Production, Concentration and Flux of Major and Trace Elements in Juniperus przewalskii Litter of the Qilian Mountains, China
Abstract
:1. Introduction
2. Materials and Methods
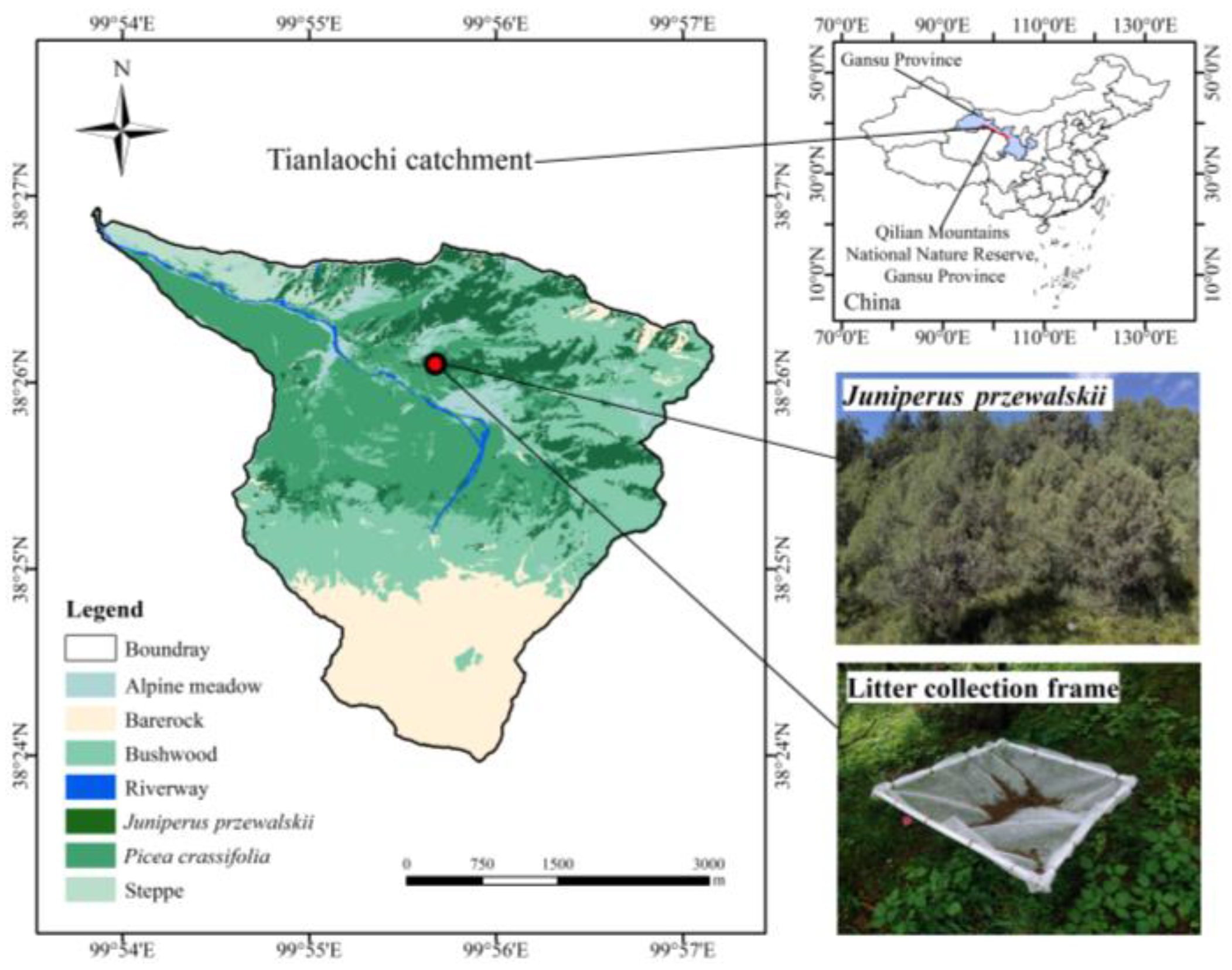
2.1. Study Site
2.2. Experimental Design
2.3. Sample Pretreatment
2.4. Chemical Analysis
2.5. Data Analysis
3. Results
3.1. Monthly Dynamics of Litter Production
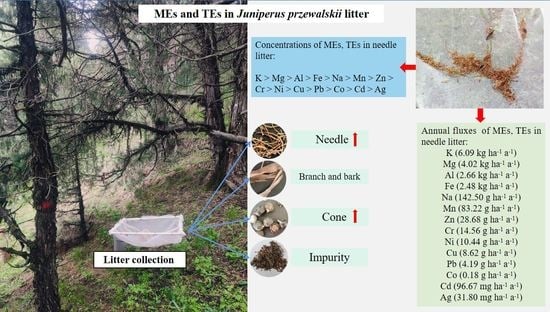
3.2. Concentrations and Fluxes of MEs in Needle Litter
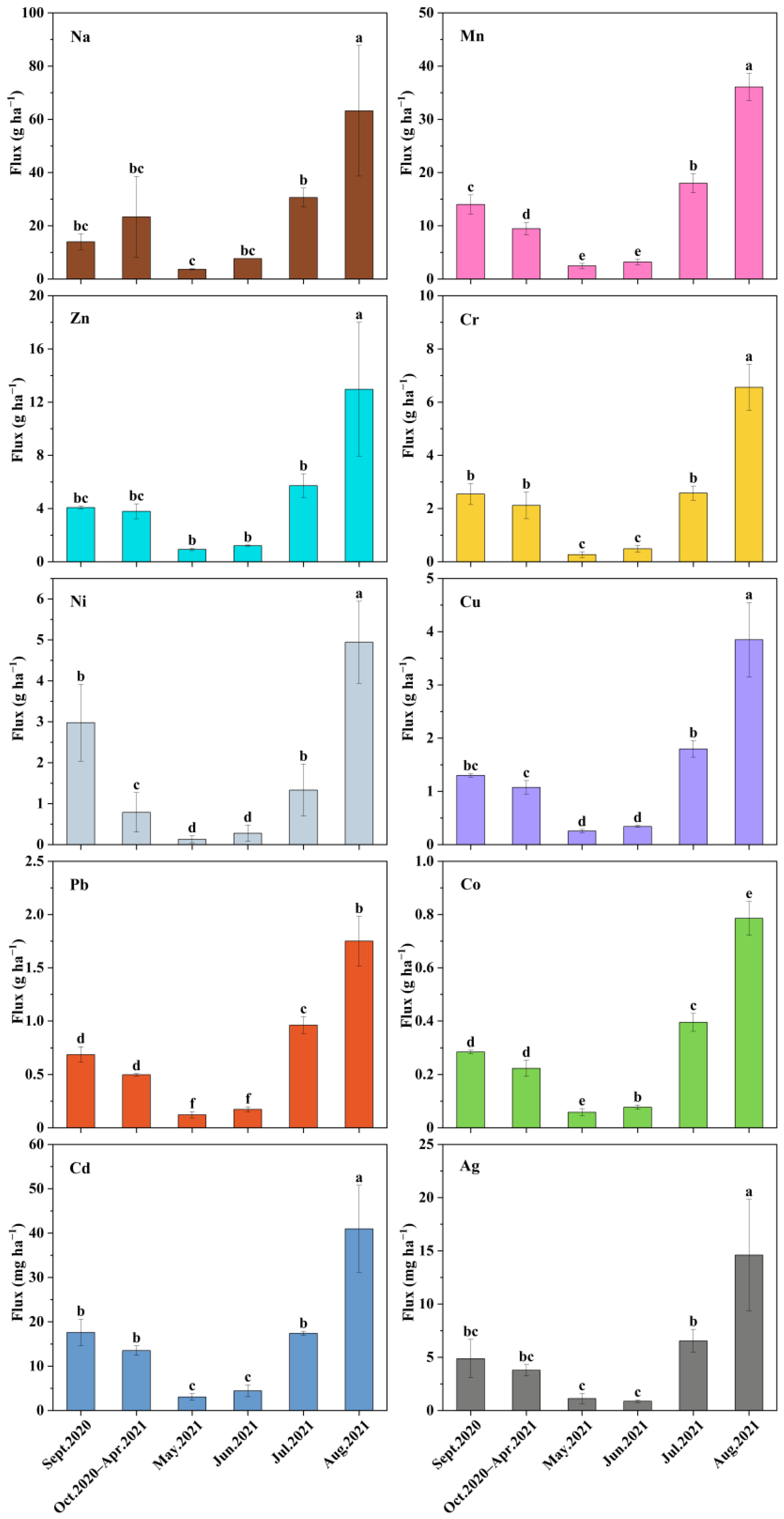
3.3. Concentrations and Fluxes of TEs in Needle Litter
4. Discussion
4.1. Litter Input and Composition
4.2. Element Concentrations and Fluxes of the Needle Litter
4.3. Novelty, Limitations, and Potential Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petraglia, A.; Cacciatori, C.; Chelli, S.; Fenu, G.; Calderisi, G.; Gargano, D.; Abeli, T.; Orsenigo, S.; Carbognani, M. Litter decomposition: Effects of temperature driven by soil moisture and vegetation type. Plant Soil 2019, 435, 187–200. [Google Scholar] [CrossRef]
- Schreeg, L.A.; Mack, M.C.; Turner, B.L. Nutrient-specific solubility patterns of leaf litter across 41 lowland tropical woody species. Ecology 2013, 94, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; Wang, X.; Chen, Y. The interaction between abiotic photodegradation and microbial decomposition under ultraviolet radiation. Global Chang. Biol. 2014, 21, 2095–2104. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, H.; Li, X.; Xie, Y.; Lei, X.; Zheng, Y.; Yang, D.; Wang, F. Spatial heterogeneity and affecting factors of litter organic carbon and total nitrogen over natural spruce-fir mixed forests in northeastern China. Catena 2019, 174, 293–300. [Google Scholar] [CrossRef]
- Wei, X.; Blanco, J.A.; Jiang, H.; Hamish Kimminsc, J.P. Effects of nitrogen deposition on carbon sequestration in Chinese fir forest ecosystems. Sci. Total Environ. 2012, 416, 351–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, G.; Chen, D.; Wu, Y.; Liu, L.; Liu, C. Spatial patterns and estimates of global forest litterfall. Ecosphere 2019, 10, e02587. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yan, W.; Zheng, W.; Liang, X.; Wang, G.; Zhu, F. Litter fall production and nutrient dynamic of Cinnamomum camphora and Pinus massoniana mixed forests in subtropics China. Acta Ecol. Sin. 2013, 33, 7707–7714. [Google Scholar]
