Effect of Litter Removal and Addition on Root Exudation and Associated Microbial N Transformation in a Pinus massoniana Plantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Root Exudation Measurements
2.4. Soil Sampling
2.5. N Mineralization and Nitrification Experiments
2.6. Laboratory Analyses
2.7. Statistical Analyses
3. Results
3.1. Litter Treatment Effects on Soil Properties and Net N Mineralization and Nitrification Rates
3.2. Litter Treatment Effects on Soil Enzyme Activities
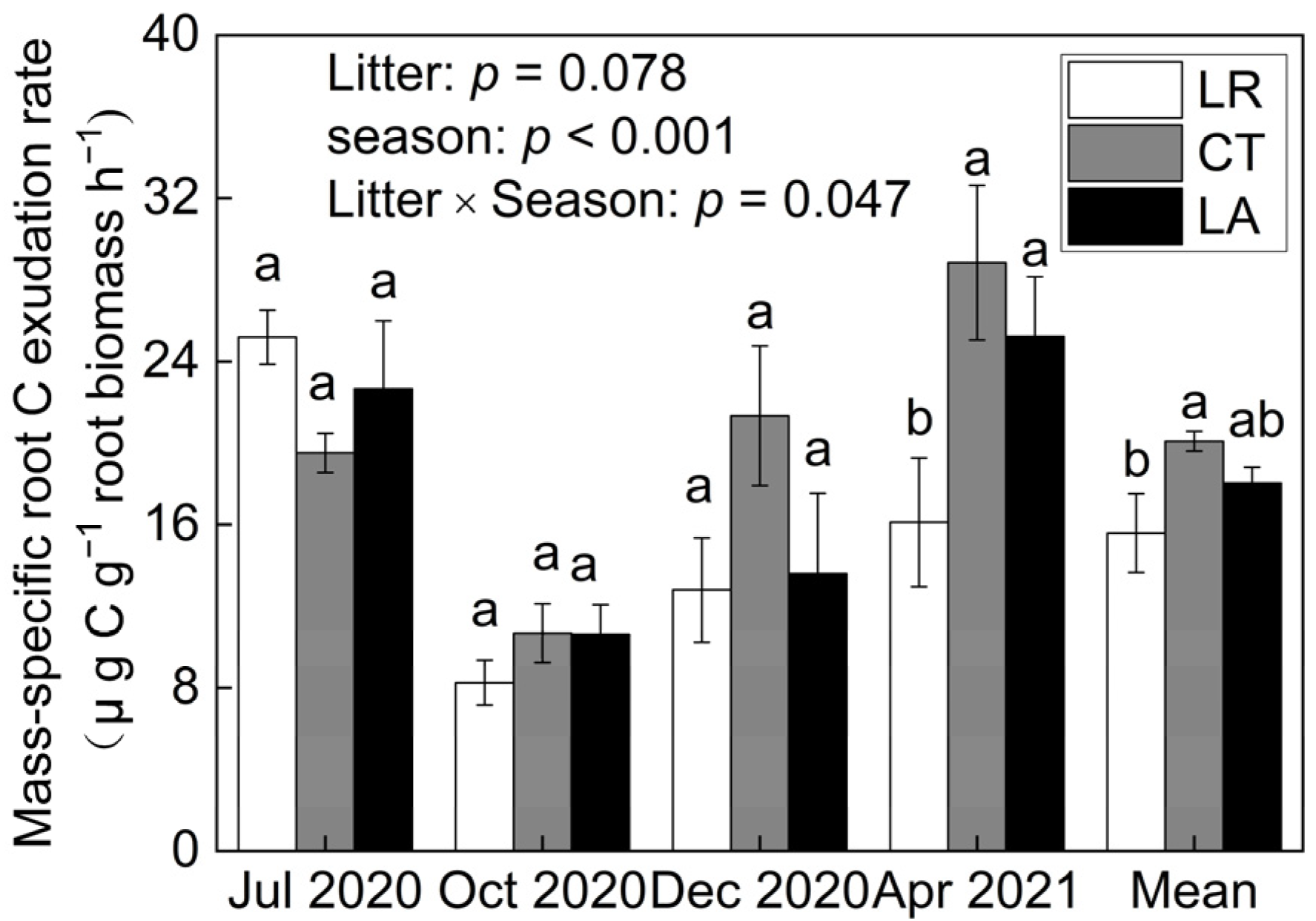
3.3. Litter Treatment Effects on Root C Exudation Rates
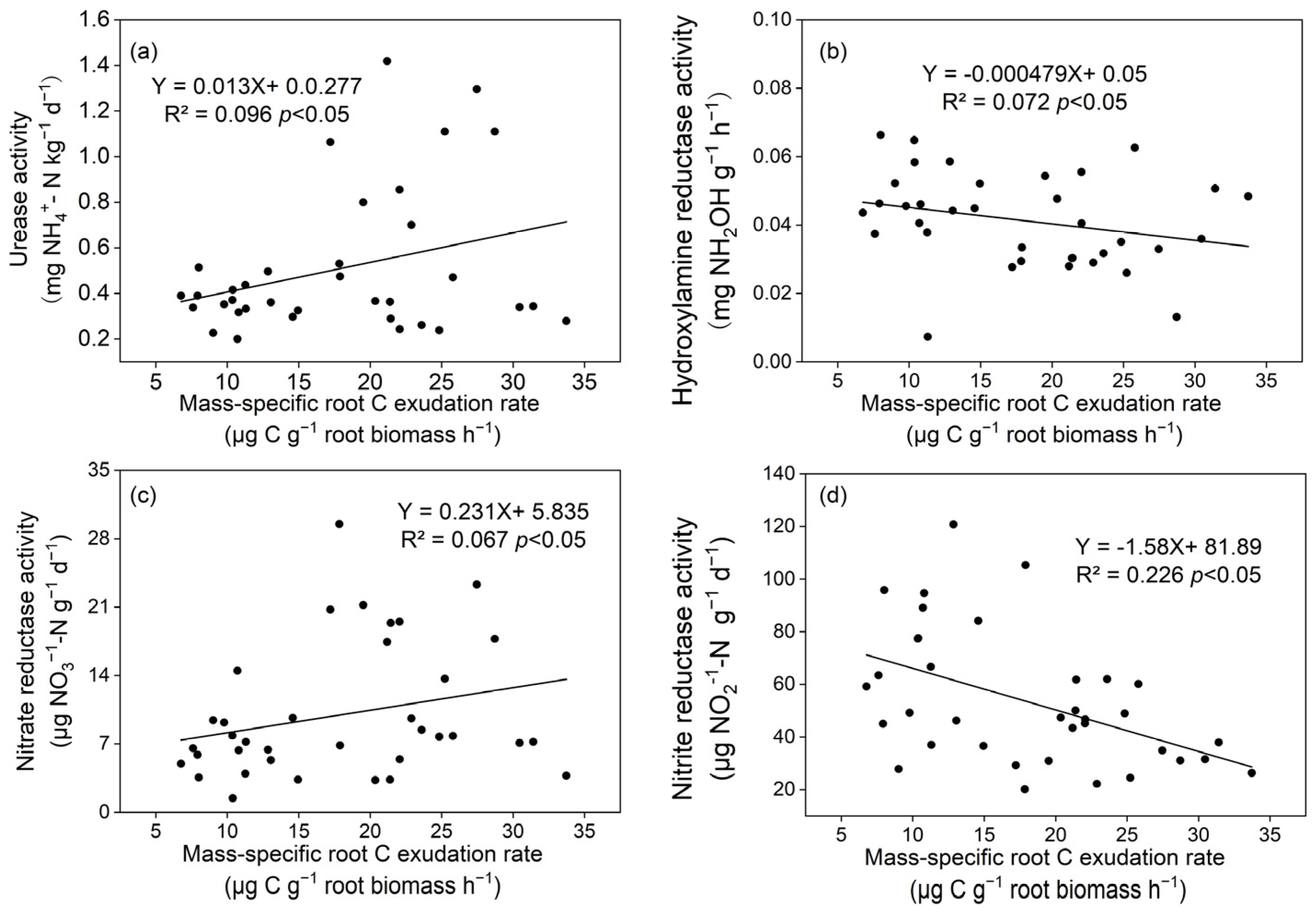
3.4. Correlations between Root C Exudation Rates and Soil Enzyme Activities and the Rates of Net N Mineralization and Nitrification
4. Discussion
4.1. Changes in Soil C and N after Litter Removal and Addition
4.2. Changes in Rates of Soil Net N Mineralization and Nitrification after Litter Removal and Addition
4.3. Changes in Root C Exudation Rates after Litter Removal and Addition
4.4. Soil N Transformations and Root Exudates
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, G.; Chen, F.; Xu, Z.; Liu, J.; Zhang, Y.; Fang, X.; Wan, S. Effects of thinning and understory removal on soil nitrogen mineralization rate and temperature-sensitivity in a moso-bamboo plantation. Acta Ecol. Sin. 2019, 39, 4106–4115. [Google Scholar]
- Sayer, E.J.; Heard, M.S.; Grant, H.K.; Marthews, T.R.; Tanner, E.V. Soil carbon release enhanced by increased tropical forest litterfall. Nat. Clim. Chang. 2011, 1, 304–307. [Google Scholar] [CrossRef]
- Sayer, E.J.; Rodtassana, C.; Sheldrake, M.; Brechet, L.M.; Ashford, O.S.; Lopez-Sangil, L.; Kerdraon-Byrne, D.; Castro, B.; Turner, B.L.; Wright, S.J. Revisiting nutrient cycling by litterfall—Insights from 15 years of litter manipulation in old-growth lowland tropical forest. Adv. Ecol. Res. 2020, 62, 173–223. [Google Scholar]
- Sayer, E.J. Using experimental manipulation to assess the roles of leaf litter in the functioning of forest ecosystems. Biol. Rev. 2006, 81, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Wieder, W.R.; Cleveland, C.C.; Taylor, P.G.; Nemergut, D.R.; Hinckley, E.-L.; Philippot, L.; Bru, D.; Weintraub, S.R.; Martin, M.; Townsend, A.R. Experimental removal and addition of leaf litter inputs reduces nitrate production and loss in a lowland tropical forest. Biogeochemistry 2012, 113, 629–642. [Google Scholar] [CrossRef]
- Fernández-Alonso, M.; Yuste, J.C.; Kitzler, B.; Ortiz, C.; Rubio, A. Changes in litter chemistry associated with global change-driven forest succession resulted in time-decoupled responses of soil carbon and nitrogen cycles. Soil Biol. Biochem. 2018, 120, 200–211. [Google Scholar] [CrossRef]
- Sayer, E.J.; Baxendale, C.; Birkett, A.J.; Bréchet, L.M.; Castro, B.; Kerdraon-Byrne, D.; Lopez-Sangil, L.; Rodtassana, C. Altered litter inputs modify carbon and nitrogen storage in soil organic matter in a lowland tropical forest. Biogeochemistry 2021, 156, 115–130. [Google Scholar] [CrossRef]
