The Distribution and Activity of the Invasive Raccoon Dog in Lithuania as Found with Country-Wide Camera Trapping
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Camera Trapping
2.3. Data Analysis
3. Results
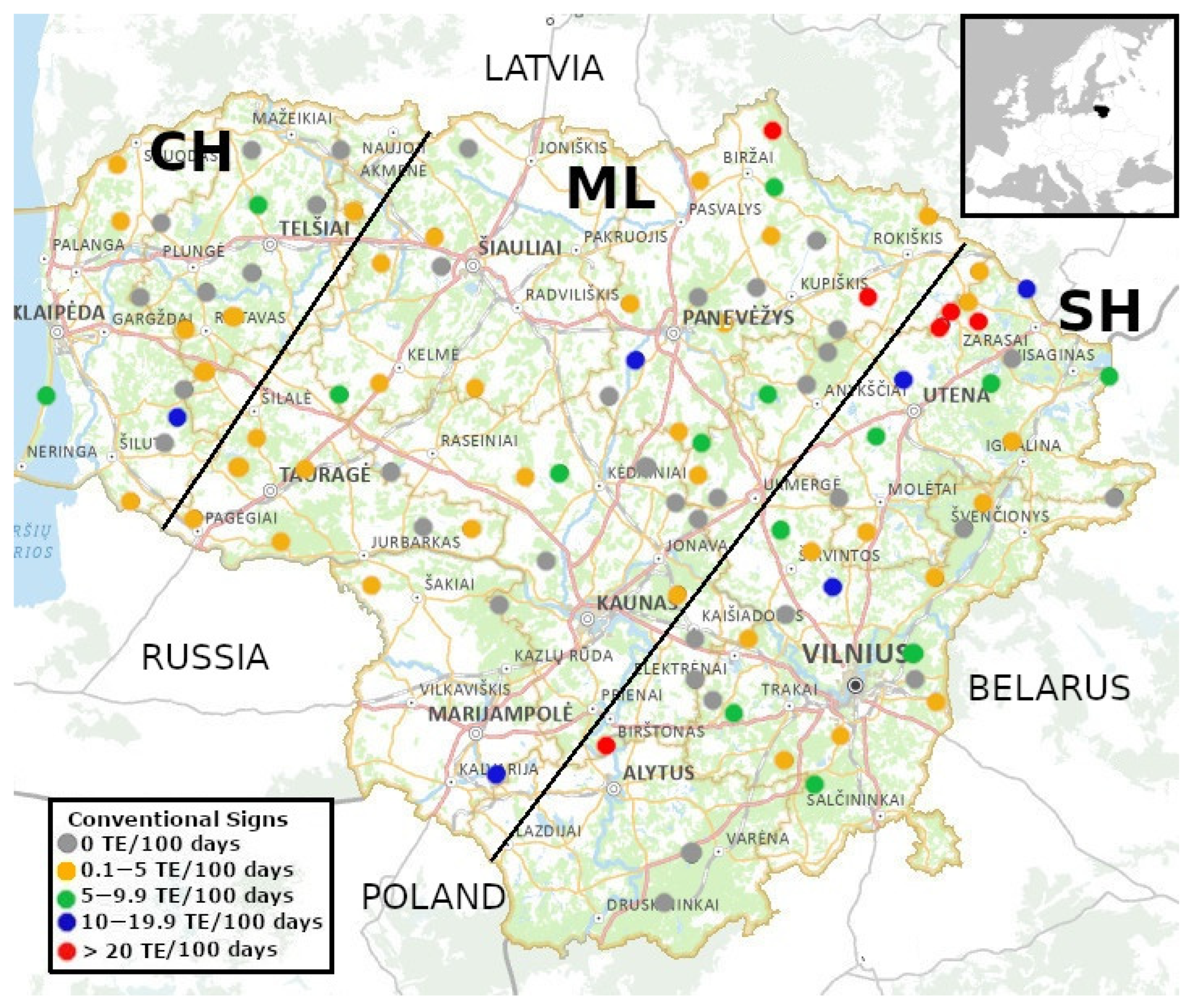
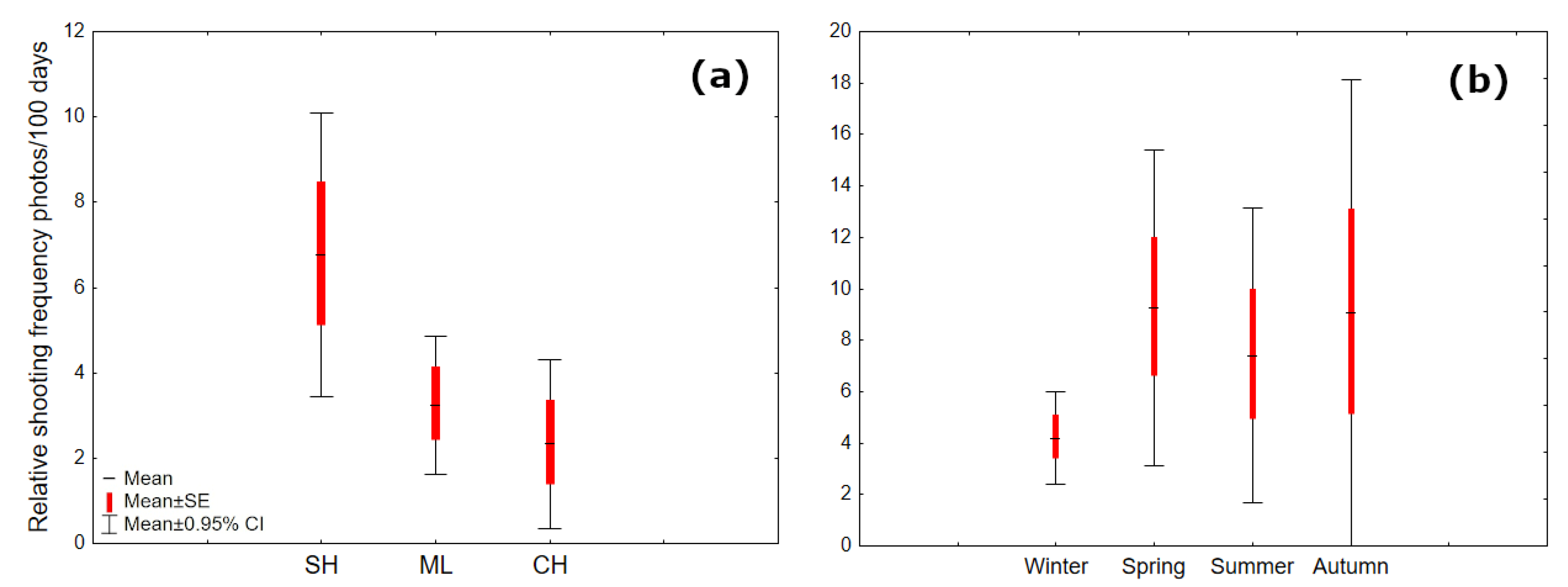
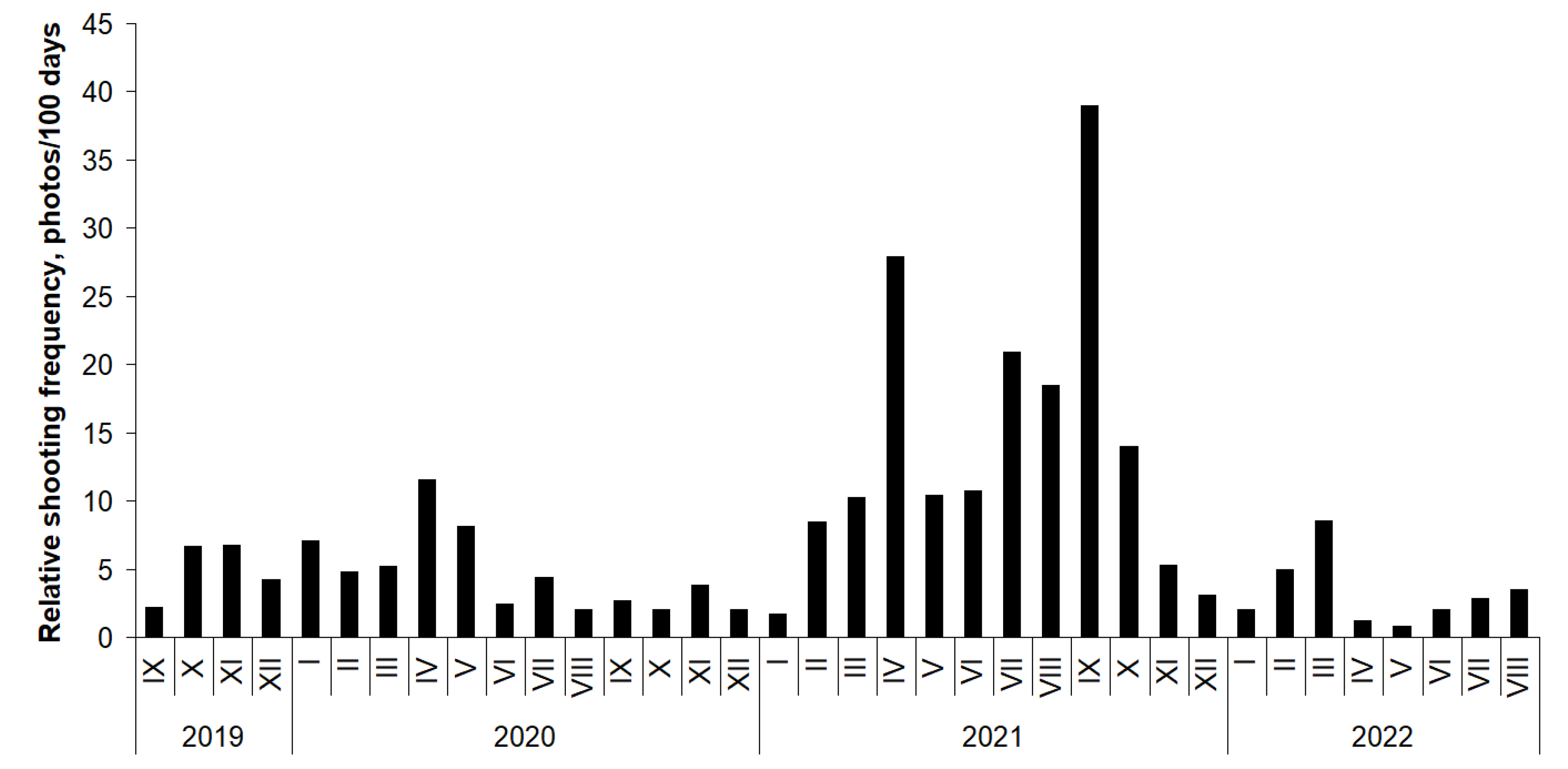
3.1. Distribution and Relative Shooting Frequency of Raccoon Dogs
3.2. Relative Abundance of Raccoon Dogs in Relation to the Presence of Large Carnivores
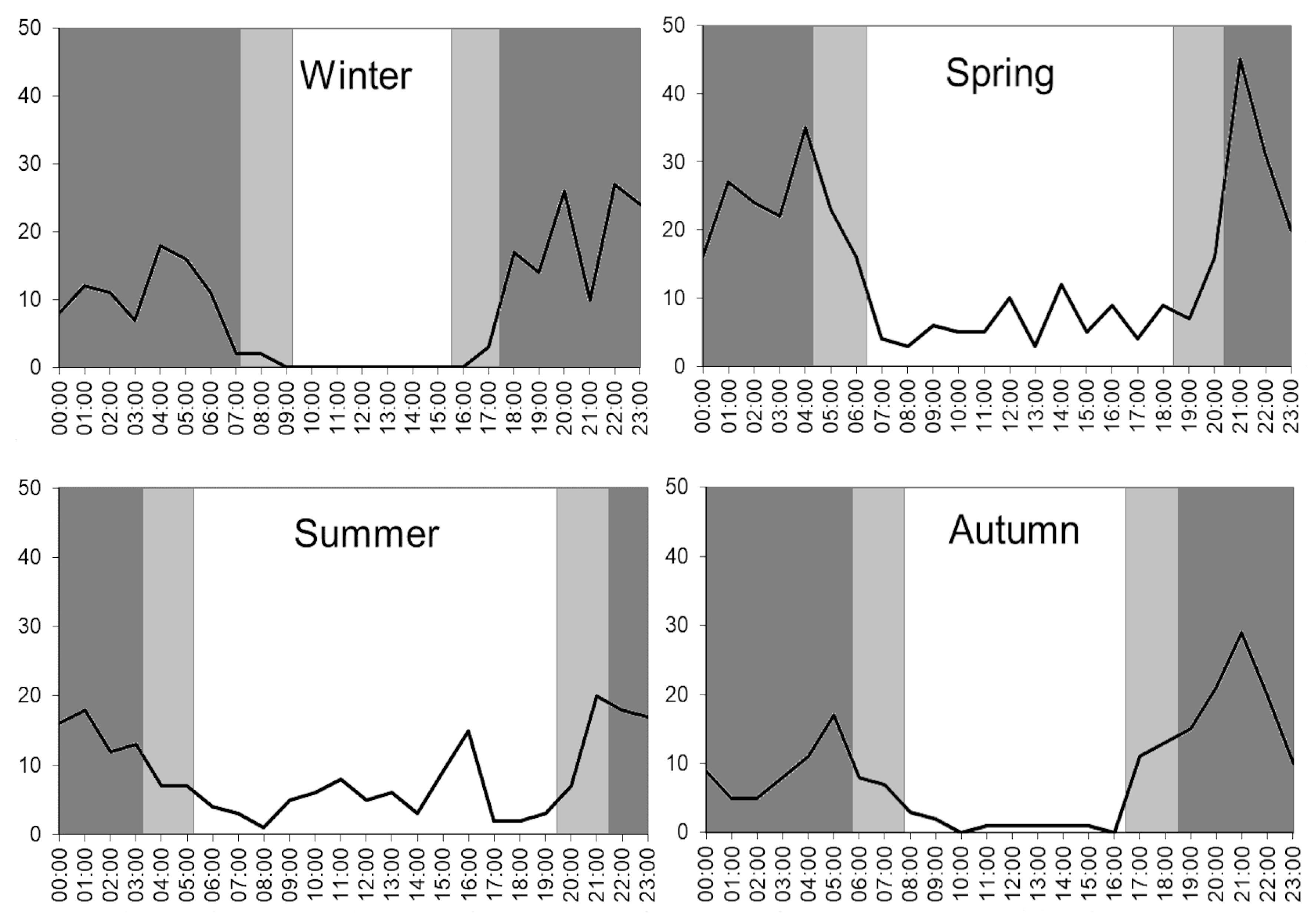
3.3. Seasonal and Daily Activity of Raccoon Dogs
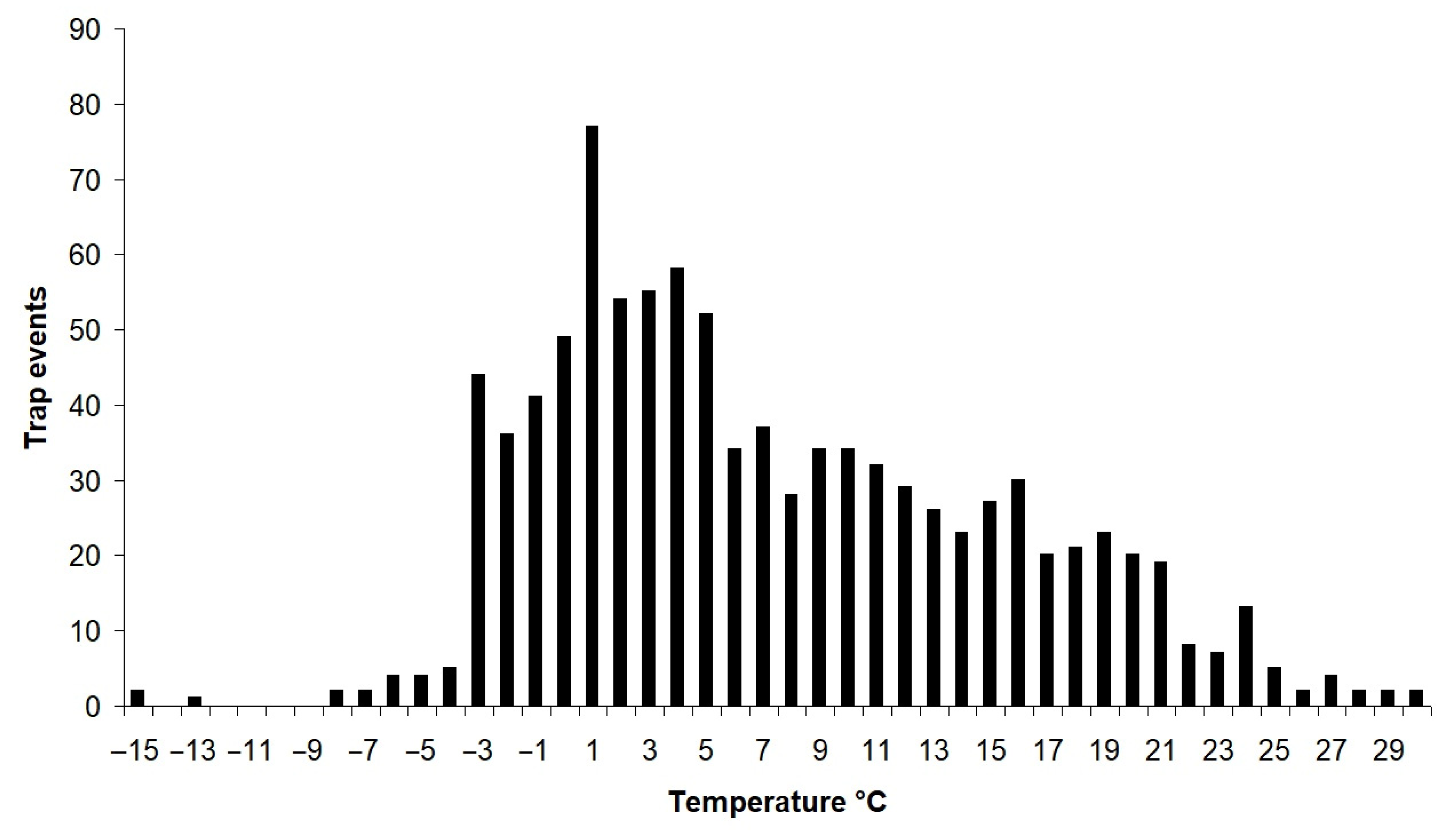
3.4. Activity of Raccoon Dogs Depending on Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tedeschi, L.; Biancolini, D.; Capinha, C.; Rondinini, C.; Essl, F. Introduction, spread, and impacts of invasive alien mammal species in Europe. Mammal. Rev. 2022, 52, 252–266. [Google Scholar] [CrossRef]
- Seebens, H.; Bacher, S.; Blackburn, T.M.; Capinha, C.; Dawson, W.; Dullinger, S.; Genovesi, P.; Hulme, P.H.; Kleunen, M.; Kühn, I.; et al. Projecting the continental accumulation of alien species through to 2050. Glob. Chang. Biol. 2021, 27, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Costello, K.E.; Lynch, S.A.; McAllen, R.; O’Riordan, R.M.; Culloty, S.C. Assessing the potential for invasive species introductions and secondary spread using vessel movements in maritime ports. Mar. Pollut. Bull. 2022, 177, 113496. [Google Scholar] [CrossRef] [PubMed]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Pauchard, A.; Alaback, P.B. Influence of elevation, land use, and landscape context on patterns of alien plant invasions along road-sides in protected areas of south-central Chile. Conserv. Biol. 2004, 18, 238–248. [Google Scholar] [CrossRef]
- Protopopova, V.V.; Shevera, M.V.; Mosyakin, S.L. Deliberate and unintentional introduction of invasive weeds: A case study of the alien flora of Ukraine. Euphytica 2006, 148, 17–33. [Google Scholar] [CrossRef]
