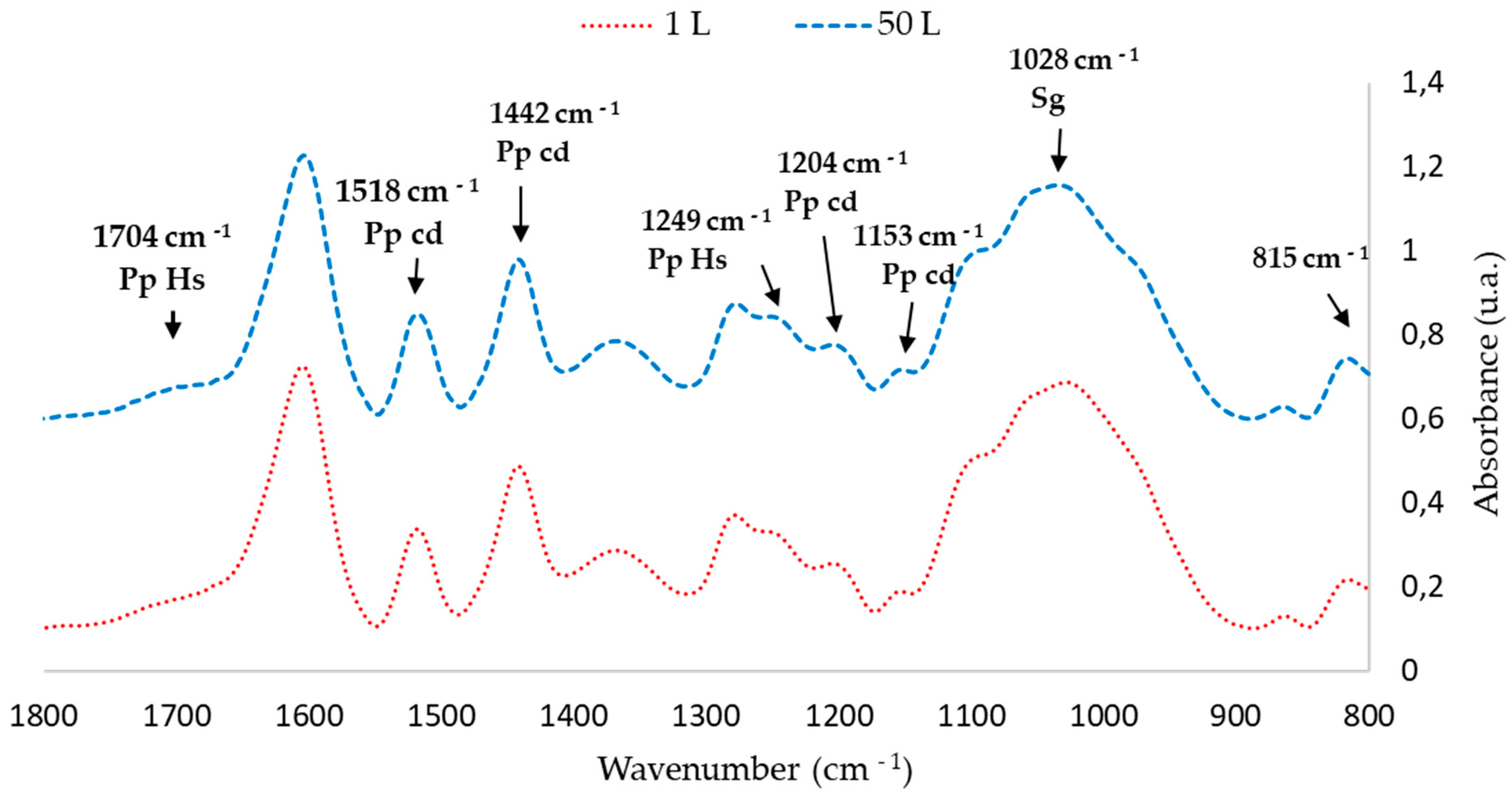
The FTIR-ATR spectra of the extractives obtained in the 1 L and 50 L tests were compared. Figure 4 and Figure 5 show that the peaks corresponding to chemical bonds characteristic of phenolic compounds in the 50 L extraction are similar to those obtained in the 1 L tests at 65 °C
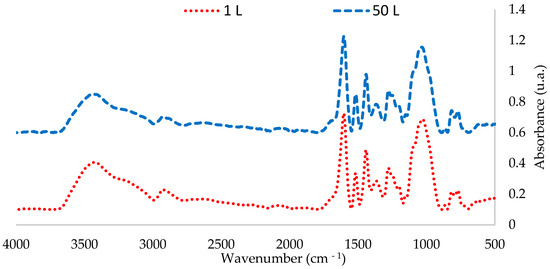
Figure 4.
FTIR spectra of P. elliottii Engelm bark extractives obtained at 1 L and 50 L extractions at 65 °C (4000–500 cm−1).
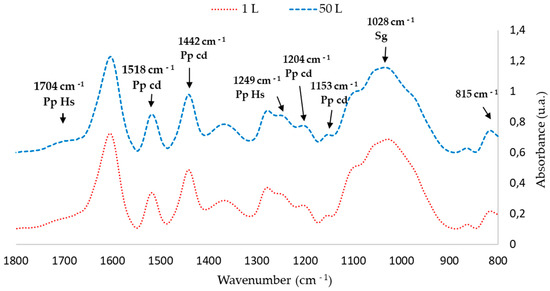
Figure 5.
Fingerprint region of Figure 4 (1800–800 cm−1).
FTIR-ATR analysis shows that scaling the extraction to 50 L does affect the total polyphenol content; however, it affected neither the characteristic pattern of the condensed polyphenols nor the intensity of the peaks (Table 6).