- Gomez, A.G.; Powers, R.F.; Singer, M.J.; Horwath, W.R. N uptake and N status in ponderosa pine as affected by soil compaction and forest floor removal. Plant Soil 2002, 242, 263–275. [Google Scholar]
- Sayer, E.J.; Wright, S.J.; Tanner, E.; Yavitt, J.B.; Turner, B.L. Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 2012, 15, 387–400. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Wieder, W.R.; Cleveland, C.C.; Townsend, A.R. Organic matter inputs shift soil enzyme activity and allocation patterns in a wet tropical forest. Biogeochemistry 2012, 114, 313–326. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Cassman, K.; Cleveland, C.; Crews, T.; Field, C.B.; Grimm, N.B.; Howarth, R.W.; Marino, R.; Martinelli, L.; Rastetter, E.B. Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry 2002, 57, 1–45. [Google Scholar] [CrossRef]
- Luo, Y.; Su, B.; Currie, W.S.; Dukes, J.S.; Finzi, A.; Hartwig, U.; Hungate, B.; McMurtrie, R.E.; Oren, R.; Parton, W.J. Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. Bioscience 2004, 54, 731–739. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liu, L.L.; Sayer, E.J. Variability of above-ground litter inputs alters soil physicochemical and biological processes: A meta-analysis of litterfall-manipulation experiments. Biogeosciences 2013, 10, 7423–7433. [Google Scholar] [CrossRef] [Green Version]
- Lyu, M.; Li, X.; Xie, J.; Homyak, P.M.; Ukonmaanaho, L.; Yang, Z.; Liu, X.; Ruan, C.; Yang, Y. Root–microbial interaction accelerates soil nitrogen depletion but not soil carbon after increasing litter inputs to a coniferous forest. Plant Soil 2019, 444, 153–164. [Google Scholar] [CrossRef]
- Deng, H.P.; Wang, G.J.; Geng, G. Response of nitrogen mineralization to litter addition and exclusion in soils of Cinnamomum camphora plantation. J. B For. Univ. 2010, 32, 47–51. [Google Scholar]
- Sayer, E.J.; Powers, J.S.; Tanner, E.V. Increased litterfall in tropical forests boosts the transfer of soil CO2 to the atmosphere. PLoS ONE 2007, 2, e1299. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Classen, A.T.; Wang, W.W.; Zhao, X.R.; Mao, B.; Zeng, D.H. Asymmetric effects of litter removal and litter addition on the structure and function of soil microbial communities in a managed pine forest. Plant Soil 2017, 414, 81–93. [Google Scholar] [CrossRef]
- Phillips, R.P.; Fahey, T.J. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 2006, 87, 1302–1313. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.P.; Finzi, A.C.; Bernhardt, E.S. Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 2011, 14, 187–194. [Google Scholar] [CrossRef]
- Yin, H.; Li, Y.; Xiao, J.; Xu, Z.; Cheng, X.; Liu, Q. Enhanced root exudation stimulates soil nitrogen transformations in a subalpine coniferous forest under experimental warming. Glob. Chang. Biol. 2013, 19, 2158–2167. [Google Scholar] [CrossRef]
