Wildfire and Climate Impacts Tree Hollow Density in a Temperate Australian Forest
Abstract
:1. Introduction
- (1)
- Tree hollow density will be highest at intermediate fire frequencies and severities which allow for hollow formation but do not reduce large tree density;
- (2)
- Basal scarring will be greatest at high fire frequencies and severities which promotes tree damage;
- (3)
- Tree hollow density and basal scarring will be greatest in warm, moist climates which facilitate hollow formation via decomposition.
2. Materials and Methods
2.1. Study Area
2.2. Site Selection
2.3. Sampling Protocol
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Fire Effects
4.2. Climate Effects
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cockle, K.L.; Martin, K.; Robledo, G. Linking fungi, trees, and hole-using birds in a Neotropical tree-cavity network: Pathways of cavity production and implications for conservation. For. Ecol. Manag. 2012, 264, 210–219. [Google Scholar] [CrossRef]
- Jackson, J.A.; Jackson, B.J. Ecological relationships between fungi and woodpecker cavity sites. Condor 2004, 106, 37–49. [Google Scholar] [CrossRef]
- Gibbons, P.; Lindenmayer, D. Tree Hollows and Wildlife Conservation in Australia; CSIRO Publishing: Melbourne, Australia, 2002. [Google Scholar]
- Gibbons, P.; Lindenmayer, D.; Barry, S.C.; Tanton, M. Hollow formation in eucalypts from temperate forests in southeastern Australia. Pac. Conserv. Biol. 2000, 6, 218. [Google Scholar] [CrossRef]
- Rose, C.L.; Marcot, B.G.; Mellen, T.K.; Ohmann, J.L.; Waddell, K.L.; Lindley, D.L.; Schreiber, B. Decaying Wood in Pacific Northwest Forests: Concepts and Tools for Habitat Management. In Wildlife-Habitat Relationships in Oregon and Washington; Johnson, D., O’Neil, T., Eds.; Oregon State University Press: Corvallis, OR, USA, 2001; pp. 580–623. [Google Scholar]
- Koch, A.J.; Munks, S.A.; Driscoll, D.; Kirkpatrick, J. Does hollow occurrence vary with forest type? A case study in wet and dry Eucalyptus obliqua forest. For. Ecol. Manag. 2008, 255, 3938–3951. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World; John Hopkins University Press: Baltimore, MD, USA, 1999; Volume 1. [Google Scholar]
- Remm, J.; Lõhmus, A. Tree cavities in forests–the broad distribution pattern of a keystone structure for biodiversity. For. Ecol. Manag. 2011, 262, 579–585. [Google Scholar] [CrossRef]
- Newton, I. The role of nest sites in limiting the numbers of hole-nesting birds: A review. Biol. Conserv. 1994, 70, 265–276. [Google Scholar] [CrossRef]
- Newton, I. Population Limitation in Birds; Academic Press: Cambridge, MA, USA, 1998. [Google Scholar]
- Bai, M.-L.; Mühlenberg, M. Sequential use of holes by birds breeding in a natural boreal forest in Mongolia. Bird Study 2008, 55, 161–168. [Google Scholar] [CrossRef] [Green Version]
- Gibbons, P.; Lindenmayer, D.; Barry, S.C.; Tanton, M. Hollow selection by vertebrate fauna in forests of southeastern Australia and implications for forest management. Biol. Conserv. 2002, 103, 1–12. [Google Scholar] [CrossRef]
- Lindenmayer, D.; Cunningham, R.; Tanton, M.; Smith, A.; Nix, H. Characteristics of hollow-bearing trees occupied by arboreal marsupials in the montane ash forests of the Central Highlands of Victoria, south-east Australia. For. Ecol. Manag. 1991, 40, 289–308. [Google Scholar] [CrossRef]
- Cornelius, C.; Cockle, K.; Politi, N.; Berkunsky, I.; Sandoval, L.; Ojeda, V.; Rivera, L.; Hunter, M., Jr.; Martin, K. Cavity-nesting birds in neotropical forests: Cavities as a potentially limiting resource. Ornitol. Neotrop. 2008, 19, 253–268. [Google Scholar]
- Heinsohn, R.; Murphy, S.; Legge, S. Overlap and competition for nest holes among eclectus parrots, palm cockatoos and sulphur-crested cockatoos. Aust. J. Zool. 2003, 51, 81–94. [Google Scholar] [CrossRef] [Green Version]
- Webb, M.H.; Wotherspoon, S.; Stojanovic, D.; Heinsohn, R.; Cunningham, R.; Bell, P.; Terauds, A. Location matters: Using spatially explicit occupancy models to predict the distribution of the highly mobile, endangered swift parrot. Biol. Conserv. 2014, 176, 99–108. [Google Scholar] [CrossRef]
- Archibald, S.; Lehmann, C.E.; Gómez-Dans, J.L.; Bradstock, R.A. Defining pyromes and global syndromes of fire regimes. Proc. Natl. Acad. Sci. USA 2013, 110, 6442–6447. [Google Scholar] [CrossRef]