Table 6.
Characterization of the extracts of P. elliottii Engelm by FTIR-ATR and effect of scaling.
Moreover, the Stiasny numbers are above 65%, therefore the extractives are suitable for the adhesive formulations.
3.4. Characterization of Adhesive Formulations
Two formaldehyde-free adhesive formulations were developed using the extractives obtained with the 50 L extractions. Gly and Hex were used as hardeners. The adhesives were analyzed through their rheological behavior, DSC and ABES.
3.4.1. Rheological Behavior
Any adhesive formulation must be viscous as to ensure adherence to wood and must have a useful life long enough to allow for industrial use. Hence, the rheological behavior and useful life of the extract on its own were evaluated at pHs of 4 and 8, with a solid content of 40%. The adhesive formulations developed with said extract plus hardeners (Gly at 12% with a pH of 4 and 6; Hex at 7% with a pH of 8 and 10) were evaluated next.
Table 7 shows that the formulation with 7% Hex at pH = 8 had a slightly lower apparent viscosity, with k = 0 and a pseudoplastic behavior with Newtonian tendency. This differs from what is found in the literature. Santos J. [44] used Castanea sativa extracts at 40% solids, using Hex (5%) at pH = 8 and measuring at 25 °C, and showed that the apparent viscosity went from 4.719 to 19.036 mPa.s from 0 to 120 min, also obtaining pseudoplastic behavior (0.89–0.83 Pa.sn). As reported by Santos et al. [11], with P. radiata extracts, the viscosity also decreases sharply from 893 to 174 mPa.s, but they do not agree that the reported behavior is pseudoplastic (0.57–0.64). When the pH increases to 10, the apparent viscosity increases to 300 from 60 min with a dilatant behavior. The 12% Gly formulation at pH = 4 displays Newtonian behavior with a tendency to dilate with time, a decreasing apparent viscosity, and that k = 0. When the pH is raised to 6, its behavior changes to pseudoplastic (0.91–0.95) while apparent viscosity increases with time. This coincides with what [45] reports for a formulation with Castanea sativa extract (40% solid content) and Gly (10%) measured at 25 °C, which also displayed pseudoplastic behavior (0.86 - 0.92) and a viscosity that increases from 5.039 mPa.s at 0 to 28.034 mPa.s at 300 min.

Table 7.
Rheological behavior of P. elliottii Engelm extract at 40% solid content on its own and with Hex and Gly as hardeners at two pHs and time at 25 °C.
Rheological tests show that the formulation with 12% Gly at pH = 6 kept its pseudoplastic behavior over time. In contrast, the useful life of the formulation with 7% Hex at pH = 10 tends to decrease over time. This variations should be accounted for, so DSC and ABES tests are necessary in order to determine the potential use of the adhesives on an industrial level.
3.4.2. DSC Analysis
The influence of pH and autocondensation reactions on the extract were evaluated. Its chemical curation depending on the hardener (Hex and Gly) was evaluated next.
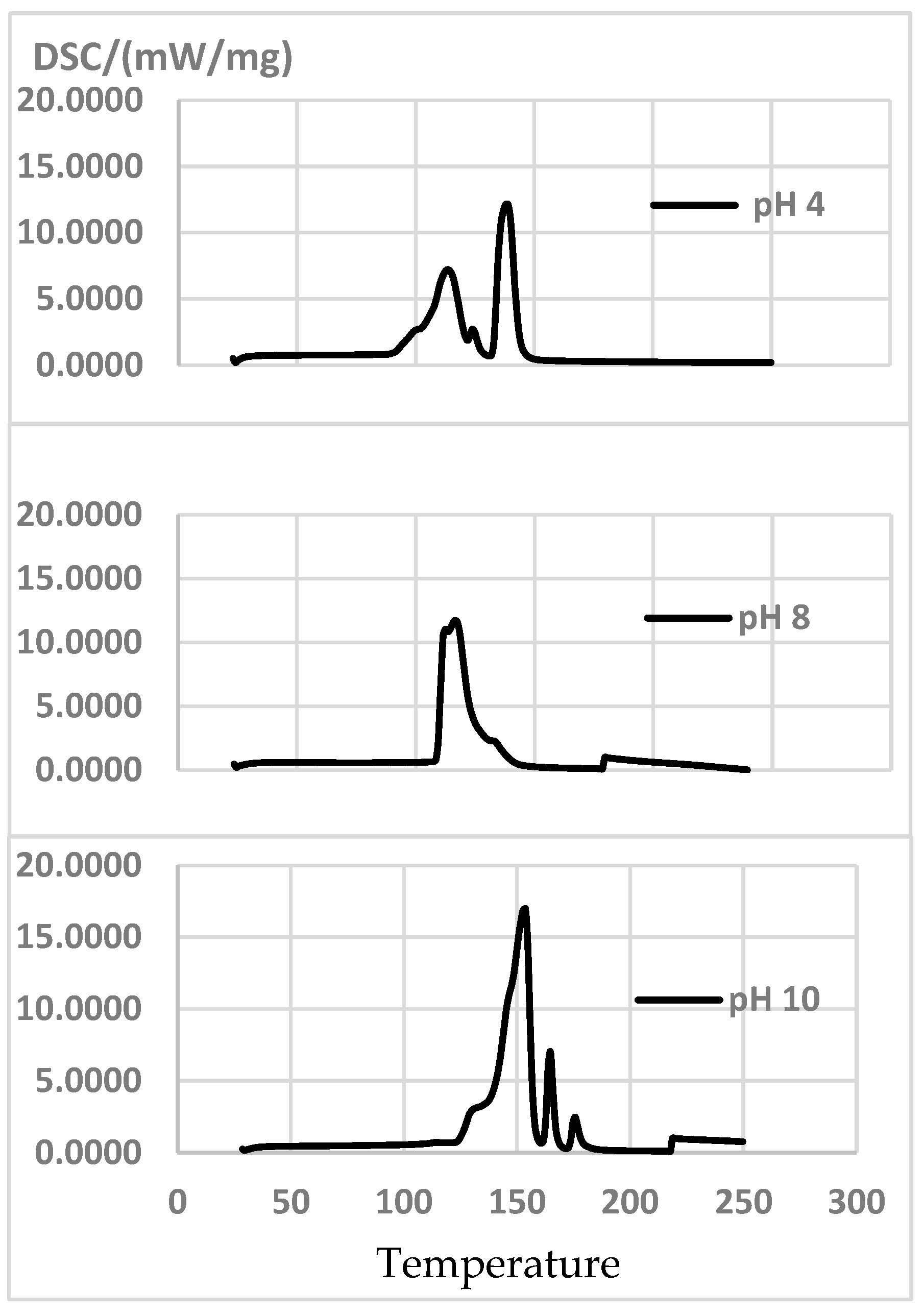
The thermograms for the extract at pH = 4 and pH = 6 peak at first at 110–118 °C, when polyphenols autocondense. This proves autocondensation temperatures increase with a higher pH [11]. If the pH is increased further, higher peaks appear as polyphenols are activated. The highest exothermic peaks are produced at pH = 10 (Figure 6). Table 8 also shows the global curing enthalpy value ∆h (J/G).