- Cheng, W.; Parton, W.J.; Gonzalez-Meler, M.A.; Phillips, R.; Asao, S.; McNickle, G.G.; Brzostek, E.; Jastrow, J.D. Synthesis and modeling perspectives of rhizosphere priming. New Phytol. 2014, 201, 31–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haichar, F.Z.; Santaella, C.; Heulin, T.; Achouak, W. Root exudates mediated interactions belowground. Soil Biol. Biochem. 2014, 77, 69–80. [Google Scholar] [CrossRef]
- Badri, D.V.; Vivanco, J.M. Regulation and function of root exudates. Plant Cell Environ. 2010, 32, 666–681. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef] [Green Version]
- Pausch, J.; Kuzyakov, Y. Carbon input by roots into the soil: Quantification of rhizodeposition from root to ecosystem scale. Global. Chang. Biol. 2018, 24, 1–12. [Google Scholar] [CrossRef]
- Finzi, A.C.; Abramoff, R.Z.; Spiller, K.S.; Brzostek, E.R.; Darby, B.A.; Kramer, M.A.; Phillips, R.P. Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Global. Chang. Biol. 2015, 21, 2082–2094. [Google Scholar] [CrossRef]
- Chen, R.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Lin, X.; Blagodatskaya, E.; Kuzyakov, Y. Soil C and N availability determine the priming effect: Microbial N mining and stoichiometric decomposition theories. Global. Chang. Biol. 2014, 20, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carrillo, S.; Álvarez-Cobelas, M.; Angeler, D.G.; Serrano-Grijalva, L.; Sánchez-Andrés, R.; Cirujano, S.; Schmid, T. Elevated atmospheric CO2 increases root exudation of carbon in wetlands: Results from the first free-air CO2 enrichment facility (FACE) in a marshland. Ecosystems 2018, 21, 852–867. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, M.; Li, D.; Yin, H.; Liu, Q. Do warming-induced changes in quantity and stoichiometry of root exudation promote soil N transformations via stimulation of soil nitrifiers, denitrifiers and ammonifiers? Eur. J. Soil Biol. 2016, 74, 60–68. [Google Scholar] [CrossRef]
- Zhao, Y. Effect and Mechanism of Root Exudates from Transgenic Bt Cotton on the Soil N Cycling. Master’ Thesis, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Subbarao, G.V.; Nakahara, K.; Ishikawa, T.; Ono, H.; Deshpande, S.P. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 2013, 366, 243–259. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nardi, P.; Akutsu, M.; Pariasca-Tanak, J.; Wissuwa, M. Effect of methyl 3-4-hydroxyphenyl propionate, a Sorghum root exudate, on N dynamic; potential nitrification activity and abundance of ammonia-oxidizing bacteria and archaea. Plant Soil 2013, 367, 627–637. [Google Scholar] [CrossRef]
- O’Sullivan, C.A.; Fillery, I.R.P.; Roper, M.M.; Richards, R.A. Identification of several wheat landraces with biological nitrification inhibition capacity. Plant Soil 2016, 404, 61–74. [Google Scholar] [CrossRef]
- LeBauer, D.S.; Treseder, K.K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Cai, Y.; Zhang, T.; He, T.; Li, J.; Li, X.; Zhao, Q. Litter removal increases the plant carbon input to soil in a Pinus massoniana plantation. Eur. J. For. Res. 2022, 141, 833–843. [Google Scholar] [CrossRef]
