Si Supply Could Alter N Uptake and Assimilation of Saplings—A 15N Tracer Study of Four Subtropical Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Growth Conditions, and Experimental Design
2.2. Plant Samplings and Biomass
2.3. Chemical Analyses
2.4. Calculations
2.5. Statistical Analysis
3. Results
3.1. Plant and Soil Properties
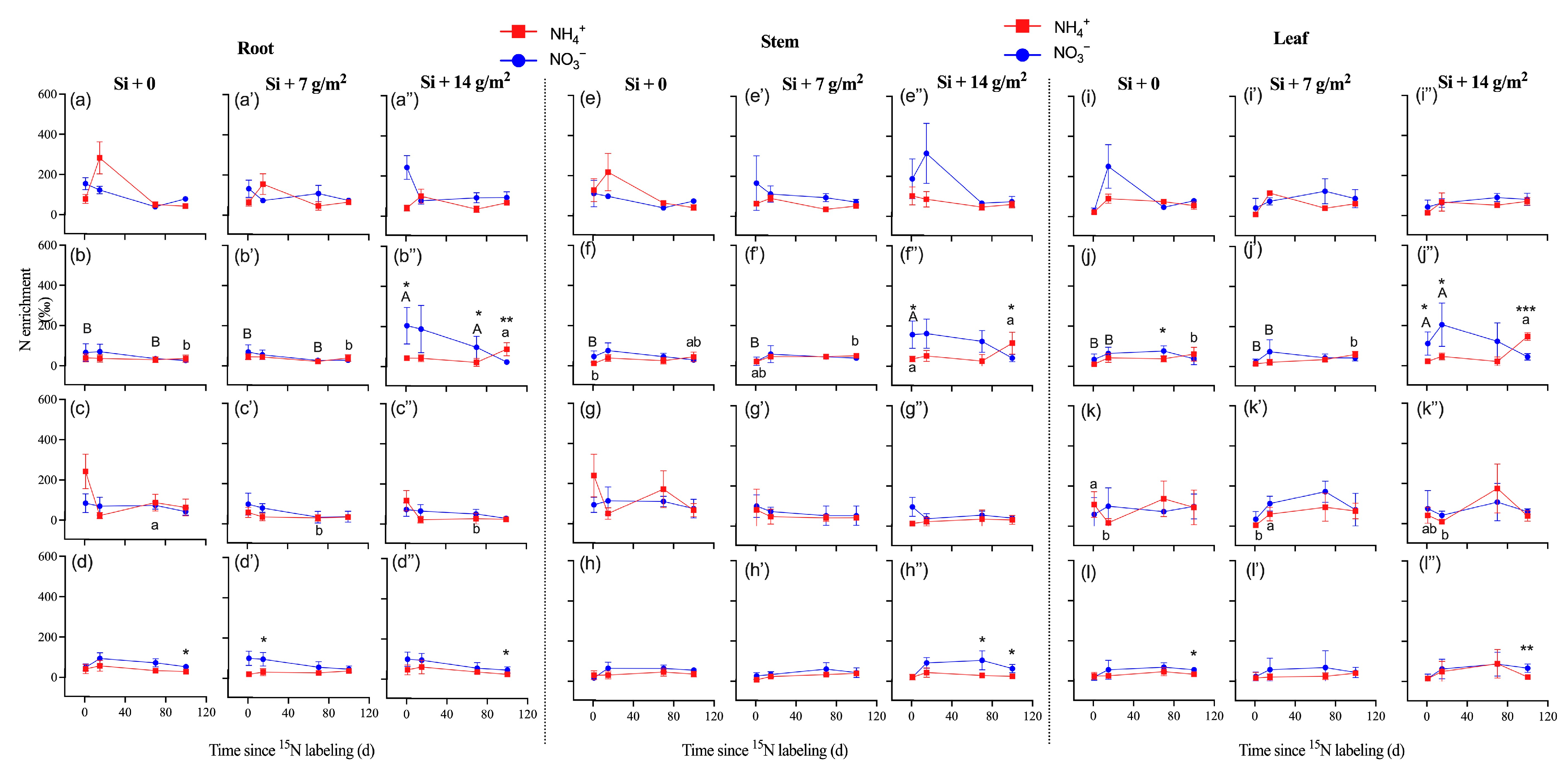
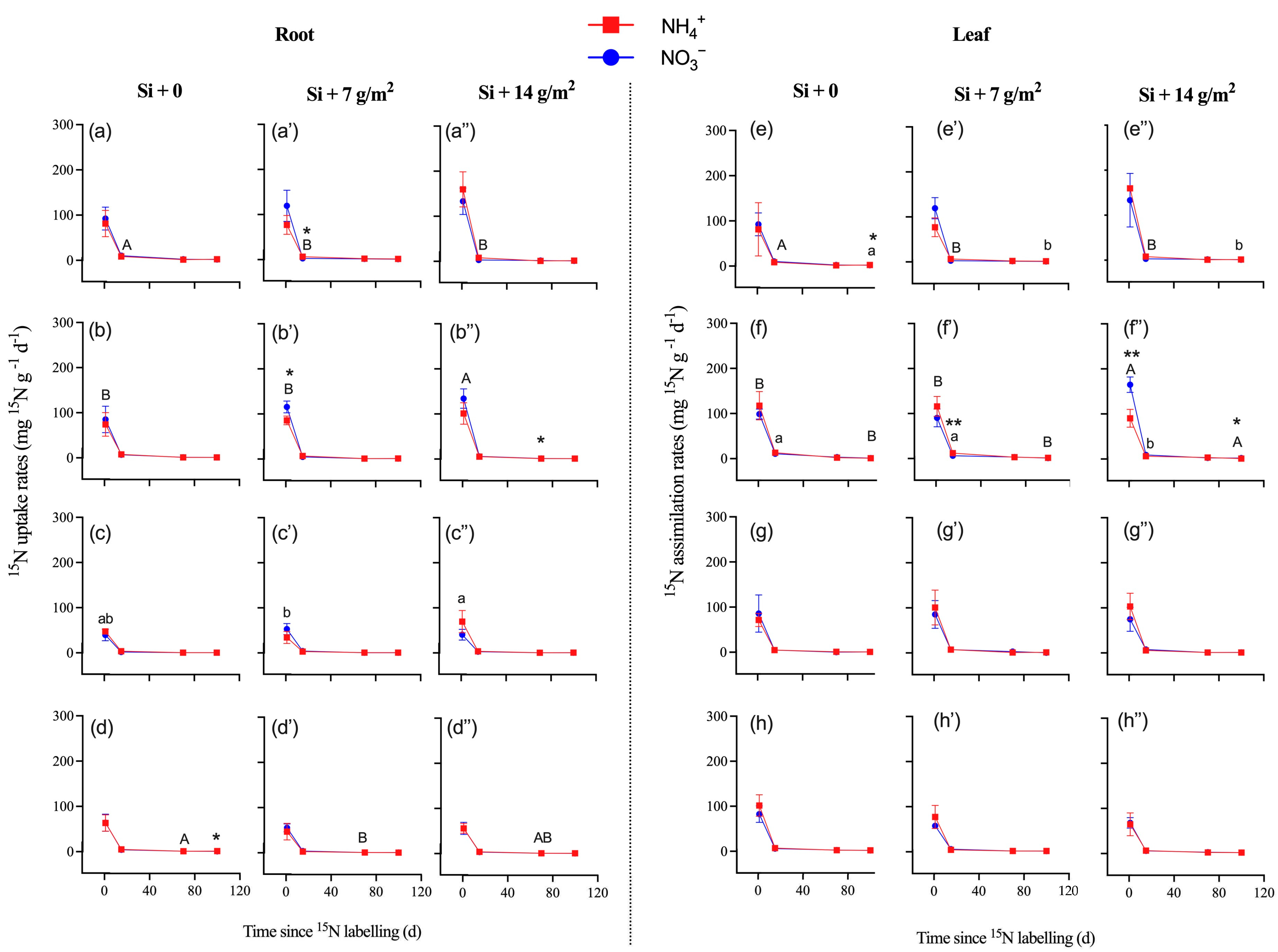
3.2. 15N Enrichment, Uptake, and Assimilation of Saplings
4. Discussion
4.1. Effects of Si Supply on Plant Properties
4.2. Effects of Si Supply on Plants’ N Uptake and Assimilation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deshmukh, R.; Sonah, H.; Belanger, R.R. New evidence defining the evolutionary path of aquaporins regulating silicon uptake in land plants. J. Exp. Bot. 2020, 71, 6775–6788. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, R.; Thakral, V.; Raturi, G.; Shinde, S.; Nikolić, M.; Tripathi, D.K.; Sonah, H.; Deshmukh, R. Significance of silicon uptake, transport, and deposition in plants. J. Exp. Bot. 2020, 71, 6703–6718. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Song, Z.; Liu, H.; Van Zwieten, L.; Song, A.; Li, Z.; Hao, Q.; Zhang, X.; Wang, H. Phytolith accumulation in broadleaf and conifer forests of northern China: Implications for phytolith carbon sequestration. Geoderma 2018, 312, 36–44. [Google Scholar] [CrossRef]
- Liu, X.; Fang, P.; Xiong, Y.; Peng, Q.; Yu, Z.; Luan, F.; Song, Q.; Fang, X.; Yang, Q.; Liu, J. Assessment of the influence of bamboo expansion on Si pools and fluxes in a disturbed subtropical evergreen broadleaved forest. Catena 2022, 213, 106136. [Google Scholar] [CrossRef]
- Cornelis, J.-T.; Delvaux, B.; Titeux, H. Contrasting silicon uptakes by coniferous trees: A hydroponic experiment on young seedlings. Plant Soil 2010, 336, 99–106. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N. Silicon uptake and accumulation in higher plants. Trends Plant Sci. 2006, 11, 392–397. [Google Scholar] [CrossRef]
- Neu, S.; Schaller, J.; Dudel, E.G. Silicon availability modifies nutrient use efficiency and content, C:N:P stoichiometry, and productivity of winter wheat (Triticum aestivum L.). Sci. Rep. 2017, 7, 40829. [Google Scholar] [CrossRef] [Green Version]
