Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Sampling
2.2. Soil and Plant Input Property Analyses
2.3. Soil Organic Matter Fractionation
2.4. Solid-State 13C CPMAS NMR Spectroscopy Analyses
2.5. Statistical Analyses
3. Results
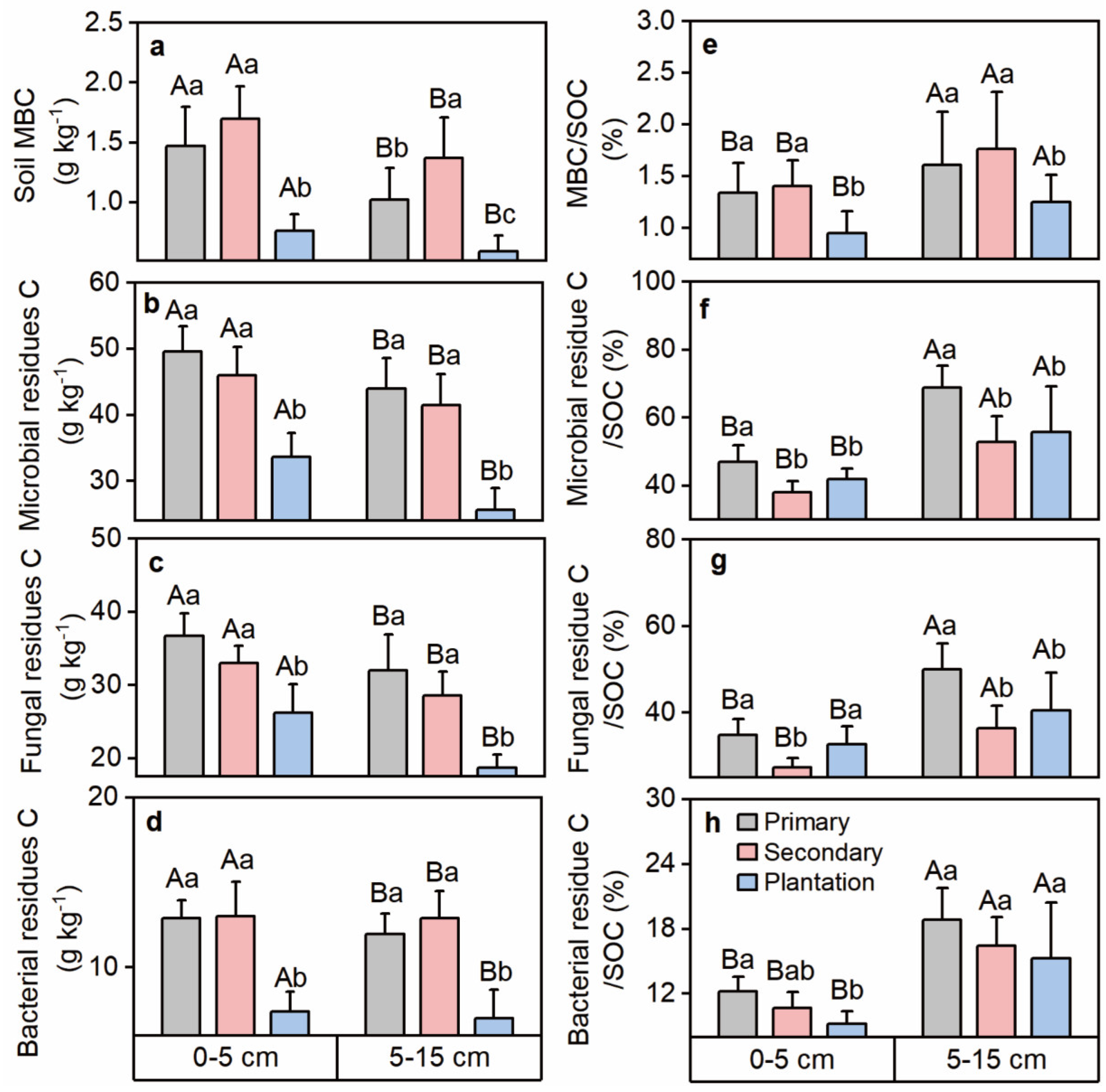
3.1. Forest Litter, Fine Roots, and Soil Properties Affected by Primary Forest Conversion
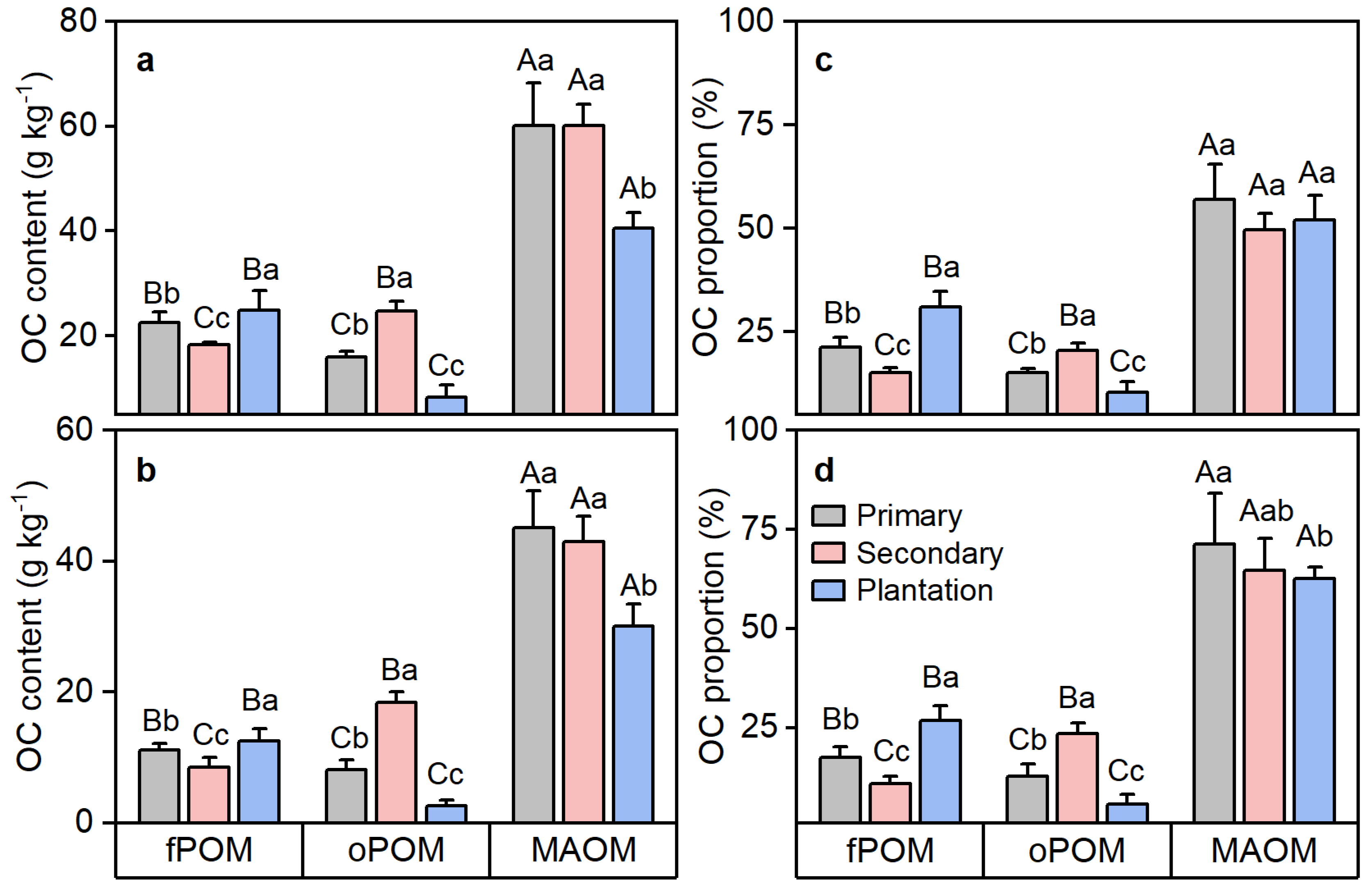
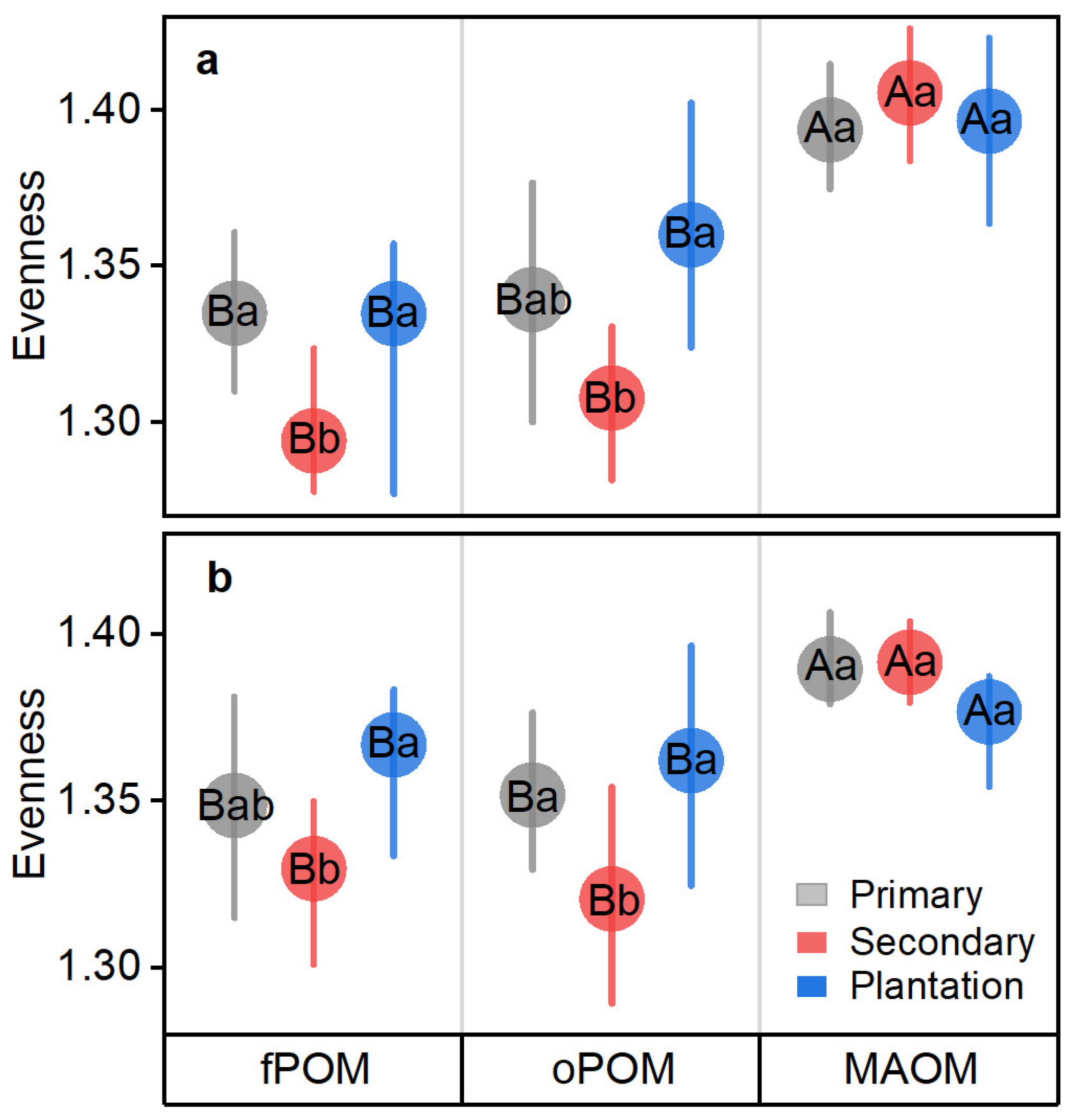
3.2. OC Components and Their Chemical Components Affected by Primary Forest Conversion
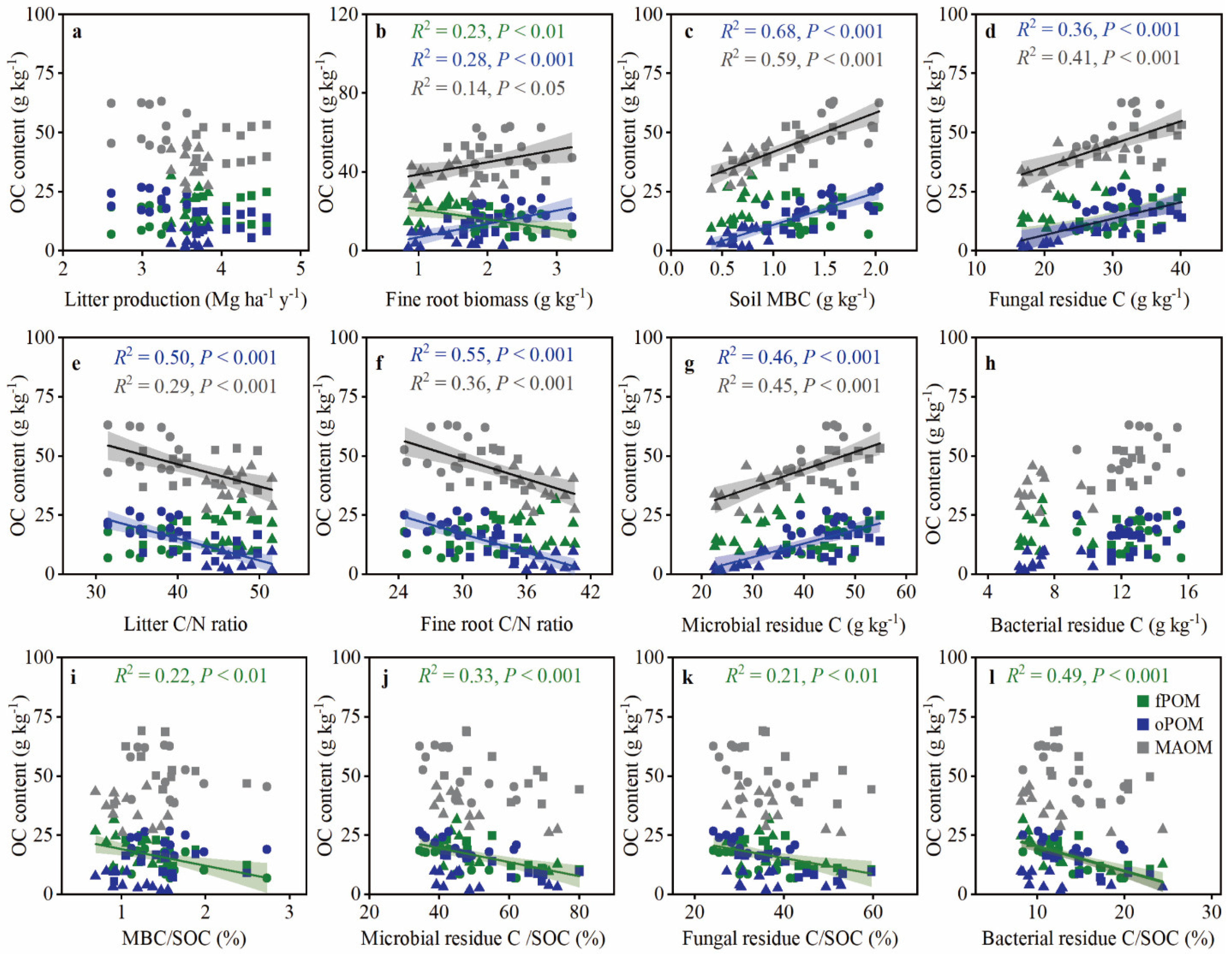
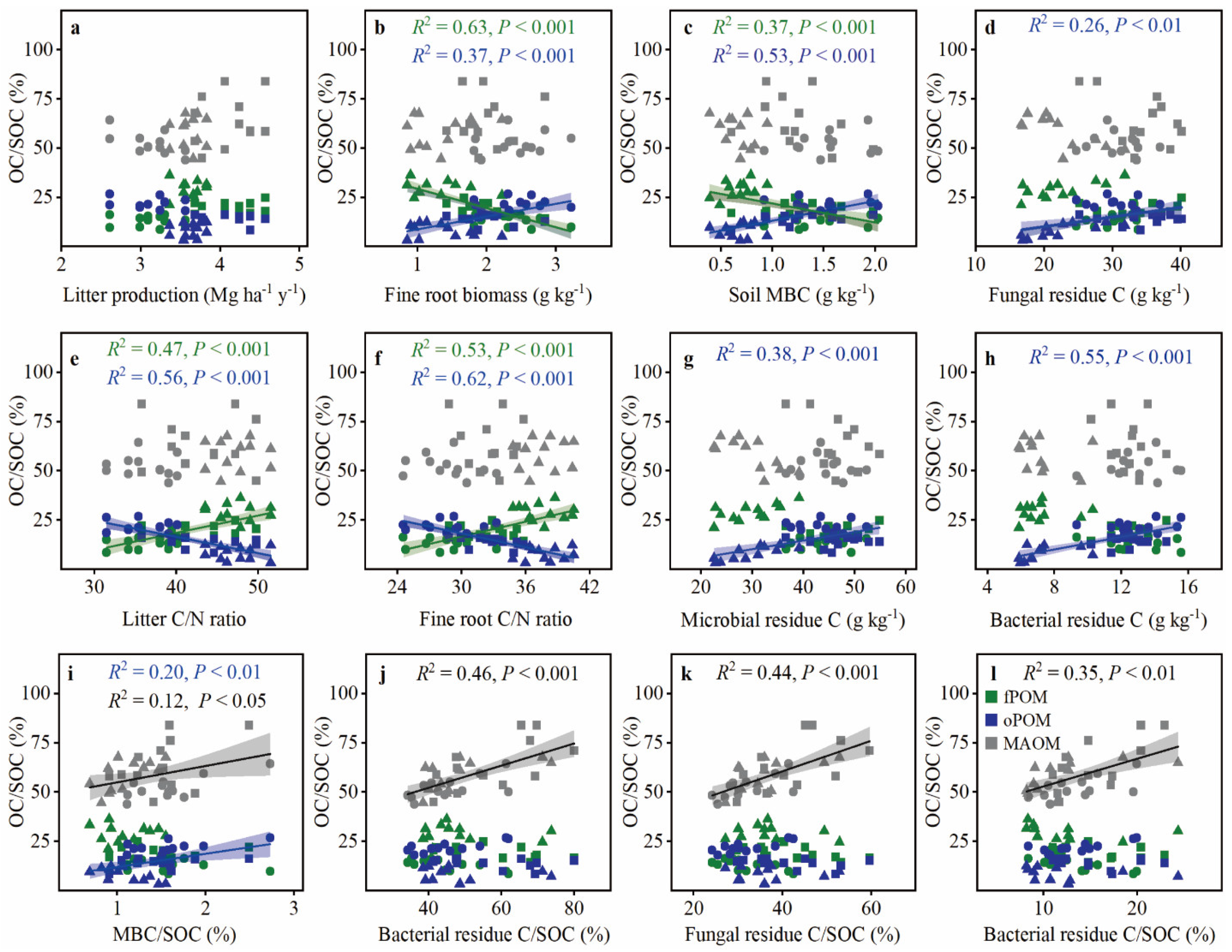
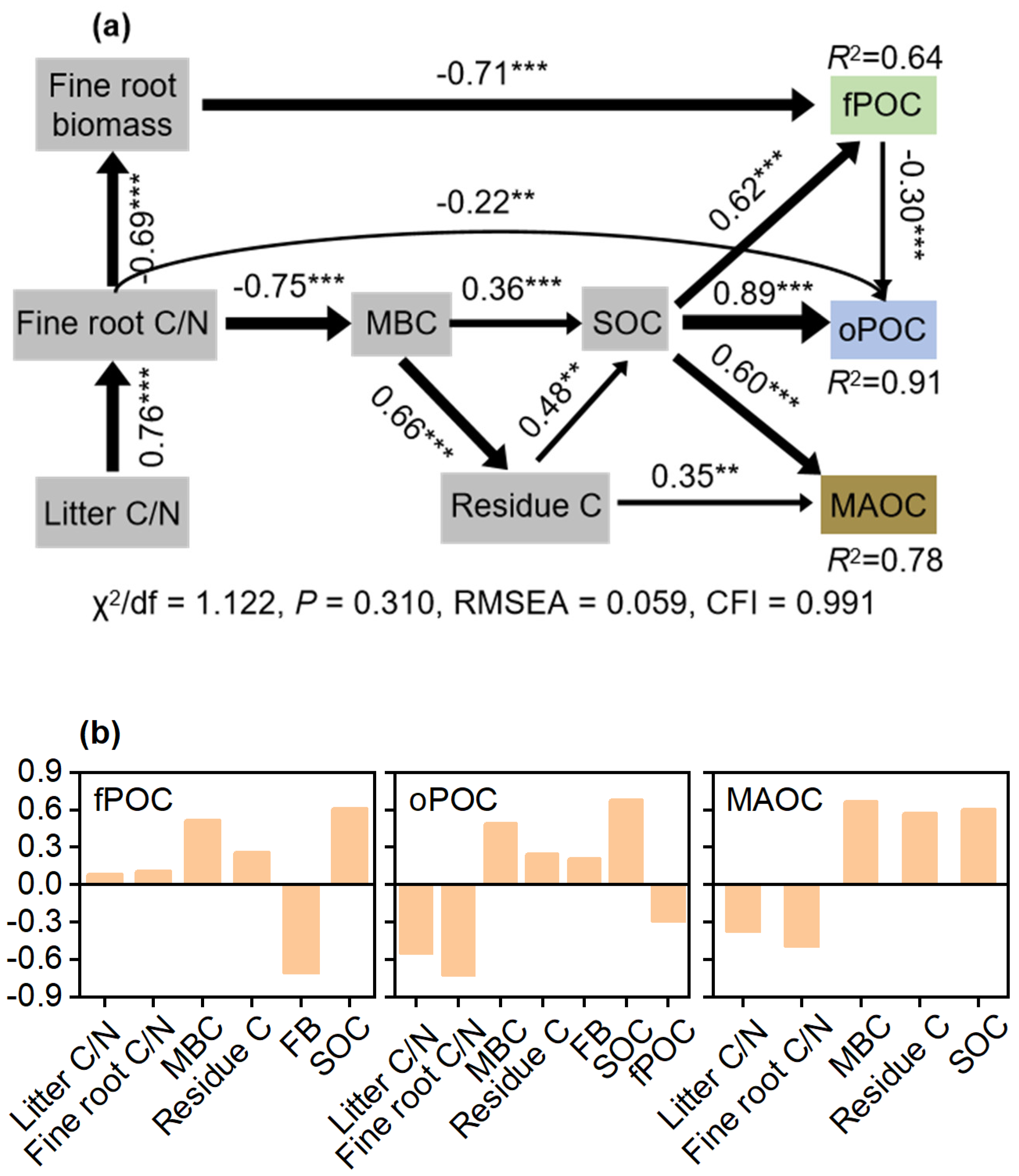
3.3. Controls on POC and MAOC Accumulation after Primary Forest Conversion
4. Discussion
4.1. Primary Forest Conversion Affects POC and MAOC through Plant Input and Soil Microbial Properties
4.2. Changed Chemical Component Evenness of POC but Not MAOC after Primary Forest Conversion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, G.; Liu, S.; Li, Z.; Zhang, D.; Tang, X.; Zhou, C.; Yan, J.; Mo, J. Old-growth forests can accumulate carbon in soils. Science 2006, 314, 1417. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A large and persistent carbon sink in the world’s forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Gibson, L.; Lee, T.M.; Koh, L.P.; Brook, B.W.; Gardner, T.A.; Barlow, J.; Peres, C.A.; Bradshaw, C.J.; Laurance, W.F.; Lovejoy, T.E.; et al. Primary forests are irreplaceable for sustaining tropical biodiversity. Nature 2011, 478, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Zhu, B. Changes in soil greenhouse gas fluxes by land use change from primary forest. Glob. Change Biol. 2020, 26, 2656–2667. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Cheng, H.; Li, Y.; Mou, Z.; Zhu, X.; Wu, W.; Zhang, J.; Kuang, L.; Wang, J.; Hui, D.; et al. Divergent accumulation of amino sugars and lignins mediated by soil functional carbon pools under tropical forest conversion. Sci. Total Environ. 2023, 881, 163204. [Google Scholar] [CrossRef]