- Culley, T.M.; Dreisilker, K.; Ryan, M.C.; Schuler, J.A.; Cavallin, N.; Gettig, R.; Havens, K.; Landel, H.; Shultz, B. The potential role of public gardens as sentinels of plant invasion. Biodivers. Conserv. 2022, 31, 1829–1844. [Google Scholar] [CrossRef]
- Toomes, A.; García-Díaz, P.; Stringham, O.C.; Ross, J.V.; Mitchell, L.; Cassey, P. Drivers of the Australian native pet trade: The role of species traits, socioeconomic attributes and regulatory systems. J. Appl. Ecol. 2022, 59, 1268–1278. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive alien plant species dynamics in the Himalayan region under climate change. Ambio 2018, 47, 697–710. [Google Scholar] [CrossRef]
- Haubrock, P.J.; Cuthbert, R.N.; Sundermann, A.; Diagne, C.; Golivets, M.; Courchamp, F. Economic costs of invasive species in Germany. NeoBiota 2021, 67, 225–246. [Google Scholar] [CrossRef]
- Kauhala, K.; Kowalczyk, R. Invasion of the raccoon dog Nyctereutes procyonoides in Europe: History of colonization, features behind its success, and threats to native fauna. Curr. Zool. 2011, 57, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Prūsaitė, J. Lithuanian Fauna Mammals; Mokslas: Vilnius, Lithuania, 1988; pp. 178–183. [Google Scholar]
- Balčiauskas, L.; Stratford, J.; Balčiauskienė, L.; Kučas, A. Roadkills as a Method to Monitor Raccoon Dog Populations. Animals 2021, 11, 3147. [Google Scholar] [CrossRef] [PubMed]
- Sutor, A.; Kauhala, K.; Ansorge, H. Diet of the raccoon dog Nyctereutes procyonoides—A canid with an opportunistic foraging strategy. Acta. Theriol. 2010, 55, 165–176. [Google Scholar] [CrossRef]
- Dahl, F.; Åhlén, P.A. Nest predation by raccoon dog Nyctereutes procyonoides in the archipelago of northern Sweden. Biol. Invasions 2019, 21, 743–755. [Google Scholar] [CrossRef]
- Myśliwy, I.; Perec-Matysiak, A.; Hildebrand, J. Invasive raccoon (Procyon lotor) and raccoon dog (Nyctereutes procyonoides) as potential reservoirs of tick-borne pathogens: Data review from native and introduced areas. Parasites Vectors 2022, 15, 126. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Asikainen, J.; Kauhala, K.; Paakkonen, T.; Nieminen, P. Seasonal rhythms of body temperature in the free-ranging raccoon dog (Nyctereutes procyonoides) with special emphasis on winter sleep. Chronobiol. Int. 2007, 24, 1095–1107. [Google Scholar] [CrossRef]