- Schaller, J.; Brackhage, C.; Gessner, M.O.; Bäuker, E.; Dudel, E.G. Silicon supply modifies C:N:P stoichiometry and growth of Phragmites australis. Plant Biol. 2012, 14, 392–396. [Google Scholar] [CrossRef]
- Teixeira, G.C.M.; Prado, R.D.M.; Rocha, A.M.S.; Piccolo, M.D.C. Root- and foliar-applied silicon modifies C:N:P ratio and increases the nutritional efficiency of pre-sprouted sugarcane seedlings under water deficit. PLoS ONE 2020, 15, e0240847. [Google Scholar] [CrossRef]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Burger, M.; Jackson, L.E. Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol. Biochem. 2003, 35, 29–36. [Google Scholar] [CrossRef]
- Javaid, A. Arbuscular Mycorrhizal Mediated Nutrition in Plants. J. Plant Nutr. 2009, 32, 1595–1618. [Google Scholar] [CrossRef]
- Greger, M.; Landberg, T.; Vaculík, M. Silicon Influences Soil Availability and Accumulation of Mineral Nutrients in Various Plant Species. Plants 2018, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-C.; Song, Z.-L.; Yang, X.-M.; Song, A.-L.; Yu, C.-X.; Wang, T.; Xia, S.; Liang, Y.-C. Impacts of silicon on biogeochemical cycles of carbon and nutrients in croplands. J. Integr. Agric. 2018, 17, 2182–2195. [Google Scholar] [CrossRef]
- Liu, H.; Shen, X.; Guo, Z. Effects of silicon addition on seed germination and seedling growth of alfalfa. Acta Prataculturae Sin. 2012, 20, 155–160. [Google Scholar]
- Song, Q.-N.; Ouyang, M.; Yang, Q.-P.; Lu, H.; Yang, G.-Y.; Chen, F.-S.; Shi, J.-M. Degradation of litter quality and decline of soil nitrogen mineralization after moso bamboo (Phyllostachys pubscens) expansion to neighboring broadleaved forest in subtropical China. Plant Soil 2016, 404, 113–124. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Deng, B.; Liu, Y.; Kong, F.; Huang, G.; Zou, Q.; Liu, Q.; Guo, X.; Fu, Y.; et al. Effects of moso bamboo (Phyllostachys edulis) invasions on soil nitrogen cycles depend on invasion stage and warming. Environ. Sci. Pollut. Res. 2017, 24, 24989–24999. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.-N.; Lu, H.; Liu, J.; Yang, J.; Yang, G.-Y.; Yang, Q.-P. Accessing the impacts of bamboo expansion on NPP and N cycling in evergreen broadleaved forest in subtropical China. Sci. Rep. 2017, 7, 40383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Huang, X.; Shi, W.; Kronzucker, H.J.; Hou, L.; Yang, H.; Song, Q.; Liu, J.; Shi, J.; Yang, Q.; et al. Coordination of nitrogen uptake and assimilation favours the growth and competitiveness of moso bamboo over native tree species in high-NH4+ environments. J. Plant Physiol. 2021, 266, 153508. [Google Scholar] [CrossRef]