- Kuzyakov, Y.; Friedel, J.K.; Stahr, K. Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 2000, 32, 1485–1498. [Google Scholar] [CrossRef]
- Wu, W.; Zhang, Y.; Wang, L.; Zhou, Y.; Chen, Y.; He, S.; Zhang, J.; Liu, Y. Litterfall and Element Return in an Abies faxoniana Forest in Tibet-A Five-Year Study. Forests 2021, 12, 1577. [Google Scholar] [CrossRef]
- Chen, L.; Zan, Q.; Li, M.; Shen, J.; Liao, W. Litter dynamics and forest structure of the introduced Sonneratia caseolaris mangrove forest in Shenzhen, China. Estuar. Coast. Shelf Sci. 2009, 85, 241–246. [Google Scholar] [CrossRef]
- Li, K.; Shi, Y.; Zhao, W.; Lin, Y.; He, Z. Litterfall production and dynamics of five coastal protective plantations. Chin. J. Appl. Environ. Biol. 2021, 28, 317–324. [Google Scholar]
- Dong, X.; Gao, P.; Zhou, R.; Li, C.; Dun, X.; Niu, X. Changing characteristics and influencing factors of the soil microbial community during litter decomposition in a mixed Quercus acutissima Carruth. and Robinia pseudoacacia L. forest in Northern China. Catena 2021, 196, 104811. [Google Scholar]
- Luan, J.; Li, S.; Wang, Y.; Ding, L.; Cai, C.; Liu, S. Decomposition of diverse litter mixtures affected by drought depends on nitrogen and soil fauna in a bamboo forest. Soil Biol. Biochem. 2022, 173, 108783. [Google Scholar] [CrossRef]
- Lin, H.; Li, Y.; Bruelheide, H.; Zhang, S.; Ren, H.; Zhang, N.; Ma, K. What drives leaf litter decomposition and the decomposer community in subtropical forests-The richness of the above-ground tree community or that of the leaf litter? Soil Biol. Biochem. 2021, 160, 108314. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Li, X.; Yang, Z.; Xiong, D.; Xu, C.; Wanek, W.; Yang, Y. Soil warming delays leaf litter decomposition but exerts no effect on litter nutrient release in a subtropical natural forest over 450 days. Geoderma 2022, 427, 116139. [Google Scholar] [CrossRef]
- Wang, C.; Dong, X.; Du, R.; Zhang, Z.; Huang, X. Changes of nutrient release and enzyme activity during the decomposition of mixed leaf litter of Larix principis-rupprechtii and broadleaved tree species. Chin. J. Appl. Ecol. 2021, 32, 1709–1716. [Google Scholar]
