Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. DNA Extraction, PCR Amplification, and Pyrosequencing
2.3. Pyrosequencing Data Analysis
2.4. Statistical Analyses from NGS Data
2.5. Prokaryotic and Actinobacterial Biomass Quantification
2.6. Pyrogenic Carbon Assessment
2.7. Satellite Monitoring of Fire
2.8. Post-Fire Vegetation Recovery
2.9. Climatic Data
3. Results
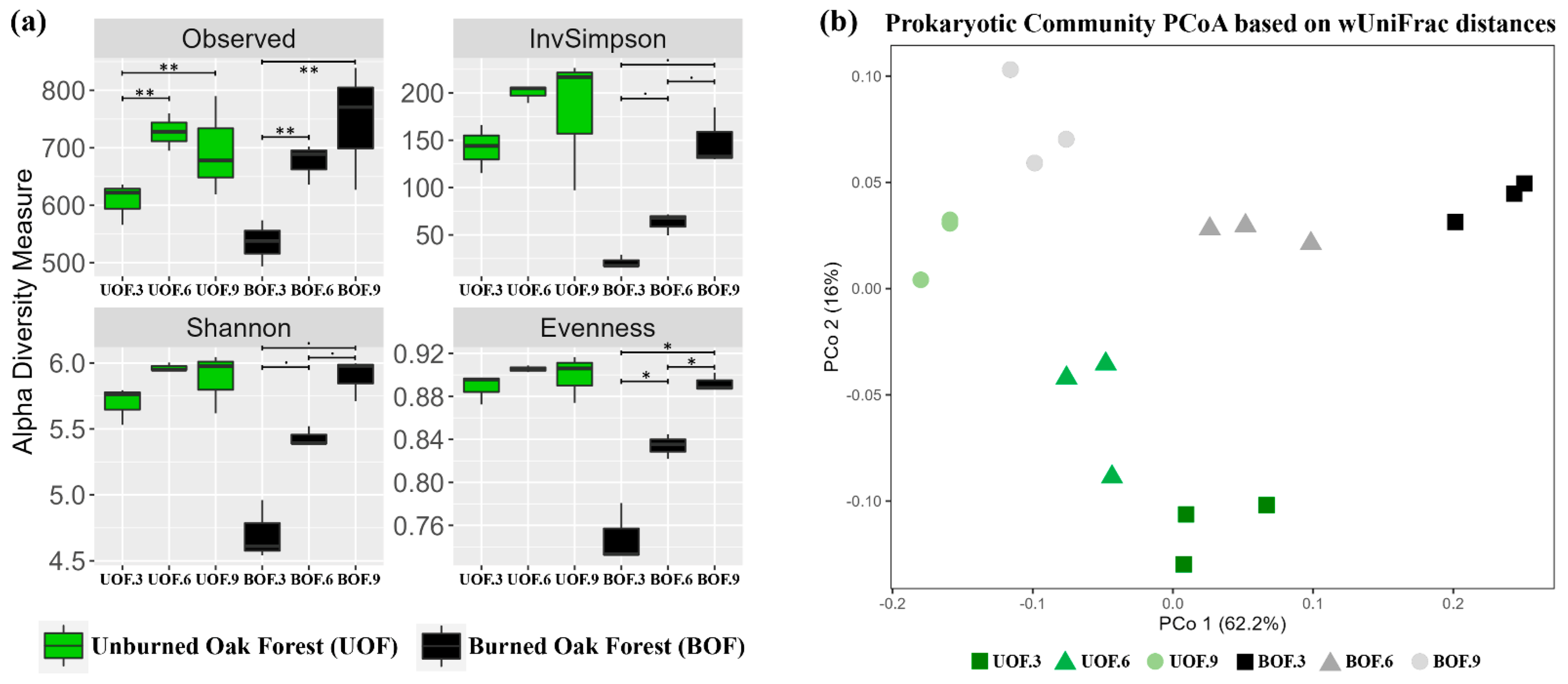
3.1. Temporal Recovery in Microbial Diversity 9 Years after Fire
3.2. Taxonomic Profiles Remained Markedly Different from Each Other
3.3. Soil Physicochemical Properties Drive the Microbial Taxonomic Profiles
3.4. Long-Term Alteration of Prokaryotic Biomass
3.5. Satellite Monitoring of Fire
3.6. Post-Fire Vegetation Recovery
3.7. Strong Changes in Rainfall Regimes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Terradas, J. Holm Oak and Holm Oak Forests: An Introduction. In Ecology of Mediterranean Evergreen Oak Forests. Ecological Studies; Rodà, F., Retana, J., Gracia, C.A., Bellot, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; Volume 137. [Google Scholar] [CrossRef]
- Cierjacks, A.; Hensen, I. Variation of stand structure and regeneration of Mediterranean holm oak along a grazing intensity gradient. Plant Ecol. 2004, 173, 215–223. [Google Scholar] [CrossRef]
- Baudena, M.; Santana, V.M.; Baeza, M.J.; Bautista, S.; Eppinga, M.B.; Hemerik, L.; Garcia Mayor, A.; Rodriguez, F.; Valdecantos, A.; Vallejo, V.R.; et al. Increased aridity drives post-fire recovery of Mediterranean forests towards open shrublands. New Phytol. 2020, 225, 1500–1515. [Google Scholar] [CrossRef] [Green Version]
- Estadística General de Incendios Forestales. Available online: https://www.miteco.gob.es/es/biodiversidad/temas/incendios-forestales/estadisticas-datos.aspx (accessed on 25 April 2023).
- Zotano, J.G.; Sánchez, J.J.M.; Martínez, F.R. El incendio de Sierra Nevada (22–24 de septiembre de 2005). Una catástrofe ecológica. In Cuadernos Geográficos; Universidad de Granada: Granada, Spain, 2005. [Google Scholar]
- Cobo-Díaz, J.F.; Fernández-González, A.J.; Villadas, P.J.; Robles, A.B.; Toro, N.; Fernández-López, M. Metagenomic Assessment of the Potential Microbial Nitrogen Pathways in the Rhizosphere of a Mediterranean Forest After a Wildfire. Microb. Ecol. 2015, 69, 895–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeager, C.M.; Northup, D.E.; Grow, C.C.; Barns, S.M.; Kuske, C.R. Changes in nitrogen-fixing and ammonia-oxidizing bacterial communities in soil of a mixed conifer forest after wildfire. Appl. Environ. Microbiol. 2005, 71, 2713–2722. [Google Scholar] [CrossRef] [Green Version]