- Moran, E.V.; Alexander, J.M. Evolutionary responses to global change: Lessons from invasive species. Ecol. Lett. 2014, 17, 637–649. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Lempiäinen, T.; Aspelund, M.; Hellstedt, P.; Ikonen, K.; Itämies, J.; Vähä, V.; Erkinaro, J.; Asikainen, J.; Kunnasranta, M.; et al. Application of change-point analysis to determine winter sleep patterns of the raccoon dog (Nyctereutes procyonoides) from body temperature recordings and a multi-faceted dietary and behavioral study of wintering. BMC Ecol. 2012, 12, 27. [Google Scholar] [CrossRef]
- Asikainen, J. Wintering Strategy of the Boreal Raccoon Dog (Nyctereutes procyonoides)—Applications to Farming Practice. Ph.D. Thesis, Eastern Finland University, Joensuu, Finland, 2013. [Google Scholar]
- Asikainen, J.; Mustoe, A.M.; Nieminen, P.; Pasanen, S.; Araja-Matilainen, H.; Hyvärinen, H. Reproduction of the raccoon dog (Nyctereutes procyonoides) after feeding or food deprivation in winter. J. Anim. Physiol. An. N. 2003, 86, 367–375. [Google Scholar] [CrossRef]
- Mustonen, A.M.; Nieminen, P. A review of the physiology of a survival expert of big freeze, deep snow, and an empty stomach: The boreal raccoon dog (Nyctereutes procyonoides). J. Comp. Physiol. B 2018, 188, 15–25. [Google Scholar] [CrossRef]
- Dahl, F.; Åhlén, P.A.; Swartström, J.; Lindström, M.; Simmelgaard Platz, M.L. Management of the Invasive Raccoon Dog Nyctereutes procyonoides in the North- European Countries; Swedish Assocation for Hunting and Wildlife Management: Nyköping, Sweden, 2013. [Google Scholar]
- Cheyne, S.M.; Stark, D.J.; Limin, S.H.; Macdonald, D.W. First estimates of population ecology and threats to Sunda clouded leopards Neofelis diardi in a peat-swamp forest, Indonesia. Endanger. Species Res. 2013, 22, 1–9. [Google Scholar] [CrossRef]
- Garrote, G.; Ayala, R.P.; Pereira, P.; Robles, F.; Guzman, N.; García, F.J.; Iglesias, M.C.; Hervás, J.; Fajardo, I.; Simón, M. Estimation of the Iberian lynx population in the Doñana area, SW Spain, using capture-recapture analysis of camera-trapping data. Eur. J. Wildl. Res. 2010, 57, 355–362. [Google Scholar] [CrossRef]