- Staelens, J.; Nachtergale, L.; Schrijver, A.D.; Vanhellemont, M.; Wuyts, K.; Verheyen, K. Spatio-temporal litterfall dynamics in a 60-year-old mixed deciduous forest. Ann. For. Sci. 2011, 68, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Chen, Y.; Chen, G. Litter production and litter elemental composition in two rehabilitated Kandelia obovata mangrove forests in Jiulongjiang Estuary, China. Mar. Environ. Res. 2013, 83, 63–72. [Google Scholar] [CrossRef]
- Wu, Q. Effects of snow depth manipulation on the releases of carbon, nitrogen and phosphorus from the foliar litter of two temperate tree species. Sci. Total Environ. 2018, 643, 1357–1365. [Google Scholar] [CrossRef]
- Tie, L.; Hu, J.; Peñuelas, J.; Sardans, J.; Wei, S.; Liu, X.; Zhou, S.; Huang, K. The amounts and ratio of nitrogen and phosphorus addition drive the rate of litter decomposition in a subtropical forest. Sci. Total Environ. 2022, 833, 155163. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Salt, D.E. The plant ionome coming into focus. Curr. Opin. Plant Biol. 2009, 12, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Richter, D.B.; Mendoza, A.; Heine, P. Four-decade responses of soil trace elements to an aggrading old-field forest: B, Mn, Zn, Cu, and Fe. Ecology 2008, 89, 2911–2923. [Google Scholar] [CrossRef] [Green Version]
- Richardson, J.B. Manganese and Mn/Ca ratios in soil and vegetation in forests across the northeastern US: Insights on spatial Mn enrichment. Sci. Total Environ. 2017, 581–582, 612–620. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y.; Song, S.; Chen, Q.; Chen, G.; Tu, L. Nitrogen addition slows litter decomposition accompanied by accelerated manganese release: A five-year experiment in a subtropical evergreen broadleaf forest. Soil Biol. Biochem. 2022, 165, 108511. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, C.; Zang, F.; Rong, Z.; Chang, Y.; Liu, Y. Spatiotemporal patterns of precipitation based on the Bayesian maximum entropy method in a typical catchment of the Heihe River watershed, northwest China. Int. J. Digit. Earth 2022, 15, 911–933. [Google Scholar] [CrossRef]
- Zang, F.; Wang, H.; Zhao, C.; Nan, Z.; Wang, S.; Yang, J.; Li, N. Atmospheric wet deposition of trace elements to forest ecosystem of the Qilian Mountains, northwest China. Catena 2021, 197, 104966. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, C.; Xu, Z.; Wang, Y.; Peng, H. Effect of vegetation on soil water retention and storage in a semi-arid alpine forest catchment. J. Arid. Land 2013, 5, 207–219. [Google Scholar] [CrossRef]