- Luo, X.; Hou, E.; Chen, J.; Li, J.; Zhang, L.; Zang, X.; Wen, D. Dynamics of carbon, nitrogen, and phosphorus stocks and stoichiometry resulting from conversion of primary broadleaf forest to plantation and secondary forest in subtropical China. Catena 2020, 193, 104606. [Google Scholar] [CrossRef]
- Lin, Z.; Li, Y.; Tang, C.; Luo, Y.; Fu, W.; Cai, X.; Li, Y.; Yue, T.; Jiang, P.; Hu, S.; et al. Converting natural evergreen broadleaf forests to intensively managed moso bamboo plantations affects the pool size and stability of soil organic carbon and enzyme activities. Biol. Fertil. Soils 2018, 54, 467–480. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Konvalina, P.; Moudrý, J.; Kopecký, M.; Hoang, T.N. Carbon Pool Dynamic and Soil Microbial Respiration Affected by Land Use Alteration: A Case Study in Humid Subtropical Area. Land 2023, 12, 459. [Google Scholar] [CrossRef]
- Lavallee, J.M.; Soong, J.L.; Cotrufo, M.F. Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. Glob. Change Biol. 2020, 26, 261–273. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Lehmann, J.; Kleber, M. The contentious nature of soil organic matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Jackson, R.D.; Cotrufo, M.F.; Sanford, G.R.; Spiesman, B.J.; Deiss, L.; Culman, S.W.; Liang, C.; Ruark, M.D. Reply to Lajtha and Silva: Agriculture and soil carbon persistence of grassland-derived Mollisols. Proc. Natl. Acad. Sci. USA 2022, 119, e2204142119. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, X.; Yao, S.; Ye, Y.; Zhang, B. Responses of soil mineral-associated and particulate organic carbon to carbon input: A meta-analysis. Sci. Total Environ. 2022, 829, 154626. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, H.; Six, J.; Hendrix, P.F. Protection of soil carbon by microaggregates within earthworm casts. Soil Biol. Biochem. 2005, 37, 251–258. [Google Scholar] [CrossRef]