- de Carvalho, J.S.; Frazão, J.J.; Prado, R.D.M.; Júnior, J.P.D.S.; Costa, M.G. Silicon modifies C:N:P stoichiometry and improves the physiological efficiency and dry matter mass production of sorghum grown under nutritional sufficiency. Sci. Rep. 2022, 12, 16082. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yu, Y.; Baerson, S.R.; Song, Y.; Liang, G.; Ding, C.; Niu, J.; Pan, Z.; Zeng, R. Interactions between nitrogen and silicon in rice and their effects on resistance toward the brown planthopper Nilaparvata lugens. Front. Plant Sci. 2017, 8, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.F.; Yamaji, N. A cooperative system of silicon transport in plants. Trends Plant Sci. 2015, 20, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Coskun, D.; Deshmukh, R.; Sonah, H.; Menzies, J.G.; Reynolds, O.; Ma, J.F.; Kronzucker, H.J.; Bélanger, R.R. The controversies of silicon’s role in plant biology. New Phytol. 2019, 221, 67–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovic, J.; Samardzic, J.; Maksimović, V.; Timotijevic, G.; Stevic, N.; Laursen, K.H.; Hansen, T.H.; Husted, S.; Schjoerring, J.K.; Liang, Y.; et al. Silicon alleviates iron deficiency in cucumber by promoting mobilization of iron in the root apoplast. New Phytol. 2013, 198, 1096–1107. [Google Scholar] [CrossRef]
- Lu, R. Methods of Soil and Agrochemical Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Zhou, X.; Wang, A.; Hobbie, E.A.; Zhu, F.; Qu, Y.; Dai, L.; Li, D.; Liu, X.; Zhu, W.; Keisuke, K. Mature conifers assimilate nitrate as efficiently as ammonium from soils in four forest plantations. New Phytol. 2021, 229, 3184–3194. [Google Scholar] [CrossRef]
- Reza Ramdan, R.; Takuji, M.; Tatsuya, A.; Arata, Y.; Shuoye, C.; Junji, S.; Yuki, T.; Toshiaki, U.; Masaru, K. Limiting silicon supply alters lignin content and structures of sorghum seedling cell walls. Plant Sci. 2022, 321, 111325. [Google Scholar]
- Klotzbücher, T.; Marxen, A.; Jahn, R.; Vetterlein, D. Silicon cycle in rice paddy fields: Insights provided by relations between silicon forms in topsoils and plant silicon uptake. Nutr. Cycl. Agroecosystems 2016, 105, 157–168. [Google Scholar] [CrossRef]