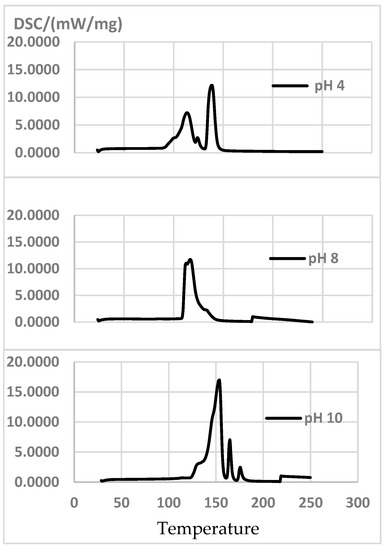
Figure 6.
DSC curves of the extracts at pH = 4, pH = 8 and pH = 10, at a heating rate of 10 °C/min.

Table 8.
Chemical curation of the extracts and the adhesives at different pHs.
With 7% Hex as the hardener, if the pH is raised from 8 to 10, the reaction temperature increases and enthalpy decreases (Figure 6). This result differs with what [11] report for P. radiata extract with 40% solid content and 7% Hex: when the pH is raised from 7 to 8, the reaction temperature decreases (from 158 °C to 139 °C) and enthalpy increases from 1125 to 1225 J/g.
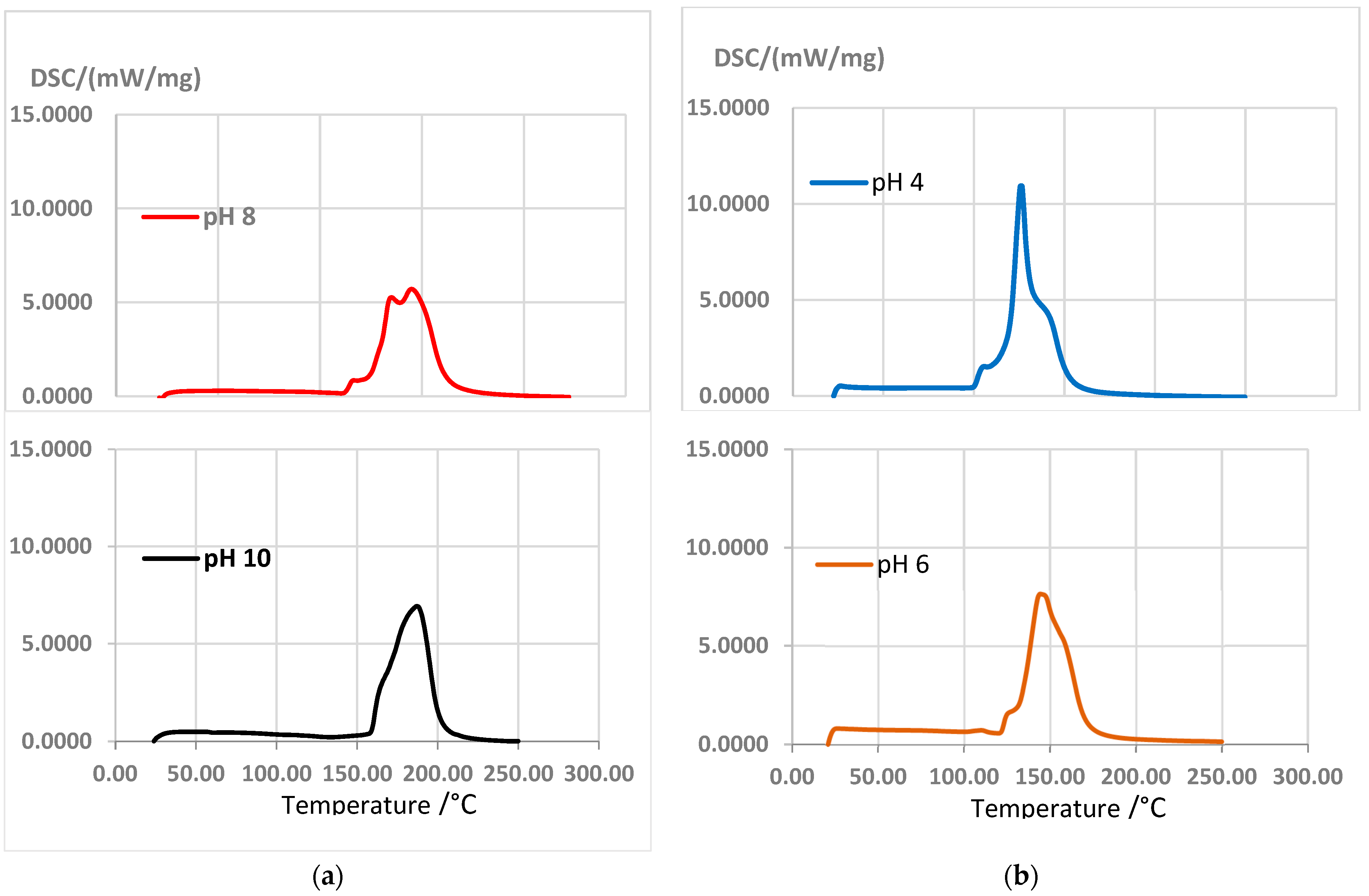
However, curing enthalpy was higher for Gly than for Hex (Figure 7); according to [19], it is due to less heat released by lowering the crosslinking and, depending on the hardener used, the variation in the curing enthalpy could be explained by taking into account that the hardening by polycondensation in adhesives with tannins can be combined with simultaneous hardening by autocondensation. According to [44], commercial resin PF (phenol–formaldehyde) produces two exothermic peaks at 114 °C and 146 °C. In comparison, the adhesive formulations with tannins and Gly 12% at pH 6 presented an exothermic peak at 144 °C; for the adhesive formulations with Hex, peaks of 170.1 °C at pH 8 and 172.3 °C at pH 10 were recorded.