- Angst, Š.; Mueller, C.W.; Cajthaml, T.; Angst, G.; Lhotáková, Z.; Bartuška, M.; Špaldoňová, A.; Frouz, J. Stabilization of soil organic matter by earthworms is connected with physical protection rather than with chemical changes of organic matter. Geoderma 2017, 289, 29–35. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A history of research on the link between (micro)aggregates, soil biota, and soil organic matter dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Change Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Ridgeway, J.R.; Morrissey, E.M.; Brzostek, E.R. Plant litter traits control microbial decomposition and drive soil carbon stabilization. Soil Biol. Biochem. 2022, 175, 108857. [Google Scholar] [CrossRef]
- Sokol, N.W.; Bradford, M.A. Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nat. Geosci. 2019, 12, 46–53. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Soil macroaggregate turnover and microaggregate formation:A mechanism for C sequestration under no-tillage agricuture. Soil Biol. Biochem. 2000, 32, 2099–2103. [Google Scholar] [CrossRef]
- Sokol, N.W.; Sanderman, J.; Bradford, M.A. Pathways of mineral-associated soil organic matter formation: Integrating the role of plant carbon source, chemistry, and point of entry. Glob. Change Biol. 2019, 25, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.; You, Y.; Guo, X.; Wu, C.; Liu, S.; Sun, O.J. Differential effects of forest-floor litter and roots on soil organic carbon formation in a temperate oak forest. Soil Biol. Biochem. 2023, 180, 109017. [Google Scholar] [CrossRef]
- Gao, F.; Cui, X.; Sang, Y.; Song, J. Changes in soil organic carbon and total nitrogen as affected by primary forest conversion. For. Ecol. Manag. 2020, 463, 118013. [Google Scholar] [CrossRef]
- Lehmann, J.; Hansel, C.M.; Kaiser, C.; Kleber, M.; Maher, K.; Manzoni, S.; Nunan, N.; Reichstein, M.; Schimel, J.P.; Torn, M.S.; et al. Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 2020, 13, 529–534. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhang, Y.; Wang, J.; Freedman, Z.B.; Liu, P.; Cong, W.; Wang, J.; Zang, R.; Liu, S. Evenness of soil organic carbon chemical components changes with tree species richness, composition and functional diversity across forests in China. Glob. Change Biol. 2023, 29, 2852–2864. [Google Scholar] [CrossRef] [PubMed]