- Sauer, D.; Saccone, L.; Conley, D.J.; Herrmann, L.; Sommer, M. Review of methodologies for extracting plant-available and amorphous Si from soils and aquatic sediments. Biogeochemistry 2006, 80, 89–108. [Google Scholar] [CrossRef]
- Georgiadis, A.; Sauer, D.; Herrmann, L.; Breuer, J.; Zarei, M.; Stahr, K. Development of a method for sequential Si extraction from soils. Geoderma 2013, 209, 251–261. [Google Scholar] [CrossRef]
- Song, Z.; Liu, H.; Li, B.; Yang, X. The production of phytolith-occluded carbon in China’s forests: Implications to biogeochemical carbon sequestration. Glob. Chang. Biol. 2013, 19, 2907–2915. [Google Scholar] [CrossRef]
- Fry, B. Stable Isotope Ecology; Springer: New York, NY, USA, 2006. [Google Scholar]
- Friedrich, U.; Falk, K.; Bahlmann, E.; Marquardt, T.; Meyer, H.; Niemeyer, T.; Schemmel, S.; von Oheimb, G.; Härdtle, W. Fate of airborne nitrogen in heathland ecosystems: A 15N tracer study. Glob. Chang. Biol. 2011, 17, 1549–1559. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Yu, C.; Qiao, N.; Xu, X.; Tian, Y.; Ouyang, H. Plant preference for NH4+ versus NO3—At different growth stages in an alpine agroecosystem. Field Crop. Res. 2017, 201, 192–199. [Google Scholar] [CrossRef]
- Sommer, M.; Kaczorek, D.; Kuzyakov, Y.; Breuer, J. Silicon pools and fluxes in soils and landscapes—A review. J. Plant Nutr. Soil Sci. 2006, 169, 310–329. [Google Scholar] [CrossRef]
- Yang, X.; Song, Z.; Yu, C.; Ding, F. Quantification of different silicon fractions in broadleaf and conifer forests of northern China and consequent implications for biogeochemical Si cycling. Geoderma 2020, 361, 114036. [Google Scholar] [CrossRef]
- Vandegeer, R.K.; Cibils-Stewart, X.; Wuhrer, R.; Hartley, S.E.; Tissue, D.T.; Johnson, S.N. Leaf silicification provides herbivore defence regardless of the extensive impacts of water stress. Funct. Ecol. 2021, 35, 1200–1211. [Google Scholar] [CrossRef]
- Katz, O.; Puppe, D.; Kaczorek, D.; Prakash, N.B.; Schaller, J. Silicon in the Soil–Plant Continuum: Intricate Feedback Mechanisms within Ecosystems. Plants 2021, 10, 652. [Google Scholar] [CrossRef]
- Song, Z.; Müllerb, K.; Yanga, X. Silicon regulation of soil organic carbon stabilization and its potential tomitigate climate change. Earth-Sci. Rev. 2018, 185, 463–475. [Google Scholar] [CrossRef]