- Lee, S.Y. Litter production and turnover of the mangrove Kandelia candel (L.) druce in a Hong Kong tidal shrimp pond. Estuar. Coast. Shelf Sci. 1989, 29, 75–87. [Google Scholar] [CrossRef]
- Sanjay, G.; Ranbeer, S.R.; Dhar, U. Patterns of litterfall and return of nutrients across anthropogenic disturbance gradients in three subalpine forests of west Himalaya, India. J. For. Res. 2009, 14, 73–80. [Google Scholar]
- Bigelow, S.W.; Canham, C.D. Litterfall as a niche construction process in a northern hardwood forest. Ecosphere 2015, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Saarsalmi, A.; Starr, M.; Hokkanen, T.; Ukonmaanaho, L.; Kukkola, M.; Nojd, P.; Sievanen, R. Predicting annual canopy litterfall production for Norway spruce (Picea abies (L.) Karst.) stands. For. Ecol. Manag. 2007, 242, 578–586. [Google Scholar] [CrossRef]
- Giaccone, E.; Colombo, N.; Acquaotta, F.; Paro, L.; Fratianni, S. Climate variations in a high altitude Alpine basin and their effects on a glacial environment (Italian Western Alps). Atmosfera 2015, 28, 117–128. [Google Scholar] [CrossRef]
- Liu, C.; Ilvesniemi, H. Variation in Litterfall-Climate relationships between coniferous and broadleaf forests in Eurasia. Global Ecol. Biogeogr. 2004, 13, 105–114. [Google Scholar] [CrossRef]
- Pausas, J.G. Litter fall and litter decomposition in Pinus sylvestris forests of the eastern Pyrenees. J. Veg. Sci. 1997, 8, 643–650. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Liu, Q.; Wu, Y.; He, H. Nutrient and litter patterns in three subalpine coniferous forests of western Sichuan, China. Pedosphere 2006, 16, 380–389. [Google Scholar] [CrossRef]
- Chave, J.; Navarrete, D.; Almeida, S.; Álvarez, E.; Aragão, L.E.O.C.; Bonal, D.; Châtelet, P.; Silva-Espejo, J.E.; Goret, J.Y.; von Hildebrand, P.; et al. Regional and seasonal patterns of litterfall in tropical South America. Biogeosciences 2010, 7, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Enright, N.J. Litterfall dynamics in a mixed conifer-angiosperm forest in northern New Zealand. J. Biogeogr. 2001, 26, 147–157. [Google Scholar] [CrossRef]
- Kotowska, M.M.; Leuschner, C.; Triadiati, T.; Hertel, D. Conversion of tropical lowland forest reduces nutrient return through litterfall, and alters nutrient use efficiency and seasonality of net primary production. Oecologia 2016, 180, 601–618. [Google Scholar] [CrossRef]