- Kögel-Knabner, I. The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter: Fourteen years on. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Song, Z.; Yang, Y.; Wang, J.; You, Y.; Zhang, X.; Shi, Z.; Nong, Y.; Ming, A.; et al. Introducing nitrogen-fixing tree species and mixing with Pinus massoniana alters and evenly distributes various chemical compositions of soil organic carbon in a planted forest in southern China. For. Ecol. Manag. 2019, 449, 117477. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, S.; Wang, S.; Luo, L.; Cao, D.; Christie, P. Molecular-Scale Investigation with ESI-FT-ICR-MS on Fractionation of Dissolved Organic Matter Induced by Adsorption on Iron Oxyhydroxides. Environ. Sci. Technol. 2016, 50, 2328–2336. [Google Scholar] [CrossRef]
- Kleber, M.; Bourg, I.C.; Coward, E.K.; Hansel, C.M.; Myneni, S.C.B.; Nunan, N. Dynamic interactions at the mineral–organic matter interface. Nat. Rev. Earth Environ. 2021, 2, 402–421. [Google Scholar] [CrossRef]
- Han, M.; Shi, B.; Jin, G. Conversion of primary mixed forest into secondary broadleaved forest and coniferous plantations: Effects on temporal dynamics of soil CO2 efflux. Catena 2018, 162, 157–165. [Google Scholar] [CrossRef]
- Shi, B.; Gao, W.; Jin, G. Effects on rhizospheric and heterotrophic respiration of conversion from primary forest to secondary forest and plantations in northeast China. Eur. J. Soil Biol. 2015, 66, 11–18. [Google Scholar] [CrossRef]
- Gao, L.; Cui, X.; Hill, P.W.; Guo, Y. Uptake of various nitrogen forms by co-existing plant species in temperate and cold-temperate forests in northeast China. Appl. Soil Ecol. 2020, 147, 103398. [Google Scholar] [CrossRef]
- Jenkinson, D. Measuring soil microbial biomass. Soil Biol. Biochem. 2004, 36, 5–7. [Google Scholar] [CrossRef]
- Sun, T.; Wang, Y.; Hui, D.; Jing, X.; Feng, W. Soil properties rather than climate and ecosystem type control the vertical variations of soil organic carbon, microbial carbon, and microbial quotient. Soil Biol. Biochem. 2020, 148, 107905. [Google Scholar] [CrossRef]
- Zhang, X.; Amelung, W. Gas chromatographic determination of muramic acid, glucosamine, mannosamine, and galactosamine in soils. Soil Biol. Biochem. 1996, 28, 1201–1206. [Google Scholar] [CrossRef]