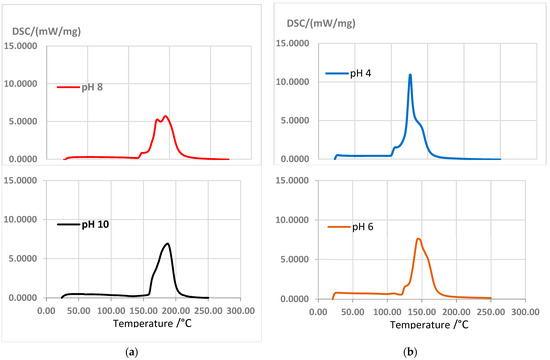
Figure 7.
DSC curves: (a) extracts + Gly 12% at pH 4 and pH 6; (b) extracts + Hex 7% at pH 8 and pH 10, at a heating rate of 10 °C/min.
The adhesives with Hex at pH 10 and pH 8 presented better behaviors than the adhesives with Gly. The reaction temperatures that produced the best outcomes were 150 °C and 160 °C, respectively.
Hex seems to require higher temperatures than Gly to reach a complete chemical cure. According to Vazquez et al. [19], this indicates a lower reaction speed at room temperature and, therefore, a longer useful life.
3.4.3. Adhesive Testing (ABES)
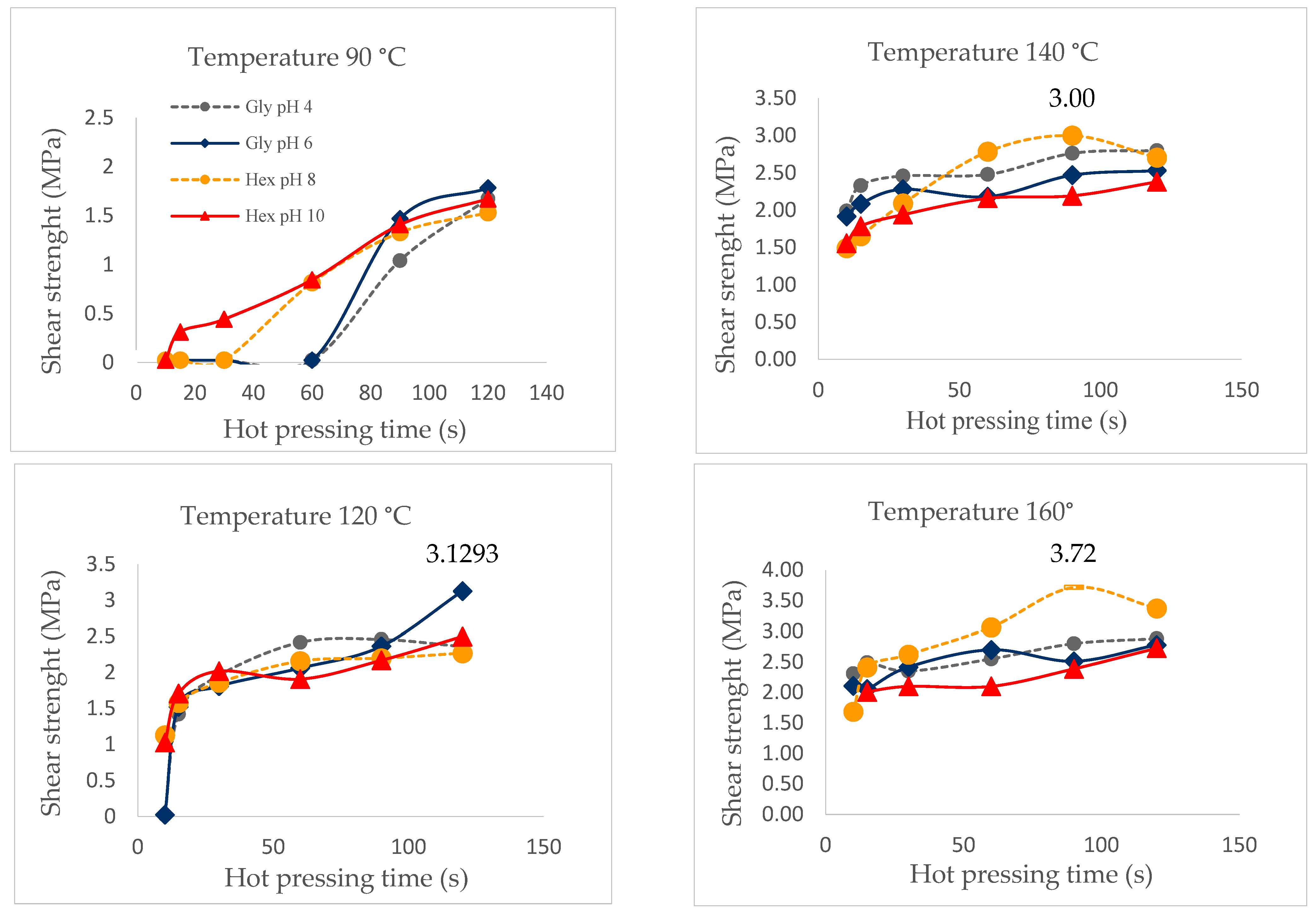
The shear strength obtained during the test depends on the wood–adhesive or wood–resin bonds formed during the curing process. The test was carried out under controlled conditions of pressing time from 15 to 120 s and pressing temperatures of 90 °C, 120 °C, 140 °C and 160 °C using both resin (different pHs) and adhesives formulations (different crosslinkers).