- Jackson, R.M.; Roe, J.D.; Wangchuk, R.; Hunter, D. Estimating snow leopard population abundance using photography and capture-recapture techniques. Wildl. Soc. Bull. 2006, 34, 772–781. [Google Scholar] [CrossRef]
- Karanth, K.U.; Nichols, J.D.; Kumar, N.S.; Hines, J.E. Assessing tiger population dynamics using photographic capture-recapture sampling. Ecology 2006, 87, 2925–2937. [Google Scholar] [CrossRef] [PubMed]
- Zarzo-Arias, A.; Penteriani, V.; Delgado, M.M.; Torre, P.P.; Garcia-Gonzalez, R.; Mateo-Sanchez, M.C.; Garcia, P.V.; Dalerum, F. Identifying potential areas of expansion for the endangered brown bear (Ursus arctos) population in the Cantabrian Mountains (NW Spain). PLoS ONE 2019, 14, e0209972. [Google Scholar] [CrossRef] [PubMed]
- Rovero, F.; Marshall, A.R. Camera trapping photographic rate as an index of density in forest ungulates. J. Appl. Ecol. 2009, 46, 1011–1017. [Google Scholar] [CrossRef]
- Diaz-Ruiz, F.; Caro, J.; Delibes-Mateos, M.; Arroyo, B.; Ferreras, P. Drivers of red fox (Vulpes vulpes) daily activity: Prey availability, human disturbance or habitat structure? J. Zool. 2015, 298, 128–138. [Google Scholar] [CrossRef]
- Sarmento, P.; Cruz, J.; Eira, C.; Fonseca, C. Evaluation of camera trapping for estimating red fox abundance. J. Wild. Manag. 2009, 73, 1207–1212. [Google Scholar] [CrossRef]
- Manzo, E.; Bartolommei, P.; Rowcliffe, J.M.; Cozzolino, R. Estimation of population density of European pine marten in central Italy using camera trapping. Acta Theriol. 2012, 57, 165–172. [Google Scholar] [CrossRef]
- Caravaggi, A.; Gatta, M.; Vallely, M.C.; Hogg, K.; Freeman, M.; Fadaei, E.; Dick, J.T.A.; Montgomery, W.I.; Reid, N.; Tosh, D.G. Seasonal and predator-prey effects on circadian activity of free-ranging mammals revealed by camera traps. PeerJ 2018, 6, e5827. [Google Scholar] [CrossRef]