- Engelking, B.; Flessa, H.; Joergensen, R.G. Shifts in amino sugar and ergosterol contents after addition of sucrose and cellulose to soil. Soil Biol. Biochem. 2007, 39, 2111–2118. [Google Scholar] [CrossRef]
- Deng, F.; Liang, C. Revisiting the quantitative contribution of microbial necromass to soil carbon pool: Stoichiometric control by microbes and soil. Soil Biol. Biochem. 2022, 165, 108486. [Google Scholar] [CrossRef]
- Appuhn, A.; Joergensen, R. Microbial colonisation of roots as a function of plant species. Soil Biol. Biochem. 2006, 38, 1040–1051. [Google Scholar] [CrossRef]
- Golchin, A.; Oades, J.M.; Skjemstad, J.O.; Clarke, P. Study of free and occluded particulate organic matter in soils by solid state 13C CP/MAS NMR spectroscopy and scanning electron microscopy. Aust. J. Soil Res. 1994, 32, 285–309. [Google Scholar] [CrossRef]
- Schmidt, M.W.I.; Knicker, H.; Hatcher, P.G.; Kögel-Knabner, I. Improvement of 13C and 15N CPMAS NMR spectra of bulk soils, particle size fractions and organic material by treatment with 10% hydrofluoric acid. Eur. J. Soil Sci. 1997, 48, 319–328. [Google Scholar] [CrossRef]
- Guo, L.B.; Gifford, R.M. Soil carbon stocks and land use change: A meta analysis. Glob. Change Biol. 2002, 8, 345–360. [Google Scholar] [CrossRef]
- Feng, J.; He, K.; Zhang, Q.; Han, M.; Zhu, B. Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems. Glob. Change Biol. 2022, 28, 3426–3440. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jin, J.; Yu, P.; Fu, W.; Morrison, L.; Lin, H.; Meng, M.; Zhou, X.; Lv, Y.; Wu, J. Converting evergreen broad-leaved forests into tea and Moso bamboo plantations affects labile carbon pools and the chemical composition of soil organic carbon. Sci. Total Environ. 2020, 711, 135225. [Google Scholar] [CrossRef]
- Yuan, Z.; Jin, X.; Xiao, W.; Wang, L.; Sun, Y.; Guan, Q.; Meshack, A.O. Comparing soil organic carbon stock and fractions under natural secondary forest and Pinus massoniana plantation in subtropical China. Catena 2022, 212, 106092. [Google Scholar] [CrossRef]