- Weber, C.F.; Lockhart, J.S.; Charaska, E.; Aho, K.; Lohse, K.A. Bacterial composition of soils in ponderosa pine and mixed conifer forests exposed to different wildfire burn severity. Soil Biol. Biochem. 2014, 69, 242–250. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Parra, A.; Laudicina, V.A.; Moreno, J.M. Post-fire soil functionality and microbial community structure in a Mediterranean shrubland subjected to experimental drought. Sci. Total Environ. 2016, 573, 1178–1189. [Google Scholar] [CrossRef]
- Whitman, T.; Whitman, E.; Woolet, J.; Flannigan, M.D.; Thompson, D.K.; Parisien, M.-A. Soil bacterial and fungal response to wildfires in the Canadian boreal forest across a burn severity gradient. Soil Biol. Biochem. 2019, 138, 107571. [Google Scholar] [CrossRef]
- Adkins, J.; Docherty, K.M.; Miesel, J.R. Copiotrophic Bacterial Traits Increase with Burn Severity One Year after a Wildfire. Front. For. Glob. Chang. 2022, 5, 873527. [Google Scholar] [CrossRef]
- Nelson, A.R.; Narrowe, A.B.; Rhoades, C.C.; Fegel, T.S.; Daly, R.A.; Roth, H.K.; Chu, R.K.; Amundson, K.K.; Young, R.B.; Steindorff, A.S.; et al. Wildfire-dependent changes in soil microbiome diversity and function. Nat. Microbiol. 2022, 7, 1419–1430. [Google Scholar] [CrossRef] [PubMed]
- Bárcenas-Moreno, G.; García-Orenes, F.; Mataix-Solera, J.; Mataix-Beneyto, J.; Bååth, E. Soil microbial recolonisation after a fire in a Mediterranean forest. Biol. Fertil. Soils 2011, 47, 261–272. [Google Scholar] [CrossRef]
- Fernandez-Gonzalez, A.J.; Martinez-Hidalgo, P.; Cobo-Diaz, J.F.; Villadas, P.J.; Martinez-Molina, E.; Toro, N.; Tringe, S.G.; Fernandez-Lopez, M. The rhizosphere microbiome of burned holm-oak: Potential role of the genus Arthrobacter in the recovery of burned soils. Sci. Rep. 2017, 7, 6008. [Google Scholar] [CrossRef] [PubMed]
- Adkins, J.; Docherty, K.M.; Gutknecht, J.L.M.; Miesel, J.R. How do soil microbial communities respond to fire in the intermediate term? Investigating direct and indirect effects associated with fire occurrence and burn severity. Sci. Total Environ. 2020, 745, 140957. [Google Scholar] [CrossRef]
- Whitman, T.; Woolet, J.; Sikora, M.; Johnson, D.B.; Whitman, E. Resilience in soil bacterial communities of the boreal forest from one to five years after wildfire across a severity gradient. Soil Biol. Biochem. 2022, 172, 108755. [Google Scholar] [CrossRef]