- Keeley, J.E.; Bond, W.J.; Bradstock, R.A.; Pausas, J.G.; Rundel, P.W. Fire in Mediterraean Ecosystems: Ecology, Evolution and Management; Cambridge University Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Adkins, M.F. A burning issue: Using fire to accelerate tree hollow formation in Eucalyptus species. Aust. For. 2006, 69, 107–113. [Google Scholar] [CrossRef]
- Pausas, J.G.; Bradstock, R.A.; Keith, D.A.; Keeley, J.E. Plant functional traits in relation to fire in crown-fire ecosystems. Ecology 2004, 85, 1085–1100. [Google Scholar] [CrossRef] [Green Version]
- Perry, D.; Lenz, M.; Watson, J. Relationships between fire, fungal rots and termite damage in Australian forest trees. Aust. For. 1985, 48, 46–53. [Google Scholar] [CrossRef]
- Wilkes, J. Host attributes affecting patterns of decay in a regrowth eucalypt forest. I. Patterns of natural decay. Holzforsch.-Int. J. Biol. Chem. Phys. Technol. Wood 1985, 39, 17–22. [Google Scholar] [CrossRef]
- Hillis, W. The role of wood characteristics in high temperature drying. Aust. For. Ind. J. 1975, 7, 60–67. [Google Scholar]
- Mackowski, C. The ontogeny of hollows in blackbutt (Eucalyptus pilularis) and its relevance to the management of forests for possums, gliders and timber. In Possums and Gliders; Smith, A., Hume, I., Eds.; Surrey Beatty & Sons Pty Ltd.: Sydney, Australia, 1984; pp. 553–567. [Google Scholar]
- Inions, G.; Tanton, M.; Davey, S. Effect of fire on the availability of hollows in trees used by the common brushtail possum, Trichosurus vulpecula Kerr, 1792, and the ringtail possum, Pseudocheirus peregrinus Boddaerts, 1785. Wildl. Res. 1989, 16, 449–458. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Blanchard, W.; McBurney, L.; Blair, D.; Banks, S.; Likens, G.E.; Franklin, J.F.; Laurance, W.F.; Stein, J.A.; Gibbons, P. Interacting factors driving a major loss of large trees with cavities in a forest ecosystem. PLoS ONE 2012, 7, e41864. [Google Scholar] [CrossRef] [Green Version]
- McLean, C.M.; Bradstock, R.; Price, O.; Kavanagh, R.P. Tree hollows and forest stand structure in Australian warm temperate Eucalyptus forests are adversely affected by logging more than wildfire. For. Ecol. Manag. 2015, 341, 37–44. [Google Scholar] [CrossRef]
- Haslem, A.; Avitabile, S.C.; Taylor, R.S.; Kelly, L.T.; Watson, S.J.; Nimmo, D.G.; Kenny, S.A.; Callister, K.E.; Spence-Bailey, L.M.; Bennett, A.F. Time-since-fire and inter-fire interval influence hollow availability for fauna in a fire-prone system. Biol. Conserv. 2012, 152, 212–221. [Google Scholar] [CrossRef]
- Hunter, J.T. Seasonality of climate drives the number of tree hollows in eastern Australia: Implications of a changing climate. Int. J. Ecol. 2015, 2015, 190637. [Google Scholar] [CrossRef] [Green Version]
- Lindenmayer, D.; Cunningham, R.; Nix, H.; Tanton, M.; Smith, A. Predicting the abundance of hollow-bearing trees in montane forests of southeastern Australia. Austral Ecol. 1991, 16, 91–98. [Google Scholar] [CrossRef]
- Bennett, A.; Lumsden, L.; Nicholls, A. Tree hollows as a resource for wildlife in remnant woodlands: Spatial and temporal patterns across the northern plains of Victoria, Australia. Pac. Conserv. Biol. 1994, 1, 222–235. [Google Scholar] [CrossRef]
- Gordon, C.E.; Bendall, E.R.; Stares, M.G.; Collins, L.; Bradstock, R.A. Aboveground carbon sequestration in dry temperate forests varies with climate not fire regime. Glob. Chang. Biol. 2018, 24, 4280–4292. [Google Scholar] [CrossRef]
- Keith, H.; Mackey, B.G.; Lindenmayer, D.B. Re-evaluation of forest biomass carbon stocks and lessons from the world’s most carbon-dense forests. Proc. Natl. Acad. Sci. USA 2009, 106, 11635–11640. [Google Scholar] [CrossRef]
- Evans, J.; Ji, F.; Lee, C.; Smith, P.; Argüeso, D.; Fita, L. Design of a regional climate modelling projection ensemble experiment–NARCliM. Geosci. Model Dev. 2014, 7, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Fick, S.E.; Hijmans, R.J. Worldclim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hammill, K.; Penman, T.; Bradstock, R. Responses of resilience traits to gradients of temperature, rainfall and fire frequency in fire-prone, Australian forests: Potential consequences of climate change. Plant Ecol. 2016, 217, 725–741. [Google Scholar] [CrossRef]
- Keith, D.A. Ocean Shores to Desert Dunes: The Native Vegetation of NSW and the ACT; Department of Environment and Conservation (NSW): Hurstville, Australia, 2004.