- Angst, G.; Mueller, K.E.; Eissenstat, D.M.; Trumbore, S.; Freeman, K.H.; Hobbie, S.E.; Chorover, J.; Oleksyn, J.; Reich, P.B.; Mueller, C.W. Soil organic carbon stability in forests: Distinct effects of tree species identity and traits. Glob. Change Biol. 2019, 25, 1529–1546. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Liang, C.; Lynch, L.; Xie, H.; Bao, X. Reforestation accelerates soil organic carbon accumulation: Evidence from microbial biomarkers. Soil Biol. Biochem. 2019, 131, 182–190. [Google Scholar] [CrossRef]
- Knops, J.M.H.; Naeem, S.; Reich, P.B. The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Glob. Change Biol. 2007, 13, 1960–1971. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Haddix, M.L.; Kroeger, M.E.; Stewart, C.E. The role of plant input physical-chemical properties, and microbial and soil chemical diversity on the formation of particulate and mineral-associated organic matter. Soil Biol. Biochem. 2022, 168, 108648. [Google Scholar] [CrossRef]
- Miltner, A.; Bombach, P.; Schmidt-Brucken, B.; Kastner, M. SOM genesis: Microbial biomass as a significant source. Biogeochemistry 2012, 111, 15. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.-R.; Mo, J.-M.; Wang, J.-X.; Makeschin, F.; Wolff, M. Soil organic carbon stock and chemical composition in four plantations of indigenous tree species in subtropical China. Ecol. Res. 2010, 25, 1071–1079. [Google Scholar] [CrossRef]
- Wickings, K.; Grandy, A.S.; Reed, S.C.; Cleveland, C.C. The origin of litter chemical complexity during decomposition. Ecol. Lett. 2012, 15, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Grandy, A.S.; Neff, J.C. Molecular C dynamics downstream: The biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci. Total Environ. 2008, 404, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, B.; Mao, J.; Sui, Y.; Cao, X. Structural convergence of maize and wheat straw during two-year decomposition under different climate conditions. Environ. Sci. Technol. 2012, 46, 7159–7165. [Google Scholar] [CrossRef]