- Zhu, X.; Zou, X.; Lu, E.; Deng, Y.; Luo, Y.; Chen, H.; Liu, W. Litterfall biomass and nutrient cycling in karst and nearby non-karst forests in tropical China: A 10-year comparison. Sci. Total Environ. 2021, 758, 143619. [Google Scholar] [CrossRef]
- Lonsdale, M.W. Predicting the Amount of Litterfall in Forests of the World. Ann. Bot. 1988, 61, 319–324. [Google Scholar] [CrossRef]
- Peixoto, K.S.; Marimon-Junior, B.H.; Cavalheiro, K.A.; Silva, N.A.; das Neves, E.C.; Freitag, R.; Mews, H.A.; Valadão, M.B.; Marimon, B.S. Assessing the effects of rainfall reduction on litterfall and the litter layer in phytophysiognomies of the Amazonia-Cerrado transition. Braz. J. Bot. 2018, 41, 589–600. [Google Scholar] [CrossRef]
- Enquist, B.J.; Brown, J.H.; West, G.B. Allometric scaling of plant energetics and population density. Nature 1998, 395, 163–165. [Google Scholar] [CrossRef]
- Zagyvai-Kiss, K.A.; Kalicz, P.; Szilágyi, J.; Gribovszki, Z. On the specific water holding capacity of litter for three forest ecosystems in the eastern foothills of the Alps. Agric. For. Meteorol. 2019, 278, 107656. [Google Scholar] [CrossRef]
- Kopittke, P.M. Role of phytohormones in aluminium rhizotoxicity. Plant Cell Environ. 2016, 39, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Kopittke, P.M.; Menzies, N.W.; Wang, P.; Blamey, F.P.C. Kinetics and nature of aluminium rhizotoxic effects: A review. J. Exp. Bot. 2016, 67, 4451–4467. [Google Scholar] [CrossRef]
- Berthelsen, B.O.; Steinnes, E.; Solberg, W.; Jingsen, L. Heavy Metal Concentrations in Plants in Relation to Atmospheric Heavy Metal Deposition. J. Environ. Qual. 1995, 24, 1018–1026. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability, and Risks of Metals; Springer: New York, NY, USA, 2001; pp. 61–89. [Google Scholar]
- Tang, R.; Luo, J.; Yang, P.; She, J.; Chen, Y.; Gong, Y.; Zhou, J. Trace metals of needles and litter in timberline forests in the Eastern of Tibetan Plateau, China. Ecol. Indic. 2014, 45, 669–676. [Google Scholar] [CrossRef]