- Phillips, R.P.; Erlitz, Y.; Bier, R.; Bernhardt, E.S. New approach for capturing soluble root exudates in forest soils. Funct. Ecol. 2008, 22, 990–999. [Google Scholar] [CrossRef]
- Sayer, E.J.; Tanner, E.V.J. Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J. Ecol. 2010, 98, 1052–1062. [Google Scholar] [CrossRef]
- Sayer, E.J.; Tanner, E.V.J.; Lacey, A.L. Effects of litter manipulation on early-stage decomposition and meso-arthropod abundance in a tropical moist forest. For. Ecol. Manag. 2006, 229, 285–293. [Google Scholar] [CrossRef]
- Wood, T.E.; Lawrence, D.; Clark, D.A.; Chazdon, R.L. Rain forest nutrient cycling and productivity in response to large-scale litter manipulation. Ecology 2009, 90, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Joergensen, R.G. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEC value. Soil Biol. Biochem. 1996, 28, 25–31. [Google Scholar] [CrossRef]
- Xu, S.; Sayer, E.J.; Eisenhauer, N.; Lu, X.; Wang, J.; Liu, C. Aboveground litter inputs determine carbon storage across soil profiles: A meta-analysis. Plant Soil. 2021, 462, 429–444. [Google Scholar] [CrossRef]
- Huang, W.; Spohn, M. Effects of long-term litter manipulation on soil carbon, nitrogen, and phosphorus in a temperate deciduous forest. Soil Biol. Biochem. 2015, 83, 12–18. [Google Scholar] [CrossRef]
- Gulde, S.; Chung, H.; Amelung, W.; Chang, J.; Six, J. Soil carbon saturation controls labile and stable carbon pool dynamics. Soil Sci. Soc. Amer. 2008, 72, 605–612. [Google Scholar] [CrossRef]
- Castellano, M.J.; Mueller, K.E.; Olk, D.C.; Sawyer, J.E.; Six, J. Integrating plant litter quality, soil organic matter stabilization, and the carbon saturation concept. Glob. Change Biol. 2015, 21, 3200–3209. [Google Scholar] [CrossRef] [Green Version]
- Hedin, L.O. Signs of saturation in the tropical carbon sink. Nature 2015, 519, 295–296. [Google Scholar] [CrossRef] [PubMed]
- Sayer, E.J.; Lopez-Sangil, L.; Crawford, J.A.; Bréchet, L.M.; Birkett, A.J.; Baxendale, C.; Castro, B.; Rodtassana, C.; Garnett, M.H.; Weiss, L.; et al. Tropical forest soil carbon stocks do not increase despite 15 years of doubled litter inputs. Sci. Rep. 2019, 9, 18030. [Google Scholar] [CrossRef] [Green Version]
- Crow, S.E.; Lajtha, K.; Bowden, R.D.; Yano, Y.; Brant, J.B.; Caldwell, B.A.; Sulzman, E.W. Increased coniferous needle inputs accelerate decomposition of soil carbon in an old-growth forest. For. Ecol. Manag. 2009, 258, 2224–2232. [Google Scholar] [CrossRef]
- Holub, S.M.; Lajtha, K.; Spears, J.D.H.; Tóth, J.A.; Crow, S.E.; Caldwell, B.A.; Papp, M.; Nagy, P.T. Organic matter manipulations have little effect on gross and net nitrogen transformations in two temperate forest mineral soils in the USA and central Europe. For. Ecol. Manag. 2005, 214, 320–330. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, Y.; Jiao, Z.; Chen, Z.; Shen, Y.; Liu, Y.; Zhang, L.; Wang, L.; Liu, S.; Wu, Q. Response of soil net nitrogen mineralization to a litter in three subalpine forests. Forests 2022, 13, 597. [Google Scholar] [CrossRef]