- Gilbert, N.A.; Clare, J.D.J.; Stenglein, J.L.; Zuckerberg, B. Abundance estimation of unmarked animals based on camera-trap data. Conserav. Biol. 2021, 35, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Bowler, M.T.; Tobler, M.W.; Endress, B.A.; Gilmore, M.P.; Anderson, M.J. Estimating mammalian species richness and occupancy in tropical forest canopies with arboreal camera traps. Remote Sens. Environ. 2017, 3, 146–157. [Google Scholar] [CrossRef]
- Hedwig, D.; Kienast, I.; Bonnet, M.; Curran, B.K.; Courage, A.; Boesch, C.; Kuhl, H.S.; King, T. A camera trap assessment of the forest mammal community within the transitional savannah-forest mosaic of the Bateke Plateau National Park, Gabon. Afr. J. Ecol. 2017, 56, 777–790. [Google Scholar] [CrossRef]
- Wearn, O.R.; Glover-Kapfer, P. Snap happy: Camera traps are an effective sampling tool when compared with alternative methods. R. Soc. Open Sci. 2019, 6, 181748. [Google Scholar] [CrossRef]
- Rovero, F.; Zimmermann, F. Camera Trapping for Wildlife Research; Pelagic Publishing: London, UK, 2016. [Google Scholar]
- Schwemmer, P.; Weiel, S.; Garthe, S. Spatio-temporal movement patterns and habitat choice of red foxes (Vulpes vulpes) and racoon dogs (Nyctereutes procyonoides) along the Wadden Sea coast. Eur. J. Wildl. Res. 2021, 67, 49. [Google Scholar] [CrossRef]
- Ogurtsov, S.S.; Zheltukhin, A.S.; Kotlov, I.P. Daily activity patterns of large and medium-sized mammals based on camera traps data in the Central Forest Nature Reserve, Valdai Upland, Russia. Nat. Conserv. Res. 2018, 3, 68–88. [Google Scholar] [CrossRef]
- Kondo, A. Interspecific burrow sharing between mammals in countryside in Japan. Mamm. Study 2018, 43, 213–218. [Google Scholar] [CrossRef]
- Nowakowski, K.; Ważna, A.; Kurek, P.; Cichocki, J.; Gabryś, G. Reproduction success in European badgers, red foxes and raccoon dogs in relation to sett cohabitation. PLoS ONE 2020, 15, e0237642. [Google Scholar] [CrossRef]
- Iwiński, M.; Zydroń, A.; Chwiałkowski, C.; Dąbrowski, T. The Use of a Camera Trap System for Monitoring the Movement of Forest Animals Through the Wildlife Crossing in Napchanie. Rocz. Ochr. Sr. 2019, 21, 157–166. [Google Scholar]