Mechanical curation was evaluated through bonding strength under controlled conditions of hot pressing for pressing times between 15 and 120 s and temperatures of 90 °C, 120 °C, 140 °C and 160 °C; for solutions of the extracts at different pHs (autocondensation reactions); and then for the adhesive formulations with the two hardeners at different pHs.
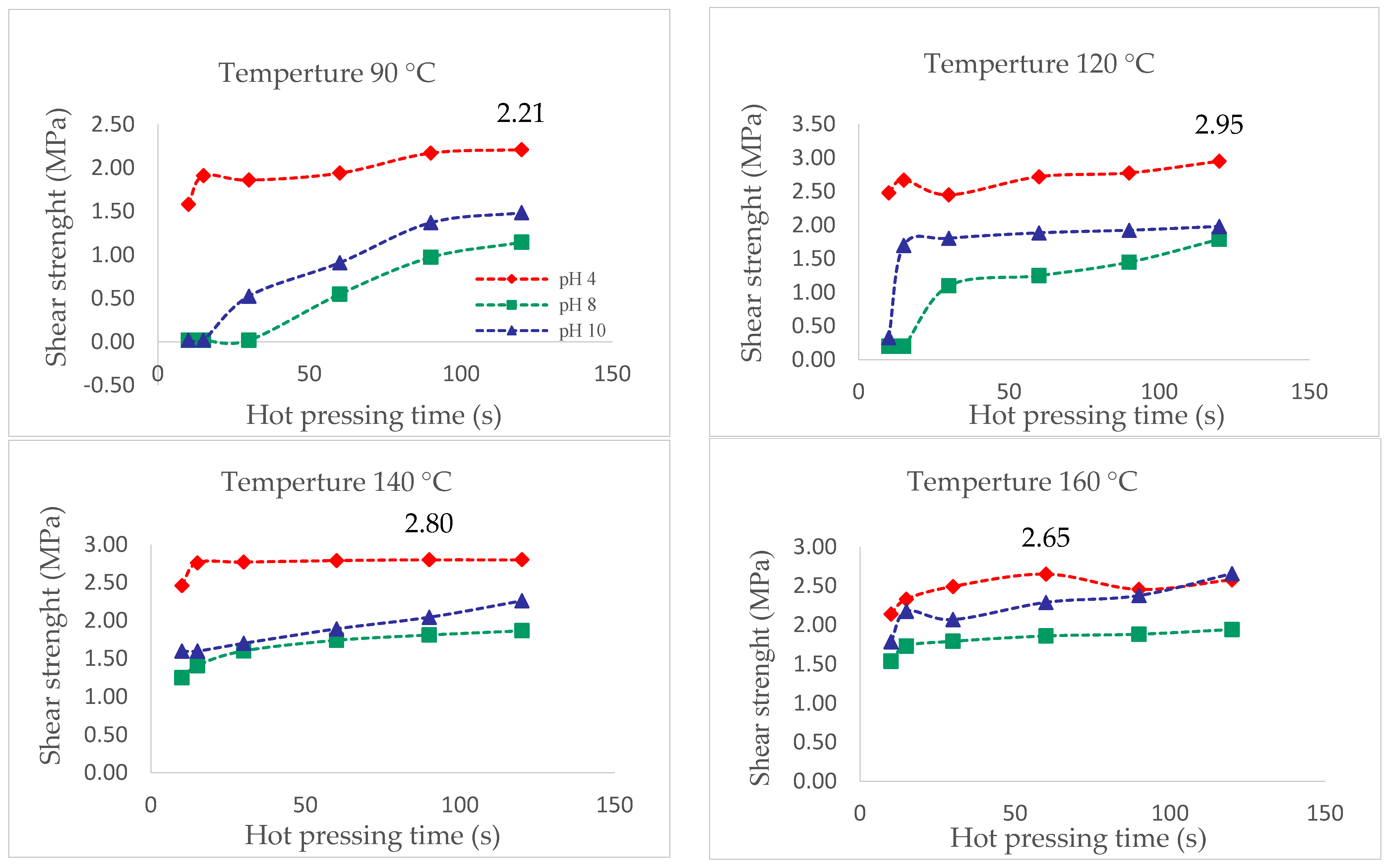
Figure 8 shows that the extract of pH = 4 presented the highest resistance to cutting for the different pressing temperatures. Above 120 °C and pH = 4, the interaction between the extract and the wood improves, as its shear strength reaches values close to 3 MPa. The values obtained are higher than those reported by [5] for P. pinaster bark aqueous extracts, which were closer to 2 MPa at a pressing temperature of 120 °C.