- Johnson, S.N.; Waterman, J.M.; Wuhrer, R.; Rowe, R.C.; Hall, C.R.; Cibils-Stewart, X. Siliceous and non-nutritious: Nitrogen limitation increases anti-herbivore silicon defences in a model grass. J. Ecol. 2021, 109, 3767–3778. [Google Scholar] [CrossRef]
- Ryalls, J.M.W.; Moore, B.D.; Johnson, S.N. Silicon uptake by a pasture grass experiencing simulated grazing is greatest under elevated precipitation. BMC Ecol. 2018, 18, 53. [Google Scholar] [CrossRef] [Green Version]
- Schoelynck, J.; Bal, K.; Backx, H.; Okruszko, T.; Meire, P.; Struyf, E. Silica uptake in aquatic and wetland macrophytes: A strategic choice between silica, lignin and cellulose? New Phytol. 2010, 186, 385–391. [Google Scholar] [CrossRef]
- Bin, Z.; Zhang, R.; Zhang, W.; Xu, D. Effects of nitrogen, phosphorus and silicon addition on leaf carbon, nitrogen, and phosphorus concentration of Elymus nutans of alpine meadow on Qinghai-Tibetan Plateau, China. Acta Ecol. Sin. 2015, 35, 4699–4706. [Google Scholar]
- Li, Z.-J.; Lin, P.; He, J.-Y.; Yang, Z.-W.; Lin, Y.-M. Silicon’s organic pool and biological cycle in moso bamboo community of Wuyishan Biosphere Reserve. J. Zhejiang Univ. B 2006, 7, 849–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Yang, Q.P.; Song, Q.N.; Yu, D.K.; Shi, J.M. Strategy of fine root expansion of Phyllostachys pubescens population into evergreen broadleaved forest. Chin. J. Plant Ecol. 2013, 37, 230–238. [Google Scholar] [CrossRef]
- Houlton, B.Z.; Sigman, D.M.; Schuur, E.A.G.; Hedin, L.O. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proc. Natl. Acad. Sci. USA 2007, 104, 8902–8906. [Google Scholar] [CrossRef] [Green Version]
- McKane, R.B.; Johnson, L.C.; Shaver, G.R.; Nadelhoffer, K.J.; Rastetter, E.B.; Fry, B.; Giblin, A.E.; Kielland, K.; Kwiatkowski, B.L.; Laundre, J.A.; et al. Resource-based niches provide a basis for plant species diversity and dominance in arctic tundra. Nature 2002, 415, 68–71. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Chang, S.X.; Xu, Q.; Li, Y.; Ma, Z.; Qin, H.; Cai, Y. Linking enhanced soil nitrogen mineralization to increased fungal decomposition capacity with Moso bamboo invasion of broadleaf forests. Sci. Total. Environ. 2021, 771, 144779. [Google Scholar] [CrossRef]