- Hammill, K.A.; Bradstock, R.A. Remote sensing of fire severity in the Blue Mountains: Influence of vegetation type and inferring fire intensity. Int. J. Wildland Fire 2006, 15, 213–226. [Google Scholar] [CrossRef]
- Bjornstad, O.N. NCF: Spatial Nonparametric Covariance Functions. R Package Version 1.1-3. 2009. Available online: http://www.CRAN.R-project.org (accessed on 1 February 2023).
- Salmona, J.; Dixon, K.M.; Banks, S.C. The effects of fire history on hollow-bearing tree abundance in montane and subalpine eucalypt forests in southeastern Australia. For. Ecol. Manag. 2018, 428, 93–103. [Google Scholar] [CrossRef]
- Australian Bureau of Meteorology. Climate Data Online. 2023. Available online: http://www.bom.gov.au/climate/data (accessed on 1 February 2023).
- Booth, T.H.; Nix, H.A.; Busby, J.R.; Hutchinson, M.F. BIOCLIM: The first species distribution modelling package, its early applications and relevance to most current MAXENT studies. Divers. Distrib. 2014, 20, 1–9. [Google Scholar] [CrossRef]
- Gibson, M. EUCLID Eucalypts of Australia. Vic. Nat. 2007, 124, 344. [Google Scholar]
- Nolan, R.H.; Rahmani, S.; Samson, S.A.; Simpson-Southward, H.M.; Boer, M.M.; Bradstock, R.A. Bark attributes determine variation in fire resistance in resprouting tree species. For. Ecol. Manag. 2020, 474, 118385. [Google Scholar] [CrossRef]
- Bendall, E.R.; Bedward, M.; Boer, M.; Clarke, H.; Collins, L.; Leigh, A.; Bradstock, R.A. Mortality and resprouting responses in forest trees driven more by tree and ecosystem characteristics than drought severity and fire frequency. For. Ecol. Manag. 2022, 509, 120070. [Google Scholar] [CrossRef]
- Wesolowski, A.; Adams, M.A.; Pfautsch, S. Insulation capacity of three bark types of temperate Eucalyptus species. For. Ecol. Manag. 2014, 313, 224–232. [Google Scholar] [CrossRef]
- Aitken, K.E.; Martin, K. Resource selection plasticity and community responses to experimental reduction of a critical resource. Ecology 2008, 89, 971–980. [Google Scholar] [CrossRef]
- Cockle, K.L.; Martin, K.; Drever, M.C. Supply of tree-holes limits nest density of cavity-nesting birds in primary and logged subtropical Atlantic forest. Biol. Conserv. 2010, 143, 2851–2857. [Google Scholar] [CrossRef]
- Vinson, S.G.; Johnson, A.P.; Mikac, K.M. Current estimates and vegetation preferences of an endangered population of the vulnerable greater glider at Seven Mile Beach National Park. Austral Ecol. 2021, 46, 303–314. [Google Scholar] [CrossRef]
- Bradstock, R.; Kenny, B. An application of plant functional types to fire management in a conservation reserve in southeastern Australia. J. Veg. Sci. 2003, 14, 345–354. [Google Scholar] [CrossRef]
- Kenny, B.; Sutherland, E.; Tasker, E.; Bradstock, R. Guidelines for Ecologically Sustainable Fire Management: NSW Biodiversity Strategy; National Parks and Wildlife Service: Sydney, Australia, 2003. Available online: www.environment.nsw.gov.au/resources/biodiversity/FireGuidelinesReport.pdf (accessed on 1 February 2023).