- Galvonaitė, A.; Valiukas, D.; Kilpys, J.; Kitrienė, Z.; Misiūnienė, M. Climate Atlas of Lithuania; Lithuanian Hydrometeorological Service under the Ministry of Environment: Vilnius, Lithuania, 2013; pp. 1–175. [Google Scholar]
- Kelly, M.J.; Holub, E.L. Camera Trapping of Carnivores: Trap Success Among Camera Types and Across Species, and Habitat Selection by Species, on Salt Pond Mountain, Giles County, Virginia. Northeast Nat. 2008, 15, 249–262. [Google Scholar] [CrossRef]
- Manly, B.F.J.; McDonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals: Statistical Design and Analysis for Field Studies, 2nd ed.; Springer: Dordrecht, The Netherlands, 2002; p. 221. [Google Scholar] [CrossRef]
- Kauhala, K. Introduced carnivores in Europe with special reference to central and northern Europe. Wildl. Biol. 1996, 2, 197–204. [Google Scholar] [CrossRef]
- Drygala, F.; Stier, N.; Zoller, H.; Bögelsack, K.; Mix, H.; Roth, M. Habitat use of the raccoon dog (Nyctereutes procyonoides) in north-eastern Germany. Mamm. Biol. 2008, 73, 371–378. [Google Scholar] [CrossRef]
- Kauhala, K.; Autila, M. Habitat preferences of the native badger and the invasive raccoon dog in southern Finland. Acta Theriol. 2010, 55, 231–240. [Google Scholar] [CrossRef]
- Saeki, M.; Johnson, P.J.; Macdonald, D.W. Movements and Habitat Selection of Raccoon Dogs (Nyctereutes procyonoides) in a Mosaic Landscape. J. Mammal. 2007, 88, 1098–1111. [Google Scholar] [CrossRef]
- Kowalczyk, R.; Zalewski, A.; Jędrzejewska, B.; Ansorge, H.; Bunevich, A.N. Reproduction and mortality of invasive raccoon dogs (Nyctereutes procyonoides) in the Białowieża Primeval Forest (eastern Poland). Ann. Zool. Fenn. 2009, 46, 291–301. [Google Scholar] [CrossRef]
- Kauhala, K.; Laukkanen, P.; Rége, I. Summer food composition and food niche overlap of the raccoon dog, red fox and badger in Finland. Ecography 1998, 21, 457–463. [Google Scholar] [CrossRef]
- Baltrūnaitė, L. Diet and Winter Habitat use of the Red Fox, Pine Marten and Raccoon Dog in Dzūkija National Park, Lithuania. Acta Zool. Litu. 2006, 16, 46–53. [Google Scholar] [CrossRef]