- Miao, D.; Peng, X.; Teng, Q.; Sardar, M.F.; Zhang, Q.; Zhu, X.; Li, Y. Different contributions of bacterial and fungal communities to nitrogen mineralization in Moso bamboo-invaded subtropical forests. J. Soils Sediments 2023, 23, 1123–1134. [Google Scholar] [CrossRef]
- Ikegami, N.; Satake, T.; Nagayama, Y.; Inubushi, K. Changes in silica in litterfall and available silica in the soil of forests invaded by bamboo species (Phyllostachys pubescens and P. bambusoides) in western Japan. Soil Sci. Plant Nutr. 2014, 60, 731–739. [Google Scholar] [CrossRef]
- Ikegami, N.; Satake, T.; Nagayama, Y.; Inubushi, K. Biogeochemical Si cycling in bamboo forests with evergreen broad-leaved forest and coniferous forest in a temperate climate. Jpn. J. For. Environ. 2015, 57, 7–17. [Google Scholar]
- Zou, N.; Shi, W.; Hou, L.; Kronzucker, H.J.; Huang, L.; Gu, H.; Yang, Q.; Deng, G.; Yang, G. Superior growth, N uptake and NH4+ tolerance in the giant bamboo Phyllostachys edulis over the broad-leaved tree Castanopsis fargesii at elevated NH4+ may underlie community succession and favor the expansion of bamboo. Tree Physiol. 2020, 40, 1606–1622. [Google Scholar] [CrossRef] [PubMed]

| Elements | Si Treatment | P. pubescens | P. bournei | S. superba | C. lanceolata | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | Leaf | Stem | Root | ||
| Si (g kg−1) | Si + 0 | 28.0 b | 17.2 b | 30.9 b | 6.2 b | 1.7 | 5.3 b | 4.2 | 2.7 | 13.1 | 4.8 | 2.9 a | 14.8 a |
| Si + 7 g/m2 | 28.7 b | 19.2 ab | 36.3 ab | 10.0 a | 2.2 | 10.0 ab | 4.8 | 2.6 | 9.5 | 3.8 | 1.8 b | 7.7 b | |
| Si + 14 g/m2 | 37.0 a | 21.8 a | 44.8 a | 9.3 a | 2.4 | 16.9 a | 4.7 | 2.3 | 9.7 | 6.6 | 1.4 b | 5.7 b | |
| C (%) | Si + 0 | 42.4 a | 45.7 a | 37.1 a | 46.9 | 45.3 | 45.2 | 46.0 | 46.4 | 44.9 | 45.4 | 45.7 | 44.7 |
| Si + 7 g/m2 | 41.5 ab | 42.7 b | 37.2 a | 46.7 | 45.2 | 45.6 | 46.5 | 45.5 | 44.8 | 45.8 | 45.6 | 45.2 | |
| Si + 14 g/m2 | 41.5 b | 42.7 b | 35.1 b | 46.7 | 45.8 | 45.3 | 46.4 | 45.2 | 44.6 | 45.3 | 45.6 | 45.6 | |
| N (%) | Si + 0 | 1.82 | 0.39 | 0.63 | 1.75 a | 0.42 | 1.30 ab | 1.68 a | 0.52 | 0.67 | 1.22 | 0.51 | 0.72 |
| Si + 7 g/m2 | 1.78 | 0.43 | 0.60 | 1.52 b | 0.45 | 1.46 a | 1.76 a | 0.55 | 0.72 | 1.26 | 0.54 | 0.78 | |
| Si + 14 g/m2 | 1.79 | 0.41 | 0.55 | 1.49 b | 0.41 | 1.22 b | 1.32 b | 0.51 | 0.80 | 1.31 | 0.51 | 0.71 | |
| P (g kg−1) | Si + 0 | 0.79 | 0.71 | 0.52 | 0.62 | 0.71 | 0.71 | 0.69 | 0.36 | 0.69 | 0.74 | 0.51 | 0.40 |