- Wang, X.; Liang, C.; Mao, J.; Jiang, Y.; Bian, Q.; Liang, Y.; Chen, Y.; Sun, B. Microbial keystone taxa drive succession of plant residue chemistry. ISME J. 2023, 17, 748–757. [Google Scholar] [CrossRef]

| Forest Type | Primary | Secondary | Plantation |
|---|---|---|---|
| Dominant tree species | 60%–75% Pinus koraiensis, 25%–40% Tilia amurensis, Acer ukurunduense, Abies nephrolepis, and others. | 60%–65% Betula latyphylla, 35%–40% Betula costata, Populus davidiana, Ulmus laciniata and others. | 100% Larix gmelinii. |
| Stand age (year) | >200 | ~65 | ~65 |
| Density (Trees ha−1) | 1512 ± 537 b | 2157 ± 586 a | 1521 ± 102 b |
| BA (m2 ha−1) | 30.1 ± 3.0 a | 29.4 ± 2.2 a | 30.7 ± 1.5 a |
| Mean DBH (cm) | 16.5 ± 1.5 a | 15.0 ± 2.1 a | 15.9 ± 0.8 a |
| Litter production (Mg ha−1 y−1) | 4.1 ± 0.1 a | 3.1 ± 0.1 b | 2.6 ± 0.07 c |
| Litter C/N | 42.9 ± 4.2 b | 36.4 ± 2.4 c | 47.2 ± 5.8 a |
| 0–5 cm soil depth | |||
| Fine root biomass (Mg ha−1) | 1.8 ± 0.2 b | 2.3 ± 0.1 a | 1.2 ± 0.1 c |
| Fine root C/N | 32.7 ± 6.3 b | 28.7 ± 8.2 c | 37.1 ± 7.4 a |
| Soil pH | 5.4 ± 0.3 a | 5.7 ± 0.4 a | 5.1 ± 0.3 a |
| Water content (%) | 78.2 ± 4.3 a | 75.4 ± 3.9 a | 72.1 ± 6.1 a |
| SOC (g kg−1) | 105.9 ± 4.6 b | 121.3 ± 8.3 a | 80.1 ± 7.4 c |
| TN (g kg−1) | 8.1 ± 1.1 b | 10.3 ± 0.3 a | 5.8 ± 0.5 c |
| Soil C/N ratio | 13.1 ± 1.8 a | 11.8 ± 1.7 b | 13.8 ± 1.1 a |
| 5–15 cm soil depth | |||
| Fine root biomass (Mg ha−1) | 2.1 ± 0.2 b | 2.6 ± 0.1 c | 1.4 ± 0.1 a |
| Fine root C/N | 32.6 ± 4.2 b | 28.6 ± 3.4 c | 38.1 ± 4.3 a |
| Soil pH | 5.5 ± 0.6 a | 5.8 ± 0.3 a | 5.4 ± 0.4 a |
| Water content (%) | 58.6 ± 9.1 a | 52.6 ± 4.8 a | 53.6 ± 5.1 a |
| SOC (g kg−1) | 63.9 ± 2.4 b | 78.9 ± 2.7 a | 47.1 ± 4.1 c |
| TN (g kg−1) | 5.0 ± 0.6 b | 6.8 ± 0.6 a | 3.6 ± 0.3 a |
| Soil C/N ratio | 12.8 ± 1.9 a | 11.6 ± 1.1 b | 13.2 ± 1.7 a |
| Alkyl C | O-Alkyl C | Aromatic C | Carboxyl C | |||
|---|---|---|---|---|---|---|
| 0–5 cm | fPOC | Primary | 17.99 ± 1.50 ab | 40.07 ± 2.59 b | 30.44 ± 2.33 b | 11.49 ± 1.43 a |
| Secondary | 16.31 ± 1.30 b | 46.31 ± 1.18 a | 26.51 ± 1.28 c | 10.88 ± 1.59 a | ||
| Plantations | 18.41 ± 1.16 a | 36.43 ± 1.39 c | 34.46 ± 1.25 a | 10.70 ± 2.25 a | ||