- Takatsuki, S.; Inaba, M.; Hashigoe, K.; Matsui, H. Opportunistic food habits of the raccoon dog–a case study on Suwazaki Peninsula, Shikoku, western Japan. Mamm. Study 2020, 46, 25–32. [Google Scholar] [CrossRef]
- Bahlke, S.Ø.; Nash, D.R.; Pedersen, J.S.; Hansen, M.S.; Chriél, M.; Pagh, S. Detecting bird’s eggs in the diet of raccoon dog (Nyctereutes procyonoides). Genet. Biodiv. J. 2021, 5, 127–139. [Google Scholar] [CrossRef]
- Sidorovich, V.E.; Solovej, I.A.; Sidorovich, A.A.; Dyman, A.A. Seasonal and annual variation in the diet of the raccoon dog Nyctereutes procyonoides in northern Belarus: The role of habitat type and family group. Acta Theriol. 2008, 53, 27–38. [Google Scholar] [CrossRef]
- Drygala, F.; Ulrike, W.; Hinrich, Z. Diet composition of the invasive raccoon dog (Nyctereutes procyonoides) and the native red fox (Vulpes vulpes) in north-east Germany. Hystrix 2013, 24, 190–194. [Google Scholar] [CrossRef]
- Selonen, V.; Brommer, J.E.; Holopainen, S.; Kauhala, K.; Krüger, H.; Poutanen, J.; Väänänen, M.V.; Laaksonen, T. Invasive species control with apex predators: Increasing presence of wolves is associated with reduced occurrence of the alien raccoon dog. Biol. Invasions 2022, 24, 3461–3474. [Google Scholar] [CrossRef]
- Diserens, T.A.; Churski, M.; Bubnicki, J.W.; Zalewski, A.; Brzeziński, M.; Kuijper, D.P.J. Wolf risk fails to inspire fear in two mesocarnivores suggesting facilitation prevails. Sci. Rep. 2022, 12, 16469. [Google Scholar] [CrossRef]
- Selva, N.; Jȩdrzejewska, B.; Jȩdrzejewski, W.; Wajrak, A. Factors affecting carcass use by a guild of scavengers in European temperate woodland. Can. J. Zool. 2005, 83, 1590–1601. [Google Scholar] [CrossRef]
- Prugh, L.R.; Sivy, K.J. Enemies with benefits: Integrating positive and negative interactions among terrestrial carnivores. Ecol. Lett. 2020, 23, 902–918. [Google Scholar] [CrossRef]
- Zoller, H.; Drygala, F. Activity patterns of the invasive raccoon dog (Nyctereutes procyonoides) in North East Germany. Folia Zool. 2013, 62, 290–296. [Google Scholar] [CrossRef]