- Soderquist, T.; Gibbons, D. Home-range of the Powerful Owl (Ninox strenua) in dry sclerophyll forest. Emu-Austral Ornithol. 2007, 107, 177–184. [Google Scholar] [CrossRef]
- Goldingay, R.; Possingham, H. Area requirements for viable populations of the Australian gliding marsupial Petaurus australis. Biol. Conserv. 1995, 73, 161–167. [Google Scholar] [CrossRef]

| Climate Variable | Fire Frequency | Fire Severity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | >3 | Total | Low | Moderate | High | Very High | Total | |
| Mean annual precipitation | ||||||||||
| 858 mm | 0 | 13 | 9 | 4 | 26 | 14 | 8 | 2 | 2 | 26 |
| 1033 mm | 4 | 17 | 9 | 8 | 38 | 5 | 12 | 14 | 7 | 38 |
| 1325 mm | 5 | 4 | 6 | 1 | 16 | 1 | 2 | 4 | 9 | 16 |
| Total | 9 | 34 | 24 | 13 | 80 | 20 | 22 | 20 | 18 | 80 |
| Mean annual temperature | ||||||||||
| 12.3 °C | 5 | 12 | 8 | 4 | 29 | 4 | 4 | 9 | 12 | 29 |
| 15.0 °C | 2 | 11 | 8 | 4 | 25 | 10 | 6 | 3 | 6 | 25 |
| 16.1 °C | 2 | 11 | 8 | 5 | 26 | 6 | 12 | 8 | 0 | 26 |
| Total | 9 | 34 | 24 | 13 | 80 | 20 | 22 | 20 | 18 | 80 |
| Variable | (a) Small-Sized Hollow | (b) Medium-Sized Hollow | (c) Large-Sized Hollow | (d) Basal Scar | ||||
|---|---|---|---|---|---|---|---|---|
| C.E. | p | C.E. | p | C.E. | p | C.E. | p | |
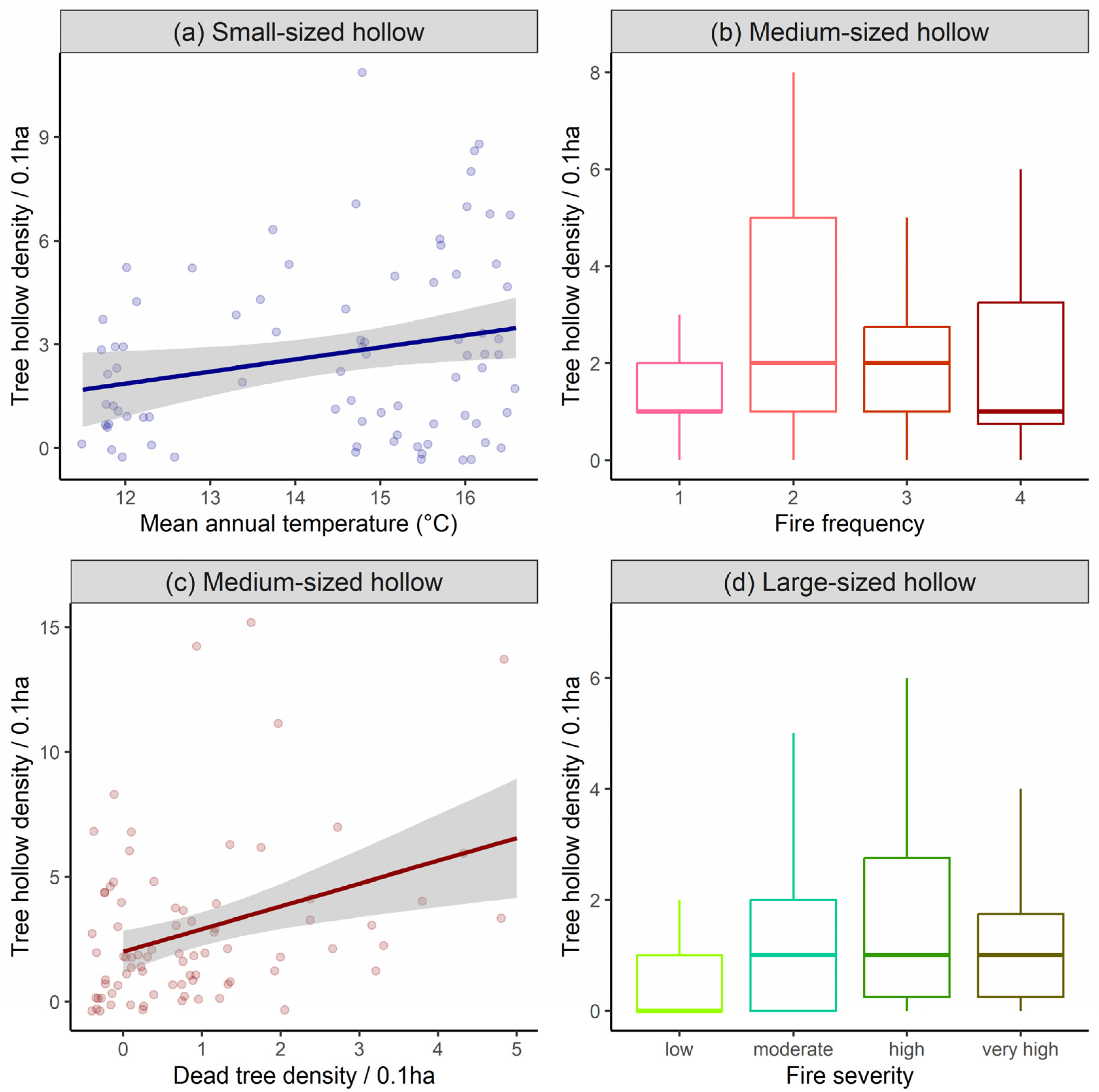
| Fire frequency: 1–2 | 0.230 ± 0.309 | 0.457 | 0.048 ± 0.334 | 0.885 | −0.493 ± 0.325 | 0.130 | 0.104 ± 0.147 | 0.478 |
| 1–3 | −0.112 ± 0.269 | 0.678 | −0.564 ± 0.285 | 0.048 * | 0.101 ± 0.292 | 0.727 | −0.077 ± 0.134 | 0.561 |
| 1 ≥ 3 | −0.003 ± 0.196 | 0.987 | 0.065 ± 0.202 | 0.745 | 0.079 ± 0.234 | 0.733 | −0.078 ± 0.099 | 0.429 |