- López-Millán, A.F.; Ellis, D.R.; Grusak, M.A. Effect of zinc and manganese supply on the activities of superoxide dismutase and carbonic anhydrase in Medicago truncatula wild type and raz mutant plants. Plant Sci. 2005, 168, 1015–1022. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Pii, Y.; Cesco, S.; Mimmo, T. Shoot ionome to predict the synergism and antagonism between nutrients as affected by substrate and physiological status. Plant Physiol. Biochem. 2015, 94, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.; Niklinska, M.; Maryanski, M. The dynamics of chemical-elements in forest litter. Ecology 1995, 76, 1393–1406. [Google Scholar] [CrossRef]
- McGee, C.J.; Fernandez, I.J.; Norton, S.A.; Stubbs, C.S. Cd, Ni, Pb, and Zn Concentrations in Forest Vegetation and Soils in Maine. Water Air Soil Pollut. 2007, 180, 141–153. [Google Scholar] [CrossRef]
- Gandois, L.; Probst, A. Localisation and mobility of trace metal in silver fir needles. Chemosphere 2012, 87, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Neumann, P.M.; Ukonmaanaho, L.; Johnson, J.; Benham, S.; Vesterdal, L.; Novotný, R.; Verstraeten, A.; Lundin, L.; Thimonier, A.; Michopoulos, P.; et al. Quantifying Carbon and Nutrient Input from Litterfall in European Forests Using Field Observations and Modeling. Global Biogeochem. Cycles 2018, 32, 784–798. [Google Scholar] [CrossRef]
- Mingorance, M.D.; Valdés, B.; Rossini-Oliva, S. Strategies of heavy metal uptake by plants growing under industrial emissions. Environ. Int. 2007, 33, 514–520. [Google Scholar] [CrossRef]
- Aznar, J.C.; Richer-Laflèche, M.; Bégin, C.; Bégin, Y. Lead Exclusion and Copper Translocation in Black Spruce Needles. Water Air Soil Pollut. 2009, 203, 139–145. [Google Scholar] [CrossRef]
- Probst, A.; Liu, H.; Fanjul, M.; Liao, B.; Hollande, E. Response of Vicia faba L. to metal toxicity on mine tailing substrate: Geochemical and morphological changes in leaf and root. Environ. Exp. Bot. 2009, 66, 297–308. [Google Scholar] [CrossRef] [Green Version]
- Mauclet, E.; Agnan, Y.; Hirst, C.; Monhonval, A.; Pereira, B.; Vandeuren, A.; Villani, M.; Ledman, J.; Taylor, M.; Jasinski, B.L.; et al. Changing sub-Arctic tundra vegetation upon permafrost degradation: Impact on foliar mineral element cycling. Biogeosciences 2022, 19, 2333–2351. [Google Scholar] [CrossRef]
- Wang, J.; Yang, S.; Zhang, B.; Liu, W.; Deng, M.; Chen, S.; Liu, L. Temporal dynamics of ultraviolet radiation impacts on litter decomposition in a semi-arid ecosystem. Plant Soil 2017, 419, 71–81. [Google Scholar] [CrossRef]