- Xiao, R.; Man, X.; Duan, B.; Cai, T. Short-term litter manipulations have strong impact on soil nitrogen dynamics in Larix gmelinii forest of northeast China. Forests 2020, 11, 1205. [Google Scholar] [CrossRef]
- Alexander, H.D.; Arthur, M.A. Increasing red maple leaf litter alters decomposition rates and nitrogen cycling in historically oak-dominated forests of the eastern U.S. Ecosystems 2014, 17, 1371–1383. [Google Scholar] [CrossRef]
- Song, Y.; Gu, X.; Yan, H.; Mao, W.; Wu, X.; Wan, Y. Dynamics of Microbes and Enzyme Activities during Litter Decomposition of Pinus massoniana Forest in Mid-subtropical Area. Environ. Sci. 2014, 35, 1151–1158. [Google Scholar]
- Hatton, P.J.; Castanha, C.; Torn, M.S.; Bird, J.A. Litter type control on soil C and N stabilization dynamics in a temperate forest. Glob. Chang. Biol. 2015, 21, 1358–1367. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, Y.; Xiao, D.; Xu, Z.; Zhang, W.; Xiao, J.; Wang, K. Dynamics of soil nitrogen availability following vegetation restoration along a climatic gradient of a subtropical karst region in China. J. Soil Sediments 2021, 21, 2167–2178. [Google Scholar] [CrossRef]
- Fontaine, S.; Hénault, C.; Aamor, A.; Bdioui, N.; Bloor, J.M.G.; Maire, V.; Mary, B.; Revaillot, S.; Maron, P.A. Fungi mediate long term sequestration of carbon and nitrogen in soil through their priming effect. Soil Biol. Biochem. 2011, 43, 86–96. [Google Scholar] [CrossRef]
- Yan, W.; Farooq, T.H.; Chen, Y.; Wang, W.; Shabbir, R.; Kumar, U.; Riaz, M.U.; Alotaibi, S.S.; Peng, Y.; Chen, X. Soil Nitrogen Transformation Process Influenced by Litterfall Manipulation in Two Subtropical Forest Types. Front. Plant Sci. 2022, 13, 923410. [Google Scholar] [CrossRef]
- Matsushima, M.; Chang, S.X. Effects of understory removal, N fertilization; and litter layer removal on soil N cycling in a 13-year-old white spruce plantation infested with Canada bluejoint grass. Plant Soil. 2007, 292, 243–258. [Google Scholar] [CrossRef]
- Ma, L.; Guo, C.; Xin, X.; Yuan, S.; Wang, R. Effects of belowground litter addition; increased precipitation and clipping on soil carbon and nitrogen mineralization in a temperate steppe. Biogeosciences 2013, 10, 7361–7372. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.; Ristaino, J.B.; Hu, S. Soil microbial biomass and activity in organic tomato farming systems: Effects of organic inputs and straw mulching. Soil Biol. Biochem. 2006, 38, 247–255. [Google Scholar] [CrossRef]
- Xiao, C.; Janssens, I.A.; Liu, P.; Zhou, Z.; Sun, O.J. Irrigation and enhanced soil carbon input effects on below-ground carbon cycling in semiarid temperate grasslands. New Phytol. 2007, 174, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B. Effects of Litter and Understory Vegetation Removal on Soil Nitrogen Mineralization in Forest Ecosystems in Different Climatic Zones of China. Master’ Thesis, Henan University, Kaifeng, China, 2019. [Google Scholar]