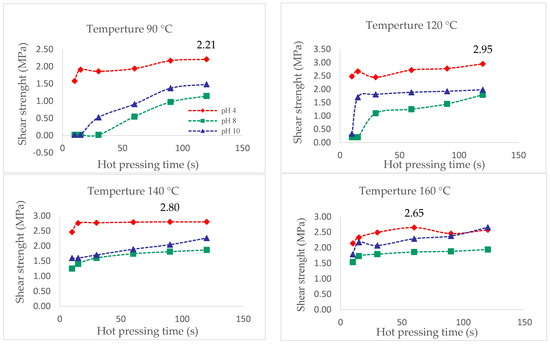
Figure 8.
Shear strength evaluation by hot pressing time of the extracts at pH 4, 8, and 10 and temperatures of 90 °C, 120 °C, 140 °C and 160 °C, measured with ABES.
Figure 9 shows that at 120 °C, there was a sharp increase in the resistance to cutting for the formulations with glyoxal at pH = 6 (3.19 MPa).
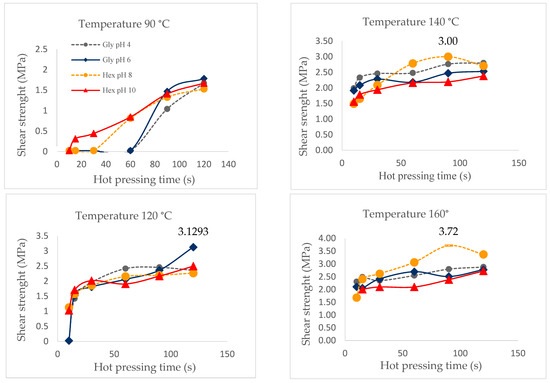
Figure 9.
Shear strength by hot pressing time of the adhesives at 90 °C, 120 °C, 140 °C and 160 °C, measured with ABES.
However, all adhesive formulations had similar behavior, with the exception of the one with hexamine at pH = 10. The best shear strength (3.72 MPa) was reached at 160 °C with hexamine at pH = 8 compared to the rest of the combinations registering differences with the values obtained in the chemical curing. According to Santos et al. [11], the chemical curation process would be dominated by the least reactive components of the extract.
3.5. Energy Content of Ash Content of the Bark
Finally, it was determined whether the P. elliottii Engelm bark could still be used for energy production after the extractions. The higher heating value (HHV) was measured under optimal conditions with and without extraction to compare the results.
Table 9 shows upper calorific power and ash percentage of bark samples before and after tannin extraction.

Table 9.
Higher heating value, ash and volatiles percentage.
Table 9 shows that extracted samples have a relatively lower heating value (18.7 MJ/kg), in line with other subproducts generated in bosque, forestry and pulp industries, such as forests planted as wood chips (19.1 MJ/kg), bark (17.1 MJ/kg), and branches [46].
Although the energy capacity of the bark decreased by 13% with the extraction of tannins, ash percentage decreased by 22.58%, which is favorable for combustion and gasification of biomass processes, since high ash percentage negatively affects equipment and yield. In a study by [14] with P. taeda L. bark from Uruguayan plantations, calorific power did not decrease significantly before and after extraction (going from 21.49 kJ/g b.s. to 21.30 kJ/g b.s.), while ash percentage decreased by 13%.
Extracting the tannins affects the properties of the bark, lowering its upper calorific power but also beneficially reducing the ash percentage. Under these conditions, extraction makes the bark better for energy production.
4. Conclusions
Extraction conditions for P. elliottii Engelm bark were optimized through yield and Stiasny number in a laboratory scale test, and could be scaled up to a pilot plant. The Stiasny number was the most relevant parameter to evaluate the use of the extract in adhesive formulations.
The best results were obtained with methanol at 65 °C for 2 h.
Quantification of total polyphenols obtained in the 50 L extractions were lower than those obtained at 1 L (40.77 mg GAE/g dry bark and 55.67 mg GAE/g dry bark, respectively), which indicates that scaling up affects the phenolic compounds.
Results show that it is possible to obtain adhesives formulations free of formaldehyde from P. elliottii Engelm bark combined with 12% Gly at a pH of 6 and a pressing temperature of 120 °C, and with 7% Hex at a pH of 8 and 10 and pressing temperatures of 140 °C and 160 °C.
Since tannin extraction does not affect the calorific power of the bark, it can still be used in biorefinery for energy generation.
Author Contributions
Conceptualization, P.S., J.S., C.F., L.H.-M. and C.M.I.; methodology, C.M.I., J.S., P.S.; formal analysis, J.S., C.M.I. and P.S.; investigation, P.S., C.M.I., J.S. and C.F.; data curation, P.S.; writing—original draft preparation, P.S., J.S., C.M.I. and L.H.-M.; writing—review and editing, P.S., J.S., C.M.I. and C.F.; visualization, P.S., C.M.I., C.F. and A.M.; supervision, C.M.I.; project administration, C.M.I. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Comisión Sectorial de Investigación Científica (CSIC), and the Universidad de la República, group ID2014 and Unidad de Desarrollo Tecnológico (UDT), the Universidad de Concepcion, proyect Basal PFB-27.
Data Availability Statement
Data is contained within the article.