| Si + 7 g/m2 | 0.57 | 0.35 | 0.53 | 0.52 | 0.35 | 0.69 | 0.73 | 0.52 | 0.84 | 0.66 | 0.46 | 0.39 | |
| Si + 14 g/m2 | 0.62 | 0.61 | 0.53 | 0.48 | 0.61 | 0.71 | 0.71 | 0.28 | 0.70 | 0.78 | 0.59 | 0.36 | |
| Tissue | Species | 1 d | 15 d | 75 d | 100 d | ||||
|---|---|---|---|---|---|---|---|---|---|
| 15NH4NO3 | NH415NO3 | 15NH4NO3 | NH415NO3 | 15NH4NO3 | NH415NO3 | 15NH4NO3 | NH415NO3 | ||
| Root | Phyllostachys pubescens | 93.0 (20.6) a | 114.2 (17.2) a | 7.5 (1.5) a | 5.0 (1.5) ab | 1.6 (0.3) a | 2.0 (0.3) a | 1.8 (0.3) a | 1.3 (0.2) a |
| Phoebe bournei | 100.4 (9.8) a | 78.7 (7.8) b | 6.7 (0.5) a | 5.9 (0.4) a | 1.1 (0.1) b | 1.2 (0.1) b | 1.1 (0.1) b | 1.0 (0.1) a | |
| Schima superba | 50.3 (6.1) b | 44.3 (7.1) c | 3.4 (0.4) b | 2.9 (0.4) c | 0.5 (0.1) c | 0.4 (0.1) c | 0.6 (0.1) c | 0.5 (0.1) b | |
| Cunninghamia lanceolata | 55.3 (5.4) b | 58.3 (5.1) bc | 3.9 (0.5) b | 3.8 (0.4) bc | 0.8 (0.1) bc | 0.9 (0.1) b | 0.8 (0.2) bc | 0.5 (0.1) b | |
| Leaf | Phyllostachys pubescens | 110.2 (26.4) ab | 157.1 (16.2) a | 10.6 (0.9) a | 9.4 (2.4) | 3.8 (0.7) a | 5.0 (0.7) a | 3.6 (0.3) a | 3.1 (0.3) a |
| Phoebe bournei | 125.4 (7.1) a | 111.4 (7.9) b | 10.7 (1.0) a | 8.7 (0.9) | 2.4 (0.2) b | 3.1 (0.2) b | 1.4 (0.1) c | 1.5 (0.2) bc | |
| Schima superba | 91.9 (9.9) ab | 82.0 (16.8) bc | 6.0 (0.8) b | 6.7 (0.7) | 1.0 (0.2) c | 1.5 (0.5) c | 1.1 (0.2) c | 0.9 (0.2) c | |
| Cunninghamia lanceolata | 81.4 (13.3) b | 69.3 (4.6) c | 6.0 (0.6) b | 5.9 (0.8) | 2.4 (0.4) b | 2.5 (0.3) bc | 2.1 (0.2) b | 1.9 (0.3) b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Tang, X.; Ran, H.; Deng, M.; Song, Q.; Yang, Q.; Huang, D.; Liu, J. Si Supply Could Alter N Uptake and Assimilation of Saplings—A 15N Tracer Study of Four Subtropical Species. Forests 2023, 14, 1353. https://doi.org/10.3390/f14071353
Liu X, Tang X, Ran H, Deng M, Song Q, Yang Q, Huang D, Liu J. Si Supply Could Alter N Uptake and Assimilation of Saplings—A 15N Tracer Study of Four Subtropical Species. Forests. 2023; 14(7):1353. https://doi.org/10.3390/f14071353
Chicago/Turabian StyleLiu, Xiaoyu, Xinghao Tang, Huan Ran, Mengyang Deng, Qingni Song, Qingpei Yang, Dongmei Huang, and Jun Liu. 2023. "Si Supply Could Alter N Uptake and Assimilation of Saplings—A 15N Tracer Study of Four Subtropical Species" Forests 14, no. 7: 1353. https://doi.org/10.3390/f14071353