- He, W.; Yuan, Y.; Zhang, Z.; Xiao, J.; Liu, Q.; Laiho, R.; Yin, H. Effect of N addition on root exudation and associated microbial N transformation under Sibiraea angustata in an alpine shrubland. Plant Soil 2021, 460, 469–481. [Google Scholar] [CrossRef]
- Wang, R.; Cavagnaro, T.R.; Jiang, Y.; Keitel, C.; Dijkstra, F.A. Carbon allocation to the rhizosphere is affected by drought and nitrogen addition. J. Ecol. 2021, 109, 3699–3709. [Google Scholar] [CrossRef]
- Tian, K.; Kong, X.; Yuan, L.; Lin, H.; He, Z.; Yao, B.; Ji, Y.; Yang, J.; Sun, S.; Tian, X. Priming effect of litter mineralization: The role of root exudate depends on its interactions with litter quality and soil condition. Plant Soil 2019, 440, 457–471. [Google Scholar] [CrossRef]
- Ataka, M.; Sun, L.; Nakaji, T.; Katayama, A.; Hiura, T. Five-year nitrogen addition affects fine root exudation and its correlation with root respiration in a dominant species, Quercus crispula, of a cool temperate forest, Japan. Tree Physiol. 2020, 40, 367–376. [Google Scholar] [CrossRef]
- Rocha, J.H.T.; Gonçalves, J.L.M.; Gava, J.L.; Godinho, T.O.; Melo, E.A.S.C.; Bazani, J.H.; Hubner, A. Forest residue maintenance increased the wood productivity of a Eucalyptus plantation over two short rotations. For. Ecol. Manag. 2016, 379, 1–10. [Google Scholar] [CrossRef]
- Darwent, M.J.; Paterson, E.; Mcdonald, A.J.S.; Tomos, A.D. Biosensor reporting of root exudation from Hordeum vulgare in relation to shoot nitrate concentration. J. Exp Bot. 2003, 54, 325–334. [Google Scholar] [CrossRef]
- Rodtassana, C.; Tanner, E.V.J. Litter removal in a tropical rain forest reduces fine root biomass and production but litter addition has few effects. Ecology 2018, 99, 735–742. [Google Scholar] [CrossRef]
- Lima, T.T.S.; Miranda, I.S.; Vasconcelos, S.S.; Vasconcelos, S.S. Effects of water and nutrient availability on fine root growth in eastern Amazonian forest regrowth, Brazil. New Phytol. 2010, 187, 622–630. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, Q.; Xiao, J.; Zhang, Z.; Yin, H. Differential responses of N benefit mediated by root exudate inputs to N addition between two subalpine forests. Rhizosphere 2021, 19, 100404. [Google Scholar] [CrossRef]
- Drake, J.E.; Gallet-Budynek, A.; Hofmockel, K.S.; Bernhardt, E.S.; Billings, S.A.; Jackson, R.B.; Johnsen, K.S.; Lichter, J.; McCarthy, H.R.; McCormack, M.L. Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol. Lett. 2011, 14, 349–357. [Google Scholar] [CrossRef]
- Phillips, R.P.; Meier, I.C.; Bernhardt, E.S.; Grandy, A.S.; Wickings, K.; Finzi, A.C. Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol. Lett. 2012, 15, 1042–1049. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Groffman, P.M.; Butterbach-Bahl, K.; Fulweiler, R.W.; Gold, A.J.; Morse, J.L.; Stander, E.K.; Tague, C.; Tonitto, C.; Vidon, P. Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 2009, 93, 49–77. [Google Scholar] [CrossRef]
- Zhuang, S.; Lin, W.; Ding, J.; Zheng, Q.; Kou, X.; Li, Q.; Li, Y. Effects of different root exudates on soil N2O emissions and isotopic signature. Sci. Agric. Sin. 2020, 53, 1860–1873. [Google Scholar]