| Fire severity: L-M | −0.065 ± 0.269 | 0.809 | 0.229 ± 0.273 | 0.403 | 0.900 ± 0.347 | 0.009 * | 0.286 ± 0.140 | 0.041 * |
| L-H | 0.114 ± 0.239 | 0.632 | −0.126 ± 0.241 | 0.601 | −0.703 ± 0.287 | 0.014 * | −0.058 ± 0.124 | 0.639 |
| L-VH | −0.168 ± 0.210 | 0.423 | 0.064 ± 0.214 | 0.762 | 0.088 ± 0.227 | 0.698 | −0.116 ± 0.105 | 0.272 |
| Mean annual precipitation | 0.118 ± 0.139 | 0.396 | −0.171 ± 0.147 | 0.245 | −0.147 ± 0.168 | 0.381 | 0.049 ± 0.063 | 0.433 |
| Mean annual temperature | 0.409 ± 0.170 | 0.016 * | 0.205 ± 0.173 | 0.237 | 0.287 ± 0.180 | 0.111 | −0.337 ± 0.081 | <0.001 * |
| Medium-sized tree density | 0.169 ± 0.142 | 0.235 | −0.155 ± 0.148 | 0.293 | 0.155 ± 0.160 | 0.333 | 0.384 ± 0.072 | <0.001 * |
| Large-sized tree density | 0.192 ± 0.134 | 0.153 | 0.047 ± 0.136 | 0.727 | 0.288 ± 0.152 | 0.057 | 0.0567 ± 0.065 | 0.387 |
| Dead tree density | 0.132 ± 0.110 | 0.230 | 0.266 ± 0.106 | 0.012 * | 0.209 ± 0.120 | 0.081 | −0.019 ± 0.068 | 0.775 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gordon, C.E.; Stares, M.G.; Bendall, E.R.; Bradstock, R.A. Wildfire and Climate Impacts Tree Hollow Density in a Temperate Australian Forest. Forests 2023, 14, 1372. https://doi.org/10.3390/f14071372
Gordon CE, Stares MG, Bendall ER, Bradstock RA. Wildfire and Climate Impacts Tree Hollow Density in a Temperate Australian Forest. Forests. 2023; 14(7):1372. https://doi.org/10.3390/f14071372
Chicago/Turabian StyleGordon, Christopher E., Mitchell G. Stares, Eli R. Bendall, and Ross A. Bradstock. 2023. "Wildfire and Climate Impacts Tree Hollow Density in a Temperate Australian Forest" Forests 14, no. 7: 1372. https://doi.org/10.3390/f14071372