- Dove, N.C.; Klingeman, D.M.; Carrell, A.A.; Cregger, M.A.; Schadt, C.W. Fire alters plant microbiome assembly patterns: Integrating the plant and soil microbial response to disturbance. New Phytol. 2021, 230, 2433–2446. [Google Scholar] [CrossRef] [PubMed]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Isobe, K.; Otsuka, S.; Sudiana, I.; Nurkanto, A.; Senoo, K. Community composition of soil bacteria nearly a decade after a fire in a tropical rainforest in East Kalimantan, Indonesia. J. Gen. Appl. Microbiol. 2009, 55, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Dangi, S.R.; Stahl, P.D.; Pendall, E.; Cleary, M.B.; Buyer, J.S. Recovery of soil microbial community structure after fire in a sagebrush-grassland ecosystem. Land Degrad. Dev. 2010, 21, 423–432. [Google Scholar] [CrossRef]
- Xiang, X.; Shi, Y.; Yang, J.; Kong, J.; Lin, X.; Zhang, H.; Zeng, J.; Chu, H. Rapid recovery of soil bacterial communities after wildfire in a Chinese boreal forest. Sci. Rep. 2014, 4, 3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Valera, E.; Verdu, M.; Navarro-Cano, J.A.; Goberna, M. Resilience to fire of phylogenetic diversity across biological domains. Mol. Ecol. 2018, 27, 2896–2908. [Google Scholar] [CrossRef] [PubMed]
- Dove, N.C.; Taş, N.; Hart, S.C. Ecological and genomic responses of soil microbiomes to high-severity wildfire: Linking community assembly to functional potential. ISME J. 2022, 16, 1853–1863. [Google Scholar] [CrossRef]
- Yuste, J.C.; Barba, J.; Fernandez-Gonzalez, A.J.; Fernandez-Lopez, M.; Mattana, S.; Martinez-Vilalta, J.; Nolis, P.; Lloret, F. Changes in soil bacterial community triggered by drought-induced gap succession preceded changes in soil C stocks and quality. Ecol. Evol. 2012, 2, 3016–3031. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [Green Version]
- Schloss, P.D. Reintroducing mothur: 10 Years Later. Appl. Environ. Microbiol. 2020, 86, e02343-19. [Google Scholar] [CrossRef] [Green Version]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurdie, P.J.; Holmes, S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Comput. Biol. 2014, 10, e1003531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kassambara, A. rstatix: Pipe-Friendly Framework for Basic Statistical Tests. Available online: https://rpkgs.datanovia.com/rstatix/ (accessed on 11 February 2023).
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- McMurdie, P.J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksanen, J.; Simpson, G.; Blanchet, F.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Solymos, P.; Stevens, M.; Szoecs, E.; et al. Vegan: Community Ecology Package. Available online: https://CRAN.R-project.org/package=vegan (accessed on 1 November 2022).