- Ikeda, T.; Uchida, K.; Matsuura, Y.; Takahashi, H.; Yoshida, T.; Kaji, K.; Koizumi, I. Seasonal and diel activity patterns of eight sympatric mammals in northern Japan revealed by an intensive camera-trap survey. PLoS ONE 2016, 11, e0163602. [Google Scholar] [CrossRef]
- Mulder, J.L. A review of the ecology of the raccoon dog (Nyctereutes procyonoides) in Europe. Lutra 2012, 55, 101–127. [Google Scholar]
- Jasiulionis, M.; Balčiauskas, L. Seasonal and daily activity patterns of mammals in the colony of great cormorants. Mammalia 2021, 85, 439–447. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Balčiauskiene, L.; Trakimas, G. Beaver influence on amphibian breeding in the agrolandscape. In The European Beaver in a New Millennium, Proceedings of 2nd European Beaver Symposium, Bialowieza, Poland, 27–30 September 2000; Carpathian Heritage Society: Krakow, Poland, 2001. [Google Scholar]
- Shibata, F.; Kawamichi, T. Decline of raccoon dog populations resulting from sarcoptic mange epizootics. Mammalia 1999, 63, 281–290. [Google Scholar] [CrossRef]
- Yoshikazu, S.; Masaaki, K. Does sika deer overabundance exert cascading effects on the raccoon dog population? J. Forest Res. 2013, 18, 121–127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jasiulionis, M.; Stirkė, V.; Balčiauskas, L. The Distribution and Activity of the Invasive Raccoon Dog in Lithuania as Found with Country-Wide Camera Trapping. Forests 2023, 14, 1328. https://doi.org/10.3390/f14071328
Jasiulionis M, Stirkė V, Balčiauskas L. The Distribution and Activity of the Invasive Raccoon Dog in Lithuania as Found with Country-Wide Camera Trapping. Forests. 2023; 14(7):1328. https://doi.org/10.3390/f14071328
Chicago/Turabian StyleJasiulionis, Marius, Vitalijus Stirkė, and Linas Balčiauskas. 2023. "The Distribution and Activity of the Invasive Raccoon Dog in Lithuania as Found with Country-Wide Camera Trapping" Forests 14, no. 7: 1328. https://doi.org/10.3390/f14071328
APA StyleJasiulionis, M., Stirkė, V., & Balčiauskas, L. (2023). The Distribution and Activity of the Invasive Raccoon Dog in Lithuania as Found with Country-Wide Camera Trapping. Forests, 14(7), 1328. https://doi.org/10.3390/f14071328