| oPOC | Primary | 17.12 ± 0.45 a | 38.71 ± 1.70 b | 31.99 ± 1.98 a | 12.19 ± 2.77 a | |
| Secondary | 16.11 ± 0.79 a | 45.69 ± 1.59 a | 26.19 ± 0.54 b | 12.01 ± 0.70 a | ||
| Plantation | 17.61 ± 0.86 a | 34.38 ± 1.93 c | 34.42 ± 1.73 a | 13.60 ± 3.23 a | ||
| MAOC | Primary | 24.87 ± 1.57 a | 36.57 ± 1.57 a | 22.86 ± 1.16 a | 15.71 ± 2.56 a | |
| Secondary | 23.61 ± 1.42 a | 34.66 ± 1.25 a | 25.00 ± 1.64 a | 16.73 ± 2.86 a | ||
| Plantation | 25.49 ± 1.11 a | 35.22 ± 1.51 a | 23.91 ± 1.02 a | 15.39 ± 3.44 a | ||
| 5–15 cm | fPOC | Primary | 20.41 ± 1.12 ab | 38.20 ± 1.88 b | 30.20 ± 1.20 b | 11.19 ± 2.19 a |
| Secondary | 17.60 ± 1.94 b | 42.20 ± 1.43 a | 28.30 ± 1.87 c | 11.90 ± 1.65 a | ||
| Plantations | 21.20 ± 1.96 a | 32.00 ± 1.22 c | 34.70 ± 1.80 a | 12.10 ± 1.99 a | ||
| oPOC | Primary | 18.70 ± 1.74 a | 36.70 ± 1.49 b | 32.60 ± 2.43 ab | 12.00 ± 1.27 a | |
| Secondary | 16.50 ± 0.50 b | 41.70 ± 1.37 a | 30.50 ± 1.45 b | 11.31 ± 1.59 a | ||
| Plantation | 19.30 ± 0.79 a | 33.30 ± 2.09 c | 34.80 ± 1.32 a | 12.61 ± 2.57 a | ||
| MAOC | Primary | 26.50 ± 1.09 a | 35.50 ± 1.19 a | 24.10 ± 1.47 a | 13.90 ± 1.49 a | |
| Secondary | 24.78 ± 1.26 a | 36.10 ± 1.31 a | 24.60 ± 1.40 a | 14.52 ± 1.47 a | ||
| Plantation | 25.51 ± 1.31 a | 36.59 ± 1.11 a | 25.54 ± 1.84 a | 12.36 ± 1.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Cui, X.; Chen, M.; Sang, Y. Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes. Forests 2023, 14, 1234. https://doi.org/10.3390/f14061234
Gao F, Cui X, Chen M, Sang Y. Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes. Forests. 2023; 14(6):1234. https://doi.org/10.3390/f14061234
Chicago/Turabian StyleGao, Fei, Xiaoyang Cui, Mengdie Chen, and Ying Sang. 2023. "Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes" Forests 14, no. 6: 1234. https://doi.org/10.3390/f14061234
APA StyleGao, F., Cui, X., Chen, M., & Sang, Y. (2023). Forest Conversion Changes Soil Particulate Organic Carbon and Mineral-Associated Organic Carbon via Plant Inputs and Microbial Processes. Forests, 14(6), 1234. https://doi.org/10.3390/f14061234