- Micro4all Workflow. Available online: https://nuriamw.github.io/micro4all/tutorial/package_workflow.html#introduction (accessed on 3 April 2023).
- Cébron, A.; Norini, M.-P.; Beguiristain, T.; Leyval, C. Real-Time PCR quantification of PAH-ring hydroxylating dioxygenase (PAH-RHDα) genes from Gram positive and Gram negative bacteria in soil and sediment samples. J. Microbiol. Methods 2008, 73, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Medina, R.; Fernández-González, A.J.; García-Rodríguez, F.M.; Villadas, P.J.; Rosso, J.A.; Fernández-López, M.; Del Panno, M.T. Exploring the effect of composting technologies on the recovery of hydrocarbon contaminated soil post chemical oxidative treatment. Appl. Soil Ecol. 2020, 150, 103459. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.-X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Key, C.; Benson, N. Landscape Assessment: Ground measure of severity, the Composite Burn Index; and Remote sensing of severity, the Normalized Burn Ratio. In FIREMON: Fire Effects Monitoring and Inventory System; Rocky Mountain Research Station: Ogden, UT, USA, 2006; pp. LA1–LA5. [Google Scholar]
- De Santis, A.; Chuvieco, E. Burn severity estimation from remotely sensed data: Performance of simulation versus empirical models. Remote Sens. Environ. 2007, 108, 422–435. [Google Scholar] [CrossRef]
- Gorelick, N.; Hancher, M.; Dixon, M.; Ilyushchenko, S.; Thau, D.; Moore, R. Google Earth Engine: Planetary-scale geospatial analysis for everyone. Remote Sens. Environ. 2017, 202, 18–27. [Google Scholar] [CrossRef]
- Foga, S.; Scaramuzza, P.L.; Guo, S.; Zhu, Z.; Dilley, R.D.; Beckmann, T.; Schmidt, G.L.; Dwyer, J.L.; Joseph Hughes, M.; Laue, B. Cloud detection algorithm comparison and validation for operational Landsat data products. Remote Sens. Environ. 2017, 194, 379–390. [Google Scholar] [CrossRef] [Green Version]
- Parks, S.A.; Holsinger, L.M.; Voss, M.A.; Loehman, R.A.; Robinson, N.P. Mean Composite Fire Severity Metrics Computed with Google Earth Engine Offer Improved Accuracy and Expanded Mapping Potential. Remote Sens. 2018, 10, 879. [Google Scholar] [CrossRef] [Green Version]
- Twele, A.; Barbosa, P. Post-Fire Vegetation Regeneration. The Case Study of the “Massif de l‘Etoile” Fire. EUR 21010 EN. 2004. JRC26771. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC26771 (accessed on 10 April 2023).
- Kennedy, R.E.; Yang, Z.; Cohen, W.B. Detecting trends in forest disturbance and recovery using yearly Landsat time series: 1. LandTrendr—Temporal segmentation algorithms. Remote Sens. Environ. 2010, 114, 2897–2910. [Google Scholar] [CrossRef]