- Giles, M.E.; Daniell, T.J.; Baggs, E.M. Compound driven differences in N2 and N2O emission from soil, the role of substrate use efficiency and the microbial community. Soil Biol. Biochem. 2017, 106, 90–98. [Google Scholar] [CrossRef]
- Langarica-Fuentes, A.; Manrubia, M.; Giles, M.E.; Mitchell, S.; Daniell, T.J. Effect of model root exudate on denitrifier community dynamics and activity at different water-filled pore space levels in a fertilised soil. Soil Biol. Biochem. 2018, 120, 70–79. [Google Scholar] [CrossRef] [Green Version]
- Zumft, W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997, 61, 533–616. [Google Scholar]
- Hu, H.W.; Chen, D.; He, J.Z. Microbial regulation of terrestrial nitrous oxide formation: Understanding the biological pathways for prediction of emission rates. Fems. Microbiol. Rev. 2015, 39, 729–749. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soil–root interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Coskun, D.; Britto, D.; Shi, W.; Kronzucker, H.J. How plant root exudates shape the nitrogen cycle. Trends Plant Sci. 2017, 22, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Nakahara, K.; Hurtado, M.P.; Ono, H.; Ito, O. Evidence for biological nitrification inhibition in Brachiaria pastures. Proc. Natl. Acad. Sci. USA 2009, 106, 17302–17307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pariasca Tanaka, J.; Nardi, P.; Wissuwa, M. Nitrification inhibition activity, a novel trait in root exudates of rice. AoB Plants 2010, 2010, plq014. [Google Scholar] [CrossRef] [PubMed]
- Subbarao, G.V.; Wang, H.Y.; Ito, O.; Nakahara, K.; Berry, W.L. NH4+ triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant Soil 2007, 290, 245–257. [Google Scholar] [CrossRef]
- Subbarao, G.V.; Kishii, M.; Nakahara, K.; Ishikawa, T.; Ban, T.; Tsujimoto, H.; George, T.S.; Berry, W.L.; Hash, C.T.; Ito, O. Biological nitrification inhibition (BNI)—Is there potential for genetic interventions in the Triticeae? Breed. Sci. 2009, 59, 529–545. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhang, F.; Liu, S.; Cao, M. Effect of allelochemicals on N2O emission from soil. Act. Sci. Circumst 1999, 19, 478–482. [Google Scholar]


| Season | Litter Treament | pH | SOC (g·kg−1) | TN (g·kg−1) | C:N | SON (g·kg−1) | NH4+-N (mg·kg−1) | NO3−-N (mg·kg−1) | NO3−-N (mg·kg−1) | MN (mg·kg−1) | MBC (mg·kg−1) | MBN (mg·kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| July 2020 | LR | 4.76 ± 0.13 a | 22.96 ± 1.78 a | 2.48 ± 0.54 a | 10.10 ± 1.96 a | 2.63 ± 0.46 a | 8.29 ± 1.51 a | 12.62 ± 1.36 a | 20.91 ± 1.55 a | 353.39 ± 101.75 a | 78.37 ± 23.70 a | |
| CT | 4.75 ± 0.04 a | 21.13 ± 0.61 a | 2.65 ± 0.46 | 8.44 ± 1.36 a | 2.46 ± 0.54 a | 9.87 ± 2.40 a | 12.66 ± 3.66 a | 22.53 ± 5.08 a | 264.95 ± 43.55 a | 72.81 ± 11.48 a | ||
| LA | 4.70 ± 0.10 a | 23.36 ± 3.00 a | 2.96 ± 0.62 a | 8.22 ± 1.01 a | 2.93 ± 0.63 a | 9.68 ± 2.11 a | 11.16 ± 4.14 a | 20.84 ± 2.05 a | 230.37 ± 74.60 a | 41.59 ± 8.85 a | ||