- Haider, F.U.; Cai, L.; Jeffrey, A.C.; Sardar, A.C.; Wu, J.; Zhang, R.; Ma, W.; Muhammad, F. Cadmium toxicity in plants: Impacts and remediation strategies. Ecotoxicol. Environ. Saf. 2021, 211, 111887. [Google Scholar] [CrossRef] [PubMed]
- Chaperon, S.; Sauvé, S. Toxicity interaction of metals (Ag, Cu, Hg, Zn) to urease and dehydrogenase activities in soils. Soil Biol. Biochem. 2007, 39, 2329–2338. [Google Scholar] [CrossRef]




| Forest Types | Needle/ Leaf | Branch | Bark | Flower | Cone | Impurity | Total | Reference |
|---|---|---|---|---|---|---|---|---|
| kg ha−1 a−1 | ||||||||
| Juniperus przewalskii * | 2640.35 ± 436.96 | 581.65 ± 59.23 | – | 626.15 ± 83.40 | 192.59 ± 37.58 | 4040.74 ± 495.96 | This study | |
| Camphor tree and masson pine mixed forest * | 3327 ± 232.30 | 1216 ± 346.63 | – | 149 ± 97.7815.71 | 391 ± 80.31 | 4634 ± 337.14 | [7] | |
| Betula pendula and Quercus robur mixed forest | 3450 | 1260 | – | 593 | – | 5512 | [17] | |
| Pinus elliottii | 7153 | 802 | 112 | 1020 | 523 | 9602 | [11] | |
| Casuarina equisetifolia | 10,491 | 963 | 3 | 653 | 530 | 12,640 | [11] | |
| Sonneratia caseolaris | 6853 | 1738 | – | – | 5310 | 13,901 | [10] | |
| Kandelia candel | 5971 | 681 | – | – | 4504 | 11,070 | [29] | |
| Betula utilis | 1311 | 1412 | – | – | 505 | 417 | 3645 | [30] |
| Kandelia obovata * | 832.27 ± 84.98 | 315.51 ± 44.60 | – | 81.29 ± 22.33 | 19.92 ± 6.01 | – | 1249.99 ± 97.78 | [18] |
| Abies pindrow | 604 | 811 | – | – | 715 | 518 | 2648 | [30] |
| Abies faxoniana | 2763 | 1154 | – | – | – | 60 | 3976 | [19] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, F.; Zang, F.; Zhao, X.; Li, N.; Nan, Z.; Wang, S.; Zhao, C. Production, Concentration and Flux of Major and Trace Elements in Juniperus przewalskii Litter of the Qilian Mountains, China. Forests 2023, 14, 1364. https://doi.org/10.3390/f14071364
Huang F, Zang F, Zhao X, Li N, Nan Z, Wang S, Zhao C. Production, Concentration and Flux of Major and Trace Elements in Juniperus przewalskii Litter of the Qilian Mountains, China. Forests. 2023; 14(7):1364. https://doi.org/10.3390/f14071364
Chicago/Turabian StyleHuang, Fangyuan, Fei Zang, Xinning Zhao, Na Li, Zhongren Nan, Shengli Wang, and Chuanyan Zhao. 2023. "Production, Concentration and Flux of Major and Trace Elements in Juniperus przewalskii Litter of the Qilian Mountains, China" Forests 14, no. 7: 1364. https://doi.org/10.3390/f14071364