- Kennedy, R.E.; Yang, Z.; Gorelick, N.; Braaten, J.; Cavalcante, L.; Cohen, W.B.; Healey, S. Implementation of the LandTrendr Algorithm on Google Earth Engine. Remote Sens. 2018, 10, 691. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Luque, A.J.; Peinó-calero, E.; Rodríguez-Brito, A.; Esteban-Parra, M.J.; Mrino-Ceballos, M.; guerrero-Alonso, P.D.; Moreno-Llorca, R.; Ros-Candeira, A.; Zamora, R. ClimaNevada: Base de datos climática del Observatorio de Cambio Global de Sierra Nevada. Ecosistemas 2021, 30, 2155. [Google Scholar] [CrossRef]
- Curiel Yuste, J.; Fernandez-Gonzalez, A.J.; Fernandez-Lopez, M.; Ogaya, R.; Penuelas, J.; Sardans, J.; Lloret, F. Strong functional stability of soil microbial communities under semiarid Mediterranean conditions and subjected to long-term shifts in baseline precipitation. Soil Biol. Biochem. 2014, 69, 223–233. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Laudicina, V.A.; Parra, A.; Albert-Belda, E.; Moreno, J.M. Drought and its legacy modulate the post-fire recovery of soil functionality and microbial community structure in a Mediterranean shrubland. Glob. Chang. Biol. 2019, 25, 1409–1427. [Google Scholar] [CrossRef]
- Villadas, P.J.; Díaz-Díaz, S.; Rodríguez-Rodríguez, A.; del Arco-Aguilar, M.; Fernández-González, A.J.; Pérez-Yépez, J.; Arbelo, C.; González-Mancebo, J.M.; Fernández-López, M.; León-Barrios, M. The Soil Microbiome of the Laurel Forest in Garajonay National Park (La Gomera, Canary Islands): Comparing Unburned and Burned Habitats after a Wildfire. Forests 2019, 10, 1051. [Google Scholar] [CrossRef] [Green Version]
- Pulido-Chavez, M.F.; Randolph, J.W.J.; Zalman, C.; Larios, L.; Homyak, P.M.; Glassman, S.I. Rapid bacterial and fungal successional dynamics in first year after chaparral wildfire. Mol. Ecol. 2023, 32, 1685–1707. [Google Scholar] [CrossRef] [PubMed]
- Gerna, D.; Clara, D.; Allwardt, D.; Mitter, B.; Roach, T. Tailored Media Are Key to Unlocking the Diversity of Endophytic Bacteria in Distinct Compartments of Germinating Seeds. Microbiol. Spectr. 2022, 10, e00172-22. [Google Scholar] [CrossRef]
- Xiao, J.; Lin, G.; Cao, Z.; Chu, S.; Cui, L.; Yang, Y.; Wu, X. A shallow constructed wetland combining porous filter material and Rotala rotundifolia for advanced treatment of municipal sewage at low HRT. Environ. Sci. Pollut. Res. Int. 2022, 30, 27593–27602. [Google Scholar] [CrossRef] [PubMed]







| EFFIS Thresholds | Severity Level |
|---|---|
| dNBR < 0.100 | Unburned/Very Low |
| 0.100 ≤ dNBR ≤ 0.255 | Low |
| 0.256 ≤ dNBR ≤ 0.419 | Moderate |
| 0.420 ≤ dNBR ≤ 0.660 | High |
| dNBR > 0.660 | Very High |
| UOF | BOF | |||
|---|---|---|---|---|
| Soil Variables | 3 yaf | 9 yaf | 3 yaf | 9 yaf |
| Clay (%) | 21.00 ± 2.08 | 14.53 ± 1.46 | 20.50 ± 1.53 | 13.5 ± 0.78 |
| Sand (%) | 45.74 ± 5.51 | 46.91 ± 5.47 | 49.54 ± 6.51 | 47.36 ± 8.67 |
| Silt (%) | 33.26 ± 4.16 | 38.56 ± 4.09 | 29.96 ± 6.56 | 39.14 ± 9.20 |
| Textural class | Loam | Loam | ||
| pH (H2O) | 6.10 ± 0.10 a | 5.77 ± 0.15 a | 7.60 ± 0.10 b | 7.23 ± 0.06 b |
| Available water (%) | 17.11 ± 2.52 | 15.22 ± 2.06 | 16.43 ± 3.75 | 19.52 ± 4.65 |