Acknowledgments
The authors acknowledge the support of ANID BASAL FB210015 CENAMAD.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Thorenz, A.; Wietschel, L.; Stindt, D.; Tuma, A. Assessment of agroforestry residue potentials for the bioeconomy in the European Union. J. Clean. Prod. 2018, 176, 348–359. [Google Scholar] [CrossRef] [PubMed]
- Tahir, P.M.; Halip, J.A.; Lee, S.H. Tannin-based bioresin as adhesives. In Lignocellulose for Future Bioeconomy; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–133. [Google Scholar]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Dieste, A.; Cabrera, M.N.; Clavijo, L.; Cassella, N. La Bioeconomía Forestal en Uruguay Desde Una Perspectiva Tecnológica; Universidad de la República: Montevideo, Uruguay, 2019. [Google Scholar]
- Santos, J.; Pereira, J.; Ferreira, N.; Paiva, N.; Ferra, J.; Magalhães, F.D.; Martins, J.M.; Dulyanska, Y.; Carvalho, L.H. Valorisation of non-timber by-products from maritime pine (Pinus pinaster, Ait) for particleboard production. Ind. Crops Prod. 2021, 168, 113581. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Zhang, M.; Li, X.; Policella, M.; Lei, T.; Gupta, A.K. Co-pyrolysis of waste tire and pine bark for syngas and char production. Fuel 2020, 274, 117878. [Google Scholar] [CrossRef]
- Arias, A.; Entrena-Barbero, E.; Feijoo, G.; Moreira, M.T. Sustainable non-isocyanate polyurethanes bio-adhesives for engineered wood panels are revealed as promising candidates to move from formaldehyde-based alternatives. J. Environ. Chem. Eng. 2022, 10, 107053. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Lee, J. Energy recovery from wood pellets and waste mulching film with minimization of harmful byproducts via thermochemical conversion with CO2 agent. Chem. Eng. J. 2022, 427, 131459. [Google Scholar] [CrossRef]
- Abilleira, F.; Varela, P.; Cancela, Á.; Álvarez, X.; Sánchez, Á.; Valero, E. Tannins extraction from Pinus pinaster and Acacia dealbata bark with applications in the industry. Ind. Crops Prod. 2021, 164, 113394. [Google Scholar] [CrossRef]
- García, D.E.; Fuentealba, C.A.; Salazar, J.P.; Pérez, M.A.; Escobar, D.; Pizzi, A. Mild hydroxypropylation of polyflavonoids obtained under pilot-plant scale. Ind. Crops Prod. 2016, 87, 350–362. [Google Scholar] [CrossRef]
- Santos, J.; Delgado, N.; Fuentes, J.; Fuentealba, C.; Vega-Lara, J.; García, D.E. Exterior grade plywood adhesives based on pine bark polyphenols and hexamine. Ind. Crops Prod. 2018, 122, 340–348. [Google Scholar] [CrossRef]
- Besserer, A.; Troilo, S.; Girods, P.; Rogaume, Y.; Brosse, N. Cascading recycling of wood waste: A review. Polymers 2021, 13, 1752. [Google Scholar] [CrossRef]
- Ministry of Livestock, Agriculture and Fisheries, General Forestry Directorate. “Forest Cartography Results 2021”. Available online: https://www.gub.uy/ministerio-ganaderia-agricultura-pesca/comunicacion/publicaciones/cartografia-nacional-forestal-2021 (accessed on 4 April 2022).
- Xavier, L.; Barrenengoa, M.; Dieste, A.; Amilivia, A.; Palombo, V.; Sabag, M.; Zecchi, B. Valorization of Pinus taeda bark: Source of phenolic compounds, tannins and fuel: Characterization, extraction conditions and kinetic modelling. Eur. J. Wood Wood Prod. 2021, 79, 1067–1085. [Google Scholar] [CrossRef]
- da Silva Araujo, E.; Lorenço, M.S.; Zidanes, U.L.; Sousa, T.B.; da Silva Mota, G.; de Oliveira Reis, V.D.N.; Gomes da Silva, M.; Mori, F.A. Quantification of the bark Myrcia eximia DC tannins from the Amazon rainforest and its application in the formulation of natural adhesives for wood. J. Clean. Prod. 2021, 280, 124324. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. [Google Scholar]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crops Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Boussetta, A.; Ablouh, E.H.; Benhamou, A.A.; Taourirte, M.; Moubarik, A. Valorization of Moroccan brown seaweeds: Elaboration of formaldehyde-free particleboards based on sodium alginate–corn-starch-Mimosa tannin wood adhesives. Int. J. Adhes. Adhes. 2021, 108, 102894. [Google Scholar] [CrossRef]
- Vázquez, G.; González-Alvarez, J.; Santos, J.; Freire, M.S.; Antorrena, G. Evaluation of potential applications for chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind. Crops Prod. 2009, 29, 364–370. [Google Scholar] [CrossRef]
- Chupin, L.; Motillon, C.; Charrier-El Bouhtoury, F.; Pizzi, A.; Charrier, B. Characterisation of maritime pine (Pinus pinaster) bark tannins extracted under different conditions by spectroscopic methods, FTIR and HPLC. Ind. Crops Prod. 2013, 49, 897–903. [Google Scholar] [CrossRef]
- Saad, H.; Khoukh, A.; Ayed, N.; Charrier, B.; Charrier-El Bouhtoury, F. Characterization of Tunisian Aleppo pine tannins for a potential use in wood adhesive formulation. Ind. Crops Prod. 2014, 61, 517–525. [Google Scholar] [CrossRef]
- Costa, N.; Pereira, J.; Martins, J.; Ferra, J.; Cruz, P.; Magalhães, F.; Mendes, A.; Carvalho, L. Alternative to latent catalysts for curing UF resins used in the production of low formaldehyde emission wood-based panels. Int. J. Adhes. Adhes. 2012, 33, 56–60. [Google Scholar] [CrossRef]