| October 2020 | LR | 5.36 ± 0.56 a | 21.90 ± 0.74 a | 1.83 ± 0.13 a | 12.12 ± 1.17 a | 1.75 ± 0.09 b | 7.43 ± 1.41 a | 6.74 ± 0.97 a | 14.16 ± 1.38 a | 312.64 ± 93.10 a | 39.05 ± 11.37 a | |
| CT | 4.97 ± 0.03 a | 24.69 ± 0.92 a | 1.77 ± 0.09 a | 14.09 ± 1.17 a | 1.82 ± 0.13 ab | 9.46 ± 0.88 a | 4.23 ± 0.22 a | 13.69 ± 0.69 a | 347.60 ± 23.08 a | 53.08 ± 6.12 a | ||
| LA | 4.90 ± 0.04 a | 24.28 ± 1.75 a | 2.29 ± 0.17 a | 10.64 ± 0.50 a | 2.27 ± 0.18 a | 8.89 ± 1.08 a | 6.44 ± 0.88 a | 15.34 ± 0.88 a | 373.68 ± 76.71 a | 55.62 ± 10.81 a | ||
| December 2020 | LR | 4.77 ± 0.08 a | 24.94 ± 3.50 a | 2.34 ± 0.30 a | 10.66 ± 0.79 a | 2.18 ± 0.02 a | 5.45 ± 0.47 a | 3.34 ± 0.73 a | 8.79 ± 0.53 b | 376.09 ± 48.64 a | 57.25 ± 10.99 a | |
| CT | 4.91 ± 0.02 a | 25.07 ± 0.65 a | 2.19 ± 0.03 a | 11.46 ± 0.24 a | 2.33 ± 0.30 a | 5.63 ± 1.33 a | 3.36 ± 0.30 a | 8.99 ± 1.42 b | 303.62 ± 19.82 a | 44.73 ± 4.49 a | ||
| LA | 4.85 ± 0.07 a | 23.44 ± 3.60 a | 2.36 ± 0.28 a | 9.99 ± 1.03 a | 2.35 ± 0.28 a | 7.67 ± 0.92 a | 6.13 ± 1.32 a | 13.80 ± 1.63 a | 303.38 ± 33.71 a | 53.29 ± 6.11 a | ||
| April 2021 | LR | 4.86 ± 0.05 a | 21.03 ± 2.77 a | 2.32 ± 0.10 a | 9.00 ± 0.80 a | 2.15 ± 0.09 a | 9.40 ± 0.70 a | 6.49 ± 1.23 a | 15.89 ± 0.75 ab | 370.25 ± 50.10 a | 52.00 ± 11.27 a | |
| CT | 4.81 ± 0.04 a | 18.27 ± 2.15 a | 2.16 ± 0.08 a | 8.40 ± 0.69 a | 2.30 ± 0.10 a | 8.74 ± 0.67 a | 5.06 ± 0.22 a | 13.79 ± 0.85 b | 357.82 ± 25.50 a | 45.18 ± 5.67 a | ||
| LA | 5.02 ± 0.16 a | 20.56 ± 0.74 a | 2.12 ± 0.07 a | 9.73 ± 0.46 a | 2.10 ± 0.07 a | 11.91 ± 1.80 a | 7.02 ± 0.97 a | 18.93 ± 1.66 a | 368.75 ± 55.30 a | 50.05 ± 6.33 a | ||
| Variance analysis of F-statistics | ||||||||||||
| Litter | 0.225 | 0.085 | 0.643 | 1.006 | 0.642 | 1.862 | 0.606 | 2.049 | 0.550 | 0.809 | ||
| Season | 1.909 | 2.507 | 2.952 | 7.079 ** | 2.888 | 4.085 * | 11.075 ** | 16.581 ** | 2.239 | 6.314 | ||
| Litter×season | 0.73 | 0.453 | 0.337 | 1.239 | 0.340 | 0.433 | 0.436 | 0.788 | 1.346 | 0.592 | ||
| Treatment | Net Mineralization Rate (mg N kg−1 d−1) | Net Nitrification Rate (mg N kg−1 d−1) |
|---|---|---|
| LR | 0.15 ± 0.03 a | 0.18 ± 0.01 a |
| CT | 0.09 ± 0.05 a | 0.03 ± 0.01 b |
| LA | 0.16 ± 0.02 a | 0.14 ± 0.03 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Zhao, Q.; Cai, Y.; Zhang, T.; Zhang, L.; He, T. Effect of Litter Removal and Addition on Root Exudation and Associated Microbial N Transformation in a Pinus massoniana Plantation. Forests 2023, 14, 1305. https://doi.org/10.3390/f14071305
Zhang C, Zhao Q, Cai Y, Zhang T, Zhang L, He T. Effect of Litter Removal and Addition on Root Exudation and Associated Microbial N Transformation in a Pinus massoniana Plantation. Forests. 2023; 14(7):1305. https://doi.org/10.3390/f14071305
Chicago/Turabian StyleZhang, Chengfu, Qingxia Zhao, Yinmei Cai, Tao Zhang, Limin Zhang, and Tengbing He. 2023. "Effect of Litter Removal and Addition on Root Exudation and Associated Microbial N Transformation in a Pinus massoniana Plantation" Forests 14, no. 7: 1305. https://doi.org/10.3390/f14071305