| EC 1 (mmhos/cm3) | 0.14 ± 0.04 | 0.12 ± 0.05 | 0.22 ± 0.03 | 0.19 ± 0.03 |
| Organic matter (%) | 7.61 ± 2.00 | 7.28 ± 2.90 | 4.54 ± 0.75 | 5.35 ± 0.69 |
| Total N (%) | 0.37 ± 0.17 | 0.37 ± 0.19 | 0.23 ± 0.04 | 0.30 ± 0.04 |
| C/N | 11.95 ± 3.69 | 11.58 ± 2.22 | 11.19 ± 0.28 | 10.17 ± 0.25 |
| Available Pi (ppm) | 8.00 ± 4.44 a | 23.00 ± 17.35 ab | 5.23 ± 0.83 a | 60.33 ± 14.05 b |
| K (ppm) | 445.23 ± 41.07 | 356.67 ± 41.63 | 330.12 ± 47.29 | 343.33 ± 47.26 |
| UOF | BOF | |||
|---|---|---|---|---|
| PAHs | 3 yaf | 9 yaf | 3 yaf | 9 yaf |
| Acenaphthene | 0.17 ± 0.16 | 0 ± 0 | 0.07 ± 0.13 | 0 ± 0 |
| Acenaphthylene | 0 ± 0 | 0.06 ± 0.06 | 0 ± 0 | 0.02 ± 0.03 |
| Anthracene | 2.65 ± 0.65 | 0.32 ± 0.25 | 2.45 ± 1.35 | 0.09 ± 0.04 |
| Benzo(g,h,i)perylene | 0.62 ± 0.40 | 0.33 ± 0.28 | 0.80 ± 0.41 | 0.16 ± 0.04 |
| Benzo-a-anthracene | 0.36 ± 0.17 | 0.19 ± 0.08 | 0.82 ± 0.28 | 0.16 ± 0.03 |
| Benzo-a-pyrene | 0.56 ± 0.24 | 0.25 ± 0.16 | 0.74 ± 0.49 | 0.12 ± 0.03 |
| Benzo-b,k-fluoranthene | 1.76 ± 1.20 | 0.86 ± 0.46 | 2.73 ± 1.21 | 0.62 ± 0.14 |
| Chrysene | 1.16 ± 0.65 | 0.47 ± 0.26 | 3.35 ± 1.54 | 0.63 ± 0.36 |
| Dibenzo(a,h)anthracene | 0.34 ± 0.23 | 0.3 ± 0.22 | 0.49 ± 0.28 | 0.10 ± 0.03 |
| Phenanthrene | 2.28 ± 0.63 | 0.82 ± 0.07 | 4.51 ± 1.25 | 0.57 ± 0.13 |
| Fluoranthene | 3.61 ± 2.05 | 1.76 ± 1.02 | 7.76 ± 3.1 | 0.88 ± 0.16 |
| Fluorene | 0.42 ± 0.09 | 0.16 ± 0.02 | 0.57 ± 0.33 | 0.12 ± 0.02 |
| Indeno(1,2,3-cd)pyrene | 0.34 ± 0.23 | 0.30 ± 0.22 | 0.51 ± 0.27 | 0.09 ± 0.01 |
| Naphthalene 1 | 1.15 ± 0.07 a | 0.56 ± 0.04 b | 2.18 ± 0.98 ab | 0.48 ± 0.05 b |
| Pyrene | 3.23 ± 1.87 | 1.54 ± 0.86 | 9.12 ± 4.10 | 0.90 ± 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-González, A.J.; Lasa, A.V.; Cobo-Díaz, J.F.; Villadas, P.J.; Pérez-Luque, A.J.; García-Rodríguez, F.M.; Tringe, S.G.; Fernández-López, M. Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils. Forests 2023, 14, 1383. https://doi.org/10.3390/f14071383
Fernández-González AJ, Lasa AV, Cobo-Díaz JF, Villadas PJ, Pérez-Luque AJ, García-Rodríguez FM, Tringe SG, Fernández-López M. Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils. Forests. 2023; 14(7):1383. https://doi.org/10.3390/f14071383
Chicago/Turabian StyleFernández-González, Antonio J., Ana V. Lasa, José F. Cobo-Díaz, Pablo J. Villadas, Antonio J. Pérez-Luque, Fernando M. García-Rodríguez, Susannah G. Tringe, and Manuel Fernández-López. 2023. "Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils" Forests 14, no. 7: 1383. https://doi.org/10.3390/f14071383
APA StyleFernández-González, A. J., Lasa, A. V., Cobo-Díaz, J. F., Villadas, P. J., Pérez-Luque, A. J., García-Rodríguez, F. M., Tringe, S. G., & Fernández-López, M. (2023). Long-Term Persistence of Three Microbial Wildfire Biomarkers in Forest Soils. Forests, 14(7), 1383. https://doi.org/10.3390/f14071383