- Zidanes, U.L.; Dias, M.C.; Lorenço, M.S.; da Silva Araujo, E.; Silva, M.J.F.; Sousa, T.B.; Rocha, S.; Ugucioni, J.C.; Denzin, G.H.; Bianchi, M.L.; et al. Preparation and characterization of tannin-based adhesives reinforced with cellulose nanofibrils for wood bonding. Holzforschung 2021, 75, 159–167. [Google Scholar] [CrossRef]
- Hoong, Y.B.; Paridah, M.T.; Loh, Y.F.; Jalaluddin, H.; Chuah, L.A. A new source of natural adhesive: Acacia mangium bark extracts co-polymerized with phenol-formaldehyde (PF) for bonding Mempisang (Annonaceae spp.) veneers. Int. J. Adhes. Adhes. 2011, 31, 164–167. [Google Scholar] [CrossRef]
- Santiago-Medina, F.; Foyer, G.; Pizzi, A.; Caillol, S.; Delmotte, L. Lignin-derived non-toxic aldehydes for ecofriendly tannin adhesives for wood panels. Int. J. Adhes. Adhes. 2016, 70, 239–248. [Google Scholar] [CrossRef]
- Ghahri, S.; Chen, X.; Pizzi, A.; Hajihassani, R.; Papadopoulos, A.N. Natural tannins as new cross-linking materials for soy-based adhesives. Polymers 2021, 13, 595. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Bordado, J.M.; Marques, A.C.; Galhano dos Santos, R. Non-formaldehyde, bio-based adhesives for use in wood-based panel manufacturing industry—A review. Polymers 2021, 13, 4086. [Google Scholar] [CrossRef] [PubMed]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier–El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crops Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Bocalandro, C.; Sanhueza, V.; Gómez-Caravaca, A.M.; González-Álvarez, J.; Fernández, K.; Roeckel, M.; Rodríguez-Estrada, M.T. Comparison of the composition of Pinus radiata bark extracts obtained at bench-and pilot-scales. Ind. Crops Prod. 2012, 38, 21–26. [Google Scholar] [CrossRef]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier-El Bouhtoury, F. Green extraction process of tannins obtained from Moroccan Acacia mollissima barks by microwave: Modeling and optimization of the process using the response surface methodology RSM. Arab. J. Chem. 2019, 12, 2668–2684. [Google Scholar] [CrossRef]
- Navidad-Murrieta, M.S.; Pérez-Larios, A.; Sanchéz-Burgos, J.A.; Ragazzo-Sánchez, J.A.; Luna-Bárcenas, G.; Sáyago-Ayerdi, S.G. Use of a taguchi design in Hibiscus sabdariffa extracts encapsulated by spray-drying. Foods 2020, 9, 128. [Google Scholar] [CrossRef]
- Gholami, M.; Shakeri, A.; Zolghadr, M.; Yamini, G. Non-Isocyanate polyurethane from the extracted tannin of sumac leaves: Synthesis, characterization, and optimization of the reaction parameters. Ind. Crops Prod. 2021, 161, 113195. [Google Scholar] [CrossRef]
- Miranda, I.; Sousa, V.; Ferreira, J.; Pereira, H. Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 2017, 12, e0179268. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Ruiz-Aquino, F.; Santiago-García, W.; Suárez-Mota, M.E.; Esquivel-Reyes, H.H.; Feria-Reyes, R.; Rutiaga-Quiñones, J.G. Development and validation of an analytical method for condensed tannin extracts obtained from the bark of four tree species using HPLC. Wood Res. 2021, 66, 171–182. [Google Scholar] [CrossRef]
- Konai, N.; Pizzi, A.; Raidandi, D.; Lagel, M.C.; L’Hostis, C.; Saidou, C.; Hamido, A.; Abdalla, S.; Bahabri, F.; Ganash, A. Aningre (Aningeria spp.) tannin extract characterization and performance as an adhesive resin. Ind. Crops Prod. 2015, 77, 225–231. [Google Scholar] [CrossRef]
- UNE-EN 14918; Biocombustibles sólidos. Determinación del Poder Calorífico. Normalizacion Española: Madrid, Spain, 2011.
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D1102-84; Standard Test Method for Ash in Wood. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM E872-82; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- Yazaki, Y.; Collins, P.J. Wood adhesives based on tannin extracts from barks of some pine and spruce species. Holz Als Roh-Und Werkst. 1994, 52, 307–310. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Tesso, T.; Dowell, F.; Wang, D. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef]
- Ku, C.S.; Mun, S.P. Characterization of proanthocyanidin in hot water extract isolated from Pinus radiata bark. Wood Sci. Technol. 2007, 41, 235. [Google Scholar] [CrossRef]
- Santos, J. Formulation and Characterization of Adhesives for Wooden Boards Using Tannins from Chestnut Shell and Eucalyptus Bark. Ph.D. Thesis, University of Santiago de Compostela, Santiago, Spain, 27 December 2013. [Google Scholar]
- Andrade, J.K.S.; Barros, R.G.C.; Rezende, Y.R.R.S.; Nogueira, J.P.; de Oliveira, C.S.; Gualberto, N.C.; Narain, N. Evaluation of bioactive compounds, phytochemicals profile and antioxidant potential of the aqueous and ethanolic extracts of some traditional fruit tree leaves used in Brazilian folk medicine. Food Res. Int. 2021, 143, 110282. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Suevos, F.; Riedl, B. Effects of Pinus pinaster bark extracts content on the cure properties of tannin-modified adhesives and on bonding of exterior grade MDF. J. Adhes. Sci. Technol. 2003, 17, 1507–1522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